Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Sheikh Mansoor.
Molecules that possess at least one atom of oxygen and have unpaired electrons are referred to as reactive oxygen species (ROS). These contain singlet hydroxyl, oxygen, and hydroperoxyl radicals. ROS are formed due to the incomplete decomposition of molecular oxygen like hydroxyl radicals (OH), hydrogen peroxide (H2O2), superoxide radical anion (O2−), and ozone (O3).
- heavy metals
- toxicity
- stress
- signaling
- ROS
- mitochondrial dysfunction
1. Introduction
Molecules that possess at least one atom of oxygen and have unpaired electrons are referred to as reactive oxygen species (ROS). These contain singlet hydroxyl, oxygen, and hydroperoxyl radicals [1,2][1][2]. ROS are formed due to the incomplete decomposition of molecular oxygen like hydroxyl radicals (OH), hydrogen peroxide (H2O2), superoxide radical anion (O2−), and ozone (O3) [3]. ROS, being essential for various signaling pathways, are generated within cells produced via a wide range of physiological and biochemical processes. In aerobic life, reactive active species tolerate an essential chemical entity. In plants, any variations in enzymes and cellular structure, nucleic acid, or proteins, increase the production of ROS. In plants, ROS scavenging pathways include enzymatic and non-enzymatic action regulating the production of ROS [4,5,6][4][5][6]. Therefore, under unfavorable conditions, the higher plants are induced to produce ROS to promote an extensive range of physiological variations. Production of ROS under the impact of drought, salinity, heavy metal toxicity, etc., leads among others, to the degradation of antioxidants, ultimately induces the gene expression of antioxidative response genes, and may damage macromolecules as lipids (lipid peroxidation) or the DNA [7,8,9,10,11][7][8][9][10][11].
Anthropogenic activities like urbanization and industrialization are accompanied by the release of heavy metal pollutants into the environment, which in turn disturbs plant development and physiology by phytotoxicity [9]. Additionally, secondary metabolites counterattack ROS to maintain balance. For example, vitamins, terpenes, and polyphenols are chief secondary metabolites to counterattack ROS and inhibit oxidative strain in plants [7,10][7][10]. Presently the heavy metal toxicity to plant physio-chemical activities are highly concerning due to its toxicity accumulation in the food chain [12,13][12][13]. To reduce oxidative stress due to heavy metals, plants have evolved several mechanisms such as increased root extraction of metals, prohibiting metal entrance into the plant, preventive toxic metal accretion, chelation by organic compounds sequestration in vacuoles, and metal binding in the cell wall to stop the entry [14] and ROS are converted to lesser toxic compounds [15,16][15][16].
Heavy metals naturally exist in the Earth’s crust with varying concentrations and are not easily broken down into less toxic compounds through metabolic processes. When present in the soil, they persist for extended periods, causing harmful impacts on both the environment and human health [17,18][17][18]. In spite of these external effects, heavy metals alter many physiological processes, nutritional status of minerals, rate of photosynthesis and respiration, enzymatic processes, and many biochemical processes. However, on exposure of plants to toxic concentrations of heavy metals, more production of reaction oxygen species (ROS) is considered the most prompt effect so far. Heavy metals interact with various components in the electron transport chain thereby altering its activity and leading to ROS generation mainly in chloroplast and mitochondria [19,20,21][19][20][21]. With the rise in ROS level, the membrane potential gets imbalanced further inducing leakage of ion channels, lipid peroxidation, and destruction of macromolecules. Moreover, the toxicity of heavy metals liberating ROS on subcellular organelles may alter depending upon certain factors like duration of stress, developmental stage, dose/concentration of particular heavy metal, and plant organ involved [22,23][22][23].
2. Mechanism of ROS Production
In living cells, metabolic processes can give rise to ROS as by-products, and their enhanced level leads to a state of oxidative burst. In plants, recent studies have demonstrated an interest in the dual function of ROS [19,26,27,28][19][24][25][26]. In addition to its detrimental effects, ROS plays a significant role in signaling molecules to regulate the process of cell growth and development, apoptosis as well as stimulation of systemic response under biotic and abiotic stress. As a result, enhanced level of ROS with disturbance in cellular redox potential leads to manifold oxidative stress responses in plants. The stress responses are modulated by the type of heavy metal, its uptake, and its concentration [29,30,31][27][28][29]. On the perception of stress signal, plant adapts various mechanisms to adapt and survive under severely adverse conditions. The generation and accumulation of ROS, hydroxyl radical, singlet oxygen, superoxide anion, and hydrogen peroxide act as a secondary industry in signaling pathways by regulating the process of stomatal closure, apoptosis, and senescence thereby affecting the plant growth and development [32,33,34][30][31][32]. On exposure to heavy metal stress, an alarming level of oxidative burst due to elevated ROS generation arises. This will further lead to the activation of transcription factors, and altered gene expression related to the defense mechanism. The condition of oxidative burst causes severe damage to macromolecules like DNA and proteins due to which the normal functioning of plants, as well as animals, gets disturbed [35,36,37][33][34][35]. The concentration of ROS is the key event in signaling, the higher conc. is mainly responsible for programmed cell death whereas it acts as a signal in mediating the stress response in lower/moderate concentrations [8,38,39][8][36][37]. The presence of oxidoreductases, specifically NADPH oxidase at the plasma membrane, is the chief source of ROS generation in plants. It accepts the electron from NADPH and stimulates the production of superoxide radicals in the apoplastic region that is further responsible for diverse biological processes as well as signal transduction. The NADPH oxidases are categorized as respiratory burst oxidase homologs (Rboh)—the fundamental enzymes required for the transfer of electrons from NADPH to O2 which thereby stimulate the generation of superoxide radical followed by dismutation into H2O2 [40][38]. As CDPKs lie upstream of NADPH oxidases, hence cause their phosphorylation on sensing heavy metal stress. This leads to the subsequent elevation of apoplastic superoxide and activation of MAPK cascade for downstream signaling [40,41,42,43][38][39][40][41]. A study proposed by [44][42] noted the NADPH oxidase activity only after 6 and 24 h of cadmium stress in plants. Whereas the activation of the MAPK cascade gene was visualized after a period of 3 h.3. Antioxidative Defense System in Plant Cell Components
To scavenge ROS, plants have a sophisticated antioxidative defense system that includes both non-enzymatic and enzymatic components [6,31,34,36][6][29][32][34]. Different organelles in plant cells, such as chloroplasts, mitochondria, and peroxisomes, have distinct ROS-generating and scavenging systems. Different cellular compartments’ ROS scavenging processes are coordinated. Potentially harmful oxygen metabolites are produced at a low level under normal settings, and there is an optimal balance between ROS production and quenching [36][34]. Several negative environmental conditions can disrupt the equilibrium between ROS production and quenching, resulting in fast increases in intracellular ROS levels. ROS are created in larger quantities in cellular compartments in the presence of transition heavy metals within the cell and many other environmental stress conditions, causing oxidative stress in plants and causing damage to cell protein, lipids, and nucleic acid [7,13,36,38,45,46,47][7][13][34][36][43][44][45]. Cells employ enzymatic (e.g., CAT, GSH, SOD, APX, GPX) and non-enzymatic antioxidants (e.g., ascorbate, carotenoids, flavonoids, phenolics) to counter ROS damage (Figure 1). It was also discovered that antioxidants such as superoxide dismutase (SOD) and glutathione reductase (GR), ascorbate peroxidase (APX), and peroxidase (POD) are produced inside plants as a result of metal build-up. Additionally, research has shown that plants create more enzymatic and non-enzymatic antioxidants as a natural defense against exposure to increased concentrations of heavy metals and other abiotic stressors [31,34,48][29][32][46]. It was also observed that antioxidants such as superoxide dismutase and glutathione reductase, ascorbate peroxidase, and peroxidase are created within the plant as a result of metal build-up. Together, these systems protect cells from oxidative stress [49][47].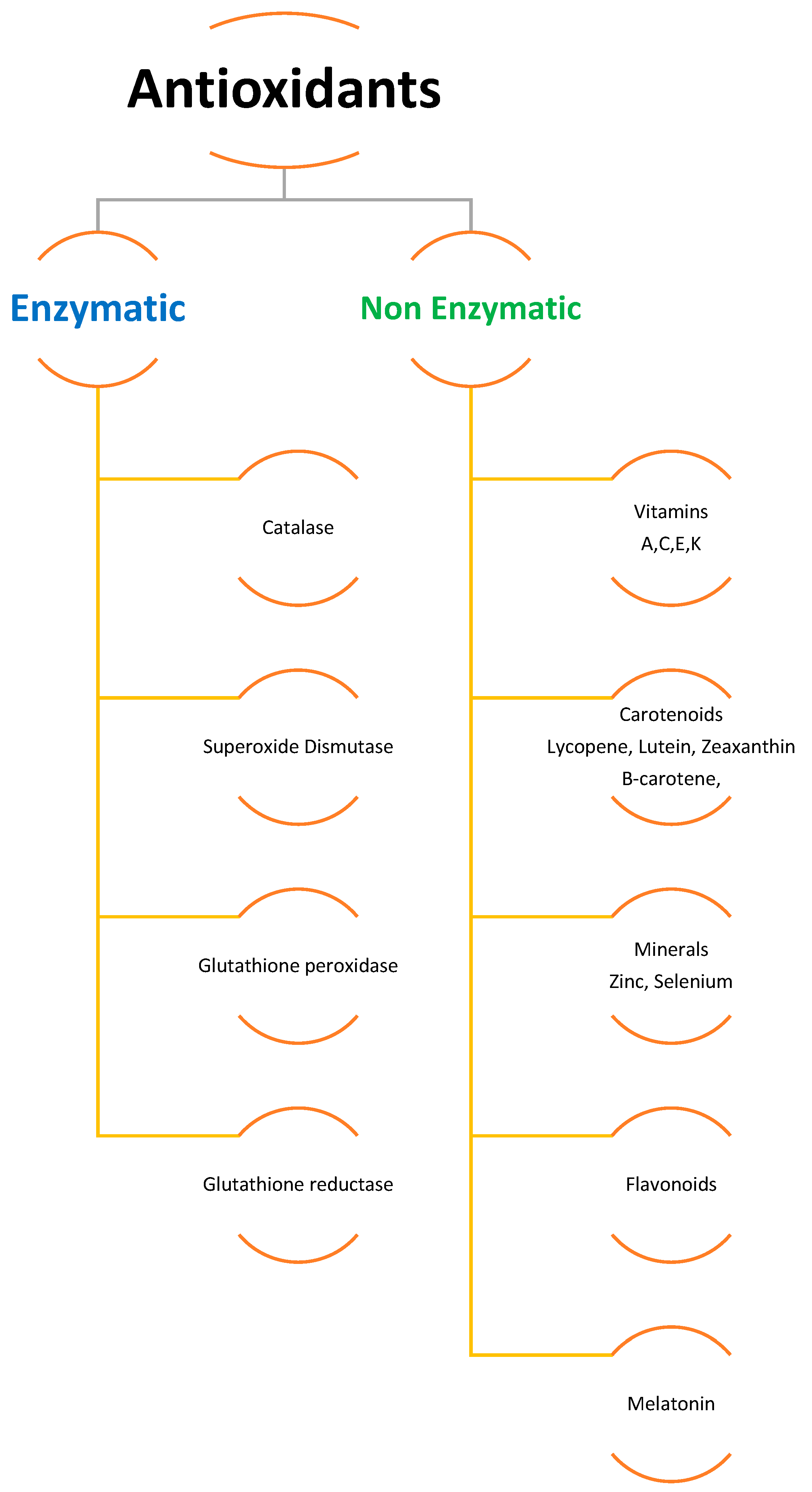
Figure 1.
Enzymatic and non-enzymatic antioxidants.
Table 1.
Enzymatic and non-enzymatic antioxidants against heavy metal stress.
| S. No | Enzymatic Antioxidants | Mode of Action |
|---|---|---|
| 1. | Superoxide Dismutase (SOD) | SOD is an enzyme that converts superoxide radicals (O2−) into hydrogen peroxide (H2O2) and oxygen (O2), preventing the accumulation of harmful superoxide radicals that can damage cells through oxidative stress. |
| 2. | Catalase | Catalase is an enzyme that transforms hydrogen peroxide (H2O2) into water and oxygen, effectively countering the potential toxicity of excess hydrogen peroxide, particularly in the presence of heavy metals. |
| 3. | Glutathione Peroxidase (GPx) | GPx is an enzyme that employs reduced glutathione (GSH) to convert hydrogen peroxide and lipid hydroperoxides into water and corresponding alcohols. Its vital role lies in safeguarding cells against oxidative damage triggered by heavy metals. |
| 4. | Peroxiredoxins | Peroxiredoxins are enzymes that neutralize peroxides, like hydrogen peroxide, using thiol groups in their active sites. They help detoxify ROS from heavy metal exposure. |
| 5. | Glutathione Reductase (GR) | GR is an enzyme that regulates reduced glutathione (GSH) levels by converting oxidized glutathione (GSSG) to its reduced form. This is crucial for upholding cellular redox equilibrium during heavy metal stress. |
| 6. | NAD(P)H Quinone Oxidoreductase 1 (NQO1) | NQO1 is an enzyme that detoxifies by reducing quinones and electrophilic substances, safeguarding cells from oxidative damage due to heavy metals and pollutants. |
| 7. | Selenium-Containing Enzymes | Selenium is in enzymes like glutathione peroxidases and thioredoxin reductases, crucial for antioxidant defense and redox regulation. They counter heavy-metal-triggered oxidative stress. |
| 8. | Cytochrome P450 Enzymes | Certain cytochrome P450 enzymes metabolize heavy metals, converting them into safer forms. This aids in detoxification and defending against heavy metal stress. |
| Non-Enzymatic Antioxidants | ||
| 9. | Glutathione (GSH) | Glutathione, a tripeptide (γ-glutamyl-cysteinyl-glycine), is a key intracellular antioxidant. It helps detoxify heavy metals by binding to them and aiding in their elimination. GSH also supports specific detoxification enzymes as a cofactor. |
| 10. | Ascorbic Acid (Vitamin C) | Vitamin C, a water-soluble antioxidant, neutralizes ROS, shielding cells from heavy-metal-triggered oxidative harm. It also indirectly boosts other antioxidants like GSH and vitamin E. |
| 11. | α-Tocopherol (Vitamin E) | Vitamin E, a lipid-soluble antioxidant, safeguards cell membranes by neutralizing lipid peroxyl radicals. It upholds membrane integrity during heavy metal stress. |
| 12. | Carotenoids | Carotenoids like β-carotene, lutein, and zeaxanthin are plant pigments with antioxidants. They counter ROS and shield cells from oxidative harm due to heavy metals. |
| 13. | Phenolic Compounds | Various phenolic compounds, such as flavonoids and polyphenols, are known for their antioxidant properties. They can scavenge ROS and chelate heavy metals, reducing their toxic effects. |
| 14. | Metal Chelators | Certain non-enzymatic antioxidants can bind to heavy metals, creating stable complexes that decrease reactivity and toxicity. Chelators like EDTA and citric acid, for instance, aid in trapping heavy metals and aiding their removal. |
| 15. | Selenium (Se) | Selenium, an essential trace element, functions as an antioxidant and can counteract heavy metal toxicity. Supplementation with selenium has been found to ease oxidative stress caused by heavy metals. |
| 16. | Melatonin | Melatonin, an indoleamine, functions as a potent antioxidant by scavenging ROS, safeguarding cells from oxidative harm due to heavy metals. |
4. Heavy Metal Stress Signaling Events in Plants
Plants in response to heavy metal stress, initiate a manifold of signaling events that are mainly comprised of recognizing the signal, activation of downstream components and thereby neutralizing the detrimental effects. These events eventually regulate the cellular response at physiological, biochemical, and molecular levels [29,36,52,63][27][34][50][61]. Heavy metal stress is known to regulate a complex of signaling cascades like calcium/calmodulin-dependent, ROS-mediated signaling, hormone signaling, and mitogen-activated protein kinases (MAPK) that further mediate the phosphorylation of certain genes [38][36]. Heavy metals enter the plant system mainly through roots via diffusion with the cell wall binding to heavy metal ions at the sites with negative charge including –COOH, -OH, and -SH. Altered cell wall constituents may lead to the destruction of the cell membrane. Moreover, the complex formed by the interaction of heavy metal and functional groups may cause the disruption of plasma membrane integrity [53,64,65,66][51][62][63][64]. On perceiving the signal, various defense responses are triggered downstream. In a proteomic study conducted by [46][44] T. Liu et al. (2014), cell walls of E. splendens under copper stress indicated 40% of cell wall proteins in abundance that are specifically involved in cell wall modulation, antioxidant defense, and many other metabolic processes. Whereas the remaining 60% of these differentially expressed proteins are inadequate and play a role in translation, cell signaling, and energy synthesis. Another group of G-proteins (Hsp70 and RAS) has demonstrated a significant role in signal transduction under copper stress. The overall study demonstrated the potential of cell walls in inducing heavy metal stress signaling responses downstream [2,67][2][65]. Furthermore, the role of cell walls in signaling is spotlighted with the findings of integral membrane proteins namely aquaporins and kinases. The aquaporin gating was detected immediately upon exposure to heavy metals in the case of onion epidermal cells regardless of the type of metal [68,69,70][66][67][68]. The findings also suggest that a high concentration of zinc downregulates specifically AQUA1 (mercury-sensitive aquaporin). As per the observations, zinc stress leads to the regulation of intracellular signaling, post-translational modifications with the relocalization of AQUA1 [71][69]. Another component is secretory vesicles present beneath the plasma membrane which also contributes to the disruption of the cell wall under heavy metal stress. Consequently, it causes cell growth inhibition; therefore, it is necessary to understand the vesicular system as well as how it affects cell wall signaling [72,73][70][71]. The interaction among intracellular trafficking proteins namely molecular motors and cytoskeletal elements, microtubules, actin filaments, and intermediate filaments is responsible for vesicle transport. A study based on vesicle trafficking in root hairs of A. thaliana demonstrated the prominent role of cytoplasmic calcium gradient with actin filaments [74][72]. Treating root hairs specifically with cadmium using FM4-64 dye as fluorescence labeling, a clear disruption of endocytosis and membrane recycling was determined in A. thaliana. To better understand the reason behind the disruption, the study was applying in vivo labeling using confocal microscopy. The results obtained demonstrated the altered longitudinal positioning of actin filaments which is modified to the transverse arrangement, thereby affecting the vesicular trafficking system. Furthermore, it was found that cadmium imitates the action of calcium by binding to gelsolin, an actin-modulating protein [75,76][73][74]. In the root hairs of A. thaliana, the disruption of calcium channels, depolymerization of actin filaments as well as diminished vesicle trafficking were the key findings [77,78][75][76]. In Funaria hygrometrica, the process of internalization of pectin in the apical tip under lead stress specifically has been evaluated, by targeting pectin epitope JIM5-P due to its high affinity for lead using FM4-64 dye and immunogold labeling. The overall study reported by [79][77] Krzesłowska, demonstrated that lead accumulation causes enhancement of vesicular trafficking with the internalization of JIM5-P in the plasma membrane as well as in different vesicles. This pectin-based signaling is regarded as a universal response in other plant species on exposure to lead stress [79,80,81][77][78][79]. After the root system, heavy metals enter the plasma membrane either by diffusion or by transporters and thereby downstream signal transduction occurs. Calcium sensors and potassium transporters are the known sensing receptors embedded in the plasma membrane of A. thaliana. Both are commonly known as transceptors [82,83][80][81]. In addition to this, NRT1 and SULTR1 are responsible for sensing nitrate and sulfur, respectively, in plants [84,85][82][83]. Apart from sensors and receptors, calcium-dependent protein kinases (CDPKs) also play a vital role in heavy metal signaling. On perceiving numerous stress responses, CDPKs transduce the signal to activate the downstream phosphorylation cascade and other proteins like NADPH oxidase, membrane channels, and transcription factors [86][84]. In leaves of Cucurbita pepo and seedlings of Setaria italica under nickel and chromium stress, respectively, overexpression of CDPKs gene expression has been detected. Moreover, CDPKs are essential components for stimulating the action of mitogen-activated protein kinases (MAPK) in the case of cadmium and copper stress by developing a close association between CDPKs and MAPKS, thereby providing a platform for subsequent signaling under heavy metal stress. MAPKs like CDPKs are localized at different cellular locations like cytosol, nucleus, microtubules, and plasma membrane. Herein the stress signal is directly transmitted to the nucleus. In Nicotiana tabacum under cadmium stress, upregulation of MAPK, as well as elevated tolerance level, has been detected [36,87,88,89,90][34][85][86][87][88]. Besides this, it also regulates the gene expression related to cell cycle events in Oryza sativa. Furthermore, in a study proposed by Xu et al. (2019) MAPK transcript was analyzed via real-time quantitative polymerase chain reaction (RT-PCR) in roots of B. papyrifera under cadmium stress. The results collected were with respect to time and clearly indicated the downregulation and upregulation of MAPK transcript at time periods of 3 h and 6 h, respectively [91,92][89][90]. This led the researchers to a clear conclusion about how MAPK’s recovery performs a significant task in downstream signaling events and also creates deleterious effects on plants on prolonged suppression of MAPK (Figure 2).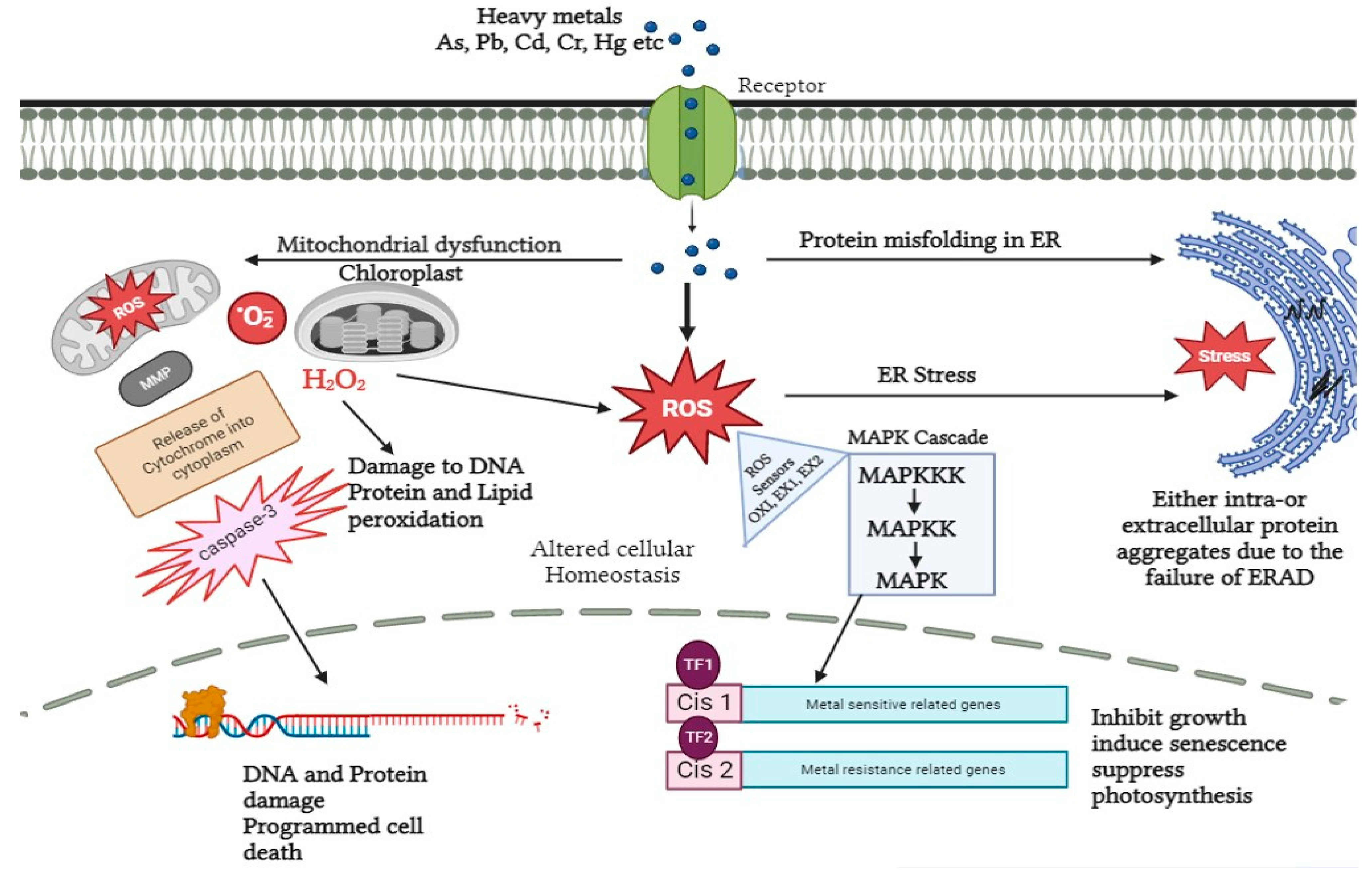
Figure 2. Signal transduction in response to heavy metal stress in plants. Heavy metals can affect ROS accumulation via dysfunction in the mitochondria and chloroplasts, which results in dysfunction in the degradation of ER-associated proteins and accumulation of aggregates. The accumulated ROS is detected by sensor (OX1, EX1, EX2) proteins and used as a signal to induce MAP Kinase cascade, which in turn causes the activation and inhibition of various downstream pathways.
5. Heavy Metal Mitigation Strategy
Heavy metal pollution including Cd, Cu, Zn, Ni, Co, Cr, Pb, and As has been detected in cultivated fields in many parts of the world. Long-term usage of phosphatic fertilizers, industrial wastewater sludge discharge, dust from anthropogenic sources, and inadequate irrigation practices in agricultural areas are all potential sources [96][94]. When plants are exposed to high quantities of heavy metals, all are potential sources of reactive oxygen species (ROS). Various metals either directly or indirectly generate ROS through Haber–Weiss reactions or overproduce ROS, causing oxidative stress in plants as an indirect result of heavy metal toxicity [6,97][6][95]. While Co, Cu, Fe, Mn, Mo, Ni, V, and Zn constitute merely a trace need for life, large concentrations of these metals can be harmful. Through absorption at the primary producer level and later consumption at the consumer level, metals are accumulated in the ecological food chain [31,72][29][70]. In aquatic environments, the plant body is exposed to these ions, and particles that are deposited on the foliar surfaces cause heavy metals to be directly integrated into the leaves [34,98][32][96]. Plants, on the other hand, have evolved a potentially useful strategy to mitigate environmental heavy metal toxicity. Low-molecular-weight thiols are produced by plants and have a great attraction to potentially deleterious metals. The two most significant low-molecular-weight biological thiols are cysteine and glutathione (GSH). GSH is a glutamate-cysteine-glycine tripeptide thiol that contains sulfur. The production of GSH is catalyzed by the ATP-dependent enzymes glutamylcysteine synthetase (GSH1) and glutathione synthetase (GSH2) [99,100][97][98]. GSH is essential for heavy metal detoxification, such as cadmium and nickel, and is a precursor to phytochelatin synthesis [21,101][21][99]. Phytochelatins are cysteine-rich polypeptides with a general structure of (-Glu-Cys) n Gly (n = 2−11) that bind heavy metals. PCs can be found in fungi and other species in addition to plants. The enzyme phytochelatin synthase catalyzes their synthesis (PCS). In the cytosol, PCs form complexes with harmful metal ions and then transport them into the vacuole [102][100]. As a result, these compounds protect plants from the harmful effects of heavy metals. Different types of environmental stresses, such as strong exposure, increasing or decreasing temperature, salinity, dehydration, nutritional deficiencies, and pathogen attack, cause the production of ROS in plants. Antioxidants, antioxidative enzymes, and other minute compounds have evolved in plants and other living beings to safely dissipate ROS [8]. Oxidative stress is caused by an imbalance between ROS generation and their removal by enzymatic and non-enzymatic processes. Photo oxidative damage to DNA, proteins, and lipids occurs as a result of increased net ROS generation, leading to cell death. ROS are also involved in pathogen defensive responses such as hypersensitive reaction and systemic acquired resistance, as well as stress hormone synthesis, desensitization, and programmed cell death [13,64,91,103,104,105,106,107,108][13][62][89][101][102][103][104][105][106].6. Heavy Metal Remediation for Plant Growth Improvement
To protect and restore soil ecosystems contaminated by heavy metals, it is crucial to understand their characteristics and remediation. Soil characterization provides insights into heavy metal speciation and bioavailability. Remediation efforts require knowledge of contamination sources, chemical properties, and associated environmental and health risks. Risk assessment is a valuable tool for the cost-effective management of contaminated sites, ensuring the preservation of public and ecosystem health [109][107]. Remediation techniques for heavy-metal-contaminated sites encompass various approaches, including physical, chemical, and biological methods. Physical methods involve actions like excavation and containment to physically remove or isolate contaminated soil. Chemical treatments employ precipitation and ion exchange to modify the chemical properties of the contaminants. Biological methods, such as phytoremediation, utilize metal-accumulating plants to extract and accumulate heavy metals from the soil [110][108]. The selection of a specific remediation method depends on several factors, including the type and concentration of the heavy metal, prevailing environmental conditions, and the desired level of remediation. Immobilization, soil washing, and phytoremediation techniques are commonly regarded as some of the most effective and well-established technologies for remediating heavy-metal-contaminated sites. Immobilization aims to reduce heavy metal mobility by altering their chemical form, while soil washing involves removing contaminants through the use of chemical or physical agents (Figure 3). Phytoremediation exploits the ability of certain plants to absorb and accumulate heavy metals, effectively mitigating contamination in the soil [111,112,113][109][110][111]. Biochar is a carbon-rich material produced from biomass through a process called pyrolysis. It has gained attention as a potential tool for heavy metal remediation in soil and water due to its unique properties. Biochar can effectively sorb heavy metals, reducing their bioavailability and mobility in the environment [114][112]. Biochar is a substance that is created through the pyrolysis process of biomass derived from sources such as agriculture, sewage, and animal wastes. Several studies have indicated that biochar has the ability to trap and store pollutants in soil. Additionally, biochar has the capacity to modify the physical and chemical properties of soil in various ways. It can enhance the soil’s ability to retain water, facilitate the uptake of water and minerals by plants, absorb heavy metals, improve soil fertility, and promote a healthy microbiome [92,115,116,117][90][113][114][115].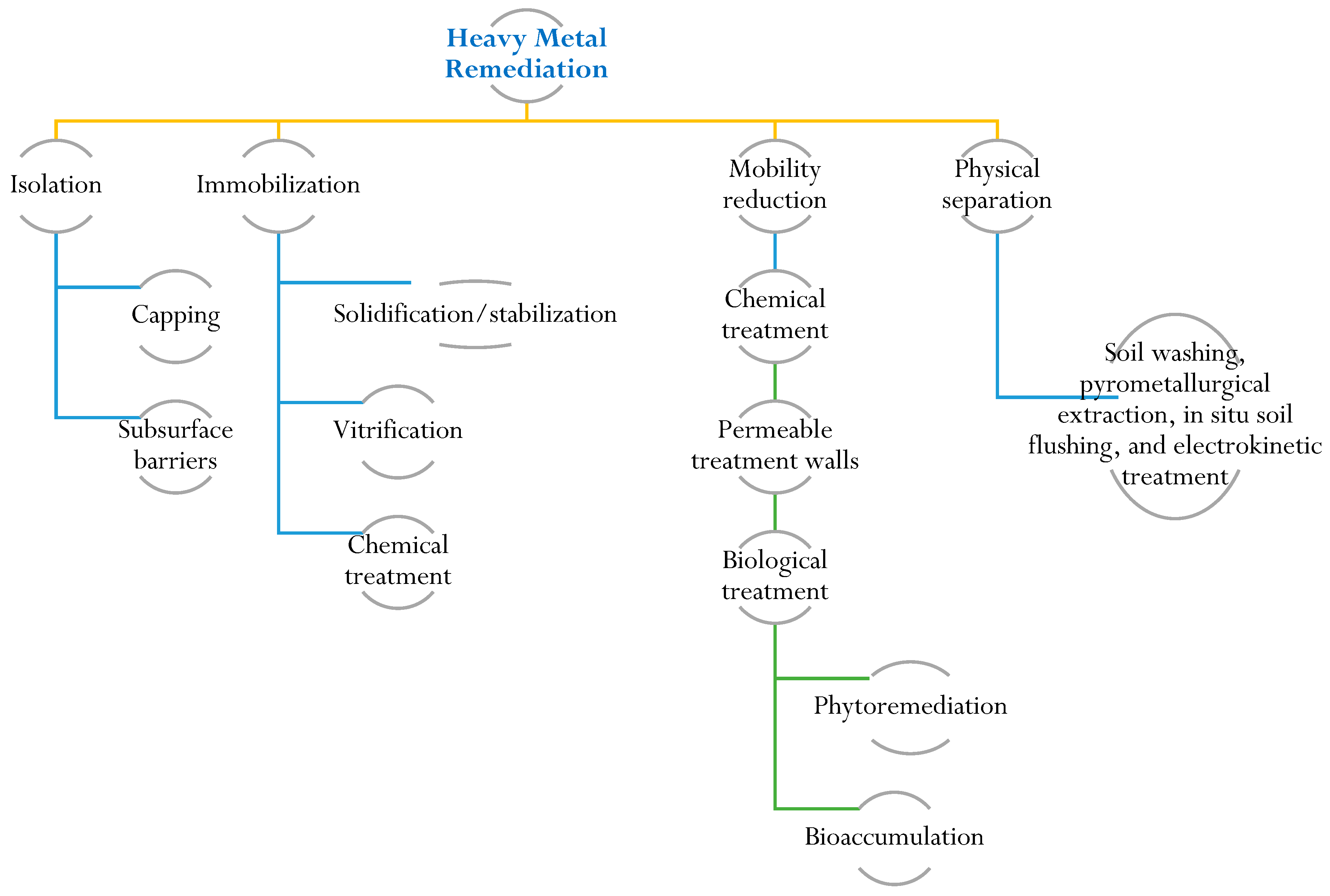
Figure 3. Illustration of remediation techniques for heavy metal contamination. Remediation techniques for heavy-metal-contaminated sites include physical, chemical, and biological methods.
References
- Gerschman, R.; Gilbert, D.L.; Nye, S.W.; Dwyer, P.; Fenn, W.O. Oxygen Poisoning and X-Irradiation: A Mechanism in Common. Science 1954, 119, 623–626.
- Liu, Y.; Zhao, H.; Yin, K.; Guo, M.; Wang, Y.; Wang, D.; Zong, H.; Xing, M. The Protective Effect of Zn2+ on As3+ Toxicity in Common Carp: Resistance to Oxidative Stress, Inhibition of Endoplasmic Reticulum Stress, Apoptosis and Autophagy. Aquaculture 2022, 546, 737375.
- Nathan, C.; Ding, A. SnapShot: Reactive Oxygen Intermediates (ROI). Cell 2010, 140, 951–951.e2.
- Jan, B.; Bhat, T.A.; Sheikh, T.A.; Wani, O.A.; Bhat, M.A.; Nazir, A.; Fayaz, S.; Mushtaq, T.; Farooq, A.; Wani, S.; et al. Agronomic Bio-Fortification of Rice and Maize with Iron and Zinc: A Review. Int. Res. J. Pure Appl. Chem. 2020, 21, 28–37.
- Mansoor, S.; Sharma, V.; Mir, M.A.; Mir, J.I.; Un Nabi, S.; Ahmed, N.; Alkahtani, J.; Alwahibi, M.S.; Masoodi, K.Z. Quantification of Polyphenolic Compounds and Relative Gene Expression Studies of Phenylpropanoid Pathway in Apple (Malus Domestica Borkh) in Response to Venturia Inaequalis Infection. Saudi J. Biol. Sci. 2020, 27, 3397–3404.
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225.
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of Secondary Metabolites and Brassinosteroids in Plant Defense Against Environmental Stresses. J. Plant Growth Regul. 2013, 32, 216–232.
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Zahid Nabi, S.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant Drought Stress Tolerance: Understanding Its Physiological, Biochemical and Molecular Mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 1912–1925.
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The Uptake and Bioaccumulation of Heavy Metals by Food Plants, Their Effects on Plants Nutrients, and Associated Health Risk: A Review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799.
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037.
- Hendrix, S.; Dard, A.; Meyer, A.J.; Reichheld, J.-P. Redox-Mediated Responses to High Temperature in Plants. J. Exp. Bot. 2023, 74, 2489–2507.
- An, J.; Jeong, S.; Moon, H.S.; Jho, E.H.; Nam, K. Prediction of Cd and Pb Toxicity to Vibrio Fischeri Using Biotic Ligand-Based Models in Soil. J. Hazard. Mater. 2012, 203–204, 69–76.
- Whiteside, J.R.; Box, C.L.; McMillan, T.J.; Allinson, S.L. Cadmium and Copper Inhibit Both DNA Repair Activities of Polynucleotide Kinase. DNA Repair 2010, 9, 83–89.
- Tang, K.; Zhan, J.-C.; Yang, H.-R.; Huang, W.-D. Changes of Resveratrol and Antioxidant Enzymes during UV-Induced Plant Defense Response in Peanut Seedlings. J. Plant Physiol. 2010, 167, 95–102.
- Álvarez, R.; Del Hoyo, A.; García-Breijo, F.; Reig-Armiñana, J.; Del Campo, E.M.; Guéra, A.; Barreno, E.; Casano, L.M. Different Strategies to Achieve Pb-Tolerance by the Two Trebouxia Algae Coexisting in the Lichen Ramalina Farinacea. J. Plant Physiol. 2012, 169, 1797–1806.
- Mangal, V.; Lal, M.K.; Tiwari, R.K.; Altaf, M.A.; Sood, S.; Kumar, D.; Bharadwaj, V.; Singh, B.; Singh, R.K.; Aftab, T. Molecular Insights into the Role of Reactive Oxygen, Nitrogen and Sulphur Species in Conferring Salinity Stress Tolerance in Plants. J. Plant Growth Regul. 2023, 42, 554–574.
- Zhao, H.; Xia, B.; Fan, C.; Zhao, P.; Shen, S. Human Health Risk from Soil Heavy Metal Contamination under Different Land Uses near Dabaoshan Mine, Southern China. Sci. Total Environ. 2012, 417–418, 45–54.
- Foucault, Y.; Lévêque, T.; Xiong, T.; Schreck, E.; Austruy, A.; Shahid, M.; Dumat, C. Green Manure Plants for Remediation of Soils Polluted by Metals and Metalloids: Ecotoxicity and Human Bioavailability Assessment. Chemosphere 2013, 93, 1430–1435.
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and Redox Signalling in the Response of Plants to Abiotic Stress: ROS and Redox Signalling in Plants. Plant Cell Environ. 2012, 35, 259–270.
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial Reactive Oxygen Species. Contribution to Oxidative Stress and Interorganellar Signaling. Plant Physiol. 2006, 141, 357–366.
- Hu, W.H.; Song, X.S.; Shi, K.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q. Changes in Electron Transport, Superoxide Dismutase and Ascorbate Peroxidase Isoenzymes in Chloroplasts and Mitochondria of Cucumber Leaves as Influenced by Chilling. Photosynthetica 2008, 46, 581–588.
- Carrasco-Gil, S.; Estebaranz-Yubero, M.; Medel-Cuesta, D.; Millán, R.; Hernández, L.E. Influence of Nitrate Fertilization on Hg Uptake and Oxidative Stress Parameters in Alfalfa Plants Cultivated in a Hg-Polluted Soil. Environ. Exp. Bot. 2012, 75, 16–24.
- Chen, F.; Gao, J.; Zhou, Q. Toxicity Assessment of Simulated Urban Runoff Containing Polycyclic Musks and Cadmium in Carassius Auratus Using Oxidative Stress Biomarkers. Environ. Pollut. 2012, 162, 91–97.
- Janků, M.; Luhová, L.; Petřivalský, M. On the Origin and Fate of Reactive Oxygen Species in Plant Cell Compartments. Antioxidants 2019, 8, 105.
- Swanson, S.; Gilroy, S. ROS in Plant Development. Physiol. Plant. 2010, 138, 384–392.
- Mansoor, S.; Sakina, A.; Mir, M.A.; Mir, J.I.; Wani, A.A.; un Nabi, S.; Alyemeni, M.N.; Chung, Y.S.; Masoodi, K.Z. Elucidating the role of reactive oxygen species metabolism and phenylpropanoid pathway during an incompatible interaction between apple-Venturia inaequalis host-pathosystem. S. Afr. J. Bot. 2023, 160, 428–436.
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive Oxygen Species and Heavy Metal Stress in Plants: Impact on the Cell Wall and Secondary Metabolism. Environ. Exp. Bot. 2019, 161, 98–106.
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679.
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208.
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and Oxidative Burst: Roots in Plant Development. Plant Divers. 2020, 42, 33–43.
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681.
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800.
- Corpas, F.J.; Gupta, D.K.; Palma, J.M. Production Sites of Reactive Oxygen Species (ROS) in Organelles from Plant Cells. In Reactive Oxygen Species and Oxidative Damage in Plants Under Stress; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–22. ISBN 978-3-319-20420-8.
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641.
- Nazir, F.; Fariduddin, Q.; Khan, T.A. Hydrogen Peroxide as a Signalling Molecule in Plants and Its Crosstalk with Other Plant Growth Regulators under Heavy Metal Stress. Chemosphere 2020, 252, 126486.
- Locato, V.; De Gara, L. Programmed Cell Death in Plants: An Overview. In Plant Programmed Cell Death; De Gara, L., Locato, V., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1743, pp. 1–8. ISBN 978-1-4939-7667-6.
- Ye, C.; Zheng, S.; Jiang, D.; Lu, J.; Huang, Z.; Liu, Z.; Zhou, H.; Zhuang, C.; Li, J. Initiation and Execution of Programmed Cell Death and Regulation of Reactive Oxygen Species in Plants. Int. J. Mol. Sci. 2021, 22, 12942.
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-Related Redox Regulation and Signaling in Plants. Semin. Cell Dev. Biol. 2018, 80, 3–12.
- Del Río, L.A.; López-Huertas, E. ROS Generation in Peroxisomes and Its Role in Cell Signaling. Plant Cell Physiol. 2016, 57, 1364–1376.
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen Peroxide Priming Modulates Abiotic Oxidative Stress Tolerance: Insights from ROS Detoxification and Scavenging. Front. Plant Sci. 2015, 6, 420.
- You, J.; Chan, Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6, 1092.
- Chmielowska-Bąk, J.; Izbiańska, K.; Ekner-Grzyb, A.; Bayar, M.; Deckert, J. Cadmium Stress Leads to Rapid Increase in RNA Oxidative Modifications in Soybean Seedlings. Front. Plant Sci. 2018, 8, 2219.
- Huang, X.; Han, B. Natural Variations and Genome-Wide Association Studies in Crop Plants. Annu. Rev. Plant Biol. 2014, 65, 531–551.
- Liu, T.; Shen, C.; Wang, Y.; Huang, C.; Shi, J. New Insights into Regulation of Proteome and Polysaccharide in Cell Wall of Elsholtzia Splendens in Response to Copper Stress. PLoS ONE 2014, 9, e109573.
- 47. Lin, Y.J.; Feng, X.H.; Feng, Y.X. Regulation of enzymatic and non-enzymatic antioxidants in rice seedlings against chromium stress through sodium hydrosulfide and sodium nitroprusside. Environ. Sci. Pollut. Res. 2023, 30, 25851–25862.
- Chmielowska-Bąk, J.; Arasimowicz-Jelonek, M.; Izbiańska, K.; Frontasyeva, M.; Zinicovscaia, I.; Guiance-Varela, C.; Deckert, J. NADPH Oxidase Is Involved in Regulation of Gene Expression and ROS Overproduction in Soybean (Glycine Max L.) Seedlings Exposed to Cadmium. Acta Soc. Bot. Pol. 2017, 86, 3551.
- Sharma, S.K.; Singh, D.; Pandey, H.; Jatav, R.B.; Singh, V.; Pandey, D. An Overview of Roles of Enzymatic and Nonenzymatic Antioxidants in Plant. In Antioxidant Defense in Plants; Aftab, T., Hakeem, K.R., Eds.; Springer Nature: Singapore, 2022; pp. 1–13. ISBN 9789811679803.
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of Enzymatic and Nonenzymatic Antioxidants in Plants during Abiotic Stress. Crit. Rev. Biotechnol. 2010, 30, 161–175.
- Amareh, R.; Kaviani, B.; Sedaghathoor, S.; Allahyari, M.S. Assessment of Some Urban Ornamental Plants in Southern Iran Revealed That They Choose One of the Two Enzymatic or Non-Enzymatic Antioxidants Defensive Strategies against Heavy Metals. Res. Sq. 2023.
- Hameed, A.; Rasool, S.; Azooz, M.M.; Hossain, M.A.; Ahanger, M.A.; Ahmad, P. Heavy Metal Stress. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 557–583. ISBN 978-0-12-803158-2.
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy Metal Stress and Some Mechanisms of Plant Defense Response. Sci. World J. 2015, 2015, 756120.
- Aina, R.; Labra, M.; Fumagalli, P.; Vannini, C.; Marsoni, M.; Cucchi, U.; Bracale, M.; Sgorbati, S.; Citterio, S. Thiol-Peptide Level and Proteomic Changes in Response to Cadmium Toxicity in Oryza sativa L. Roots. Environ. Exp. Bot. 2007, 59, 381–392.
- Anjum, N.A.; Umar, S.; Singh, S.; Nazar, R.; Khan, N.A. Sulfur Assimilation and Cadmium Tolerance in Plants. In Sulfur Assimilation and Abiotic Stress in Plants; Khan, N.A., Singh, S., Umar, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 271–302. ISBN 978-3-540-76325-3.
- Wang, Y.; Wang, Z.; Geng, S.; Du, H.; Chen, B.; Sun, L.; Wang, G.; Sha, M.; Dong, T.; Zhang, X.; et al. Identification of the GDP-L-Galactose Phosphorylase Gene as a Candidate for the Regulation of Ascorbic Acid Content in Fruits of Capsicum annuum L. Int. J. Mol. Sci. 2023, 24, 7529.
- Tao, C.; Jin, X.; Zhu, L.; Xie, Q.; Wang, X.; Li, H. Genome-Wide Investigation and Expression Profiling of APX Gene Family in Gossypium Hirsutum Provide New Insights in Redox Homeostasis Maintenance during Different Fiber Development Stages. Mol. Genet. Genom. 2018, 293, 685–697.
- Muñoz, P.; Munné-Bosch, S. Vitamin E in Plants: Biosynthesis, Transport, and Function. Trends Plant Sci. 2019, 24, 1040–1051.
- Jańczak-Pieniążek, M.; Cichoński, J.; Michalik, P.; Chrzanowski, G. Effect of Heavy Metal Stress on Phenolic Compounds Accumulation in Winter Wheat Plants. Molecules 2022, 28, 241.
- Hoque, M.N.; Tahjib-Ul-Arif, M.; Hannan, A.; Sultana, N.; Akhter, S.; Hasanuzzaman, M.; Akter, F.; Hossain, M.S.; Sayed, M.A.; Hasan, M.T.; et al. Melatonin Modulates Plant Tolerance to Heavy Metal Stress: Morphological Responses to Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 11445.
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant Mitochondria Synthesize Melatonin and Enhance the Tolerance of Plants to Drought Stress. J. Pineal Res. 2017, 63, e12429.
- Yang, H.; Fang, R.; Luo, L.; Yang, W.; Huang, Q.; Yang, C.; Hui, W.; Gong, W.; Wang, J. Potential Roles of Melatonin in Mitigating the Heavy Metals Toxicity in Horticultural Plants. Sci. Hortic. 2023, 321, 112269.
- Keyster, M.; Niekerk, L.-A.; Basson, G.; Carelse, M.; Bakare, O.; Ludidi, N.; Klein, A.; Mekuto, L.; Gokul, A. Decoding Heavy Metal Stress Signalling in Plants: Towards Improved Food Security and Safety. Plants 2020, 9, 1781.
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy Metal Stress and Responses in Plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828.
- Kumar, S.; Trivedi, P.K. Heavy Metal Stress Signaling in Plants. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 585–603. ISBN 978-0-12-803158-2.
- Mondal, S. Heavy Metal Stress–Induced Activation of Mitogen-Activated Protein Kinase Signalling Cascade in Plants. Plant Mol. Biol. Report. 2022, 41, 15–26.
- Loix, C.; Huybrechts, M.; Vangronsveld, J.; Gielen, M.; Keunen, E.; Cuypers, A. Reciprocal Interactions between Cadmium-Induced Cell Wall Responses and Oxidative Stress in Plants. Front. Plant Sci. 2017, 8, 1867.
- Afzal, Z.; Howton, T.; Sun, Y.; Mukhtar, M. The Roles of Aquaporins in Plant Stress Responses. J. Dev. Biol. 2016, 4, 9.
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of Aquaporins in Plants under Stress. Biol. Res. 2018, 51, 4.
- Lamalakshmi Devi, E.; Kumar, S.; Basanta Singh, T.; Sharma, S.K.; Beemrote, A.; Devi, C.P.; Chongtham, S.K.; Singh, C.H.; Yumlembam, R.A.; Haribhushan, A.; et al. Adaptation Strategies and Defence Mechanisms of Plants During Environmental Stress. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 359–413. ISBN 978-3-319-68716-2.
- Ariani, A.; Barozzi, F.; Sebastiani, L.; Di Toppi, L.S.; Di Sansebastiano, G.P.; Andreucci, A. AQUA1 Is a Mercury Sensitive Poplar Aquaporin Regulated at Transcriptional and Post-Translational Levels by Zn Stress. Plant Physiol. Biochem. 2019, 135, 588–600.
- De Caroli, M.; Furini, A.; DalCorso, G.; Rojas, M.; Di Sansebastiano, G.-P. Endomembrane Reorganization Induced by Heavy Metals. Plants 2020, 9, 482.
- Kosakivska, I.V.; Babenko, L.M.; Romanenko, K.O.; Korotka, I.Y.; Potters, G. Molecular Mechanisms of Plant Adaptive Responses to Heavy Metals Stress. Cell Biol. Int. 2021, 45, 258–272.
- Cai, G.; Parrotta, L.; Cresti, M. Organelle Trafficking, the Cytoskeleton, and Pollen Tube Growth: Organelle Trafficking in Pollen Tubes. J. Integr. Plant Biol. 2015, 57, 63–78.
- Xing, S.; Mehlhorn, D.G.; Wallmeroth, N.; Asseck, L.Y.; Kar, R.; Voss, A.; Denninger, P.; Schmidt, V.A.F.; Schwarzländer, M.; Stierhof, Y.-D.; et al. Loss of GET Pathway Orthologs in Arabidopsis thaliana Causes Root Hair Growth Defects and Affects SNARE Abundance. Proc. Natl. Acad. Sci. USA 2017, 114, E1544–E1553.
- Zhao, X.; Zhang, X.; Qu, Y.; Li, R.; Baluška, F.; Wan, Y. Mapping of Membrane Lipid Order in Root Apex Zones of Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 1151.
- Fan, J.-L.; Wei, X.-Z.; Wan, L.-C.; Zhang, L.-Y.; Zhao, X.-Q.; Liu, W.-Z.; Hao, H.-Q.; Zhang, H.-Y. Disarrangement of Actin Filaments and Ca2+ Gradient by CdCl2 Alters Cell Wall Construction in Arabidopsis thaliana Root Hairs by Inhibiting Vesicular Trafficking. J. Plant Physiol. 2011, 168, 1157–1167.
- Ge, W.; Jiao, Y.Q.; Sun, B.L.; Qin, R.; Jiang, W.S.; Liu, D.H. Cadmium-Mediated Oxidative Stress and Ultrastructural Changes in Root Cells of Poplar Cultivars. S. Afr. J. Bot. 2012, 83, 98–108.
- Krzesłowska, M.; Lenartowska, M.; Samardakiewicz, S.; Bilski, H.; Woźny, A. Lead Deposited in the Cell Wall of Funaria Hygrometrica Protonemata Is Not Stable—A Remobilization Can Occur. Environ. Pollut. 2010, 158, 325–338.
- Lin, W.; Tang, W.; Anderson, C.T.; Yang, Z. FERONIA’s Sensing of Cell Wall Pectin Activates ROP GTPase Signaling in Arabidopsis. Plant Biol. 2018, 22, 269647.
- Rabęda, I.; Bilski, H.; Mellerowicz, E.J.; Napieralska, A.; Suski, S.; Woźny, A.; Krzesłowska, M. Colocalization of Low-Methylesterified Pectins and Pb Deposits in the Apoplast of Aspen Roots Exposed to Lead. Environ. Pollut. 2015, 205, 315–326.
- Chu, L.; Offenborn, J.N.; Steinhorst, L.; Wu, X.N.; Xi, L.; Li, Z.; Jacquot, A.; Lejay, L.; Kudla, J.; Schulze, W.X. Plasma Membrane Calcineurin B-like Calcium-ion Sensor Proteins Function in Regulating Primary Root Growth and Nitrate Uptake by Affecting Global Phosphorylation Patterns and Microdomain Protein Distribution. New Phytol. 2021, 229, 2223–2237.
- Wang, X.; Chen, J.; Ge, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Mur, L.A.J.; Jiang, D. The Different Root Apex Zones Contribute to Drought Priming Induced Tolerance to a Reoccurring Drought Stress in Wheat. Crop. J. 2021, 9, 1088–1097.
- Mendoza-Cózatl, D.G.; Gokul, A.; Carelse, M.F.; Jobe, T.O.; Long, T.A.; Keyster, M. Keep Talking: Crosstalk between Iron and Sulfur Networks Fine-Tunes Growth and Development to Promote Survival under Iron Limitation. J. Exp. Bot. 2019, 70, 4197–4210.
- Xuan, W.; Beeckman, T.; Xu, G. Plant Nitrogen Nutrition: Sensing and Signaling. Curr. Opin. Plant Biol. 2017, 39, 57–65.
- Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H. The Arabidopsis Calcium-Dependent Protein Kinases (CDPKs) and Their Roles in Plant Growth Regulation and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 1900.
- Boro, P.; Chattopadhyay, S. Crosstalk between MAPKs and GSH under Stress: A Critical Review. J. Biosci. 2022, 47, 71.
- Mazaheri-Tirani, M.; Dayani, S. In Vitro Effect of Zinc Oxide Nanoparticles on Nicotiana Tabacum Callus Compared to ZnO Micro Particles and Zinc Sulfate (ZnSO4). Plant Cell Tissue Organ Cult. 2020, 140, 279–289.
- Peng, D.; Wang, W.; Liu, A.; Zhang, Y.; Li, X.; Wang, G.; Jin, C.; Guan, C.; Ji, J. Comparative Transcriptome Combined with Transgenic Analysis Reveal the Involvement of Salicylic Acid Pathway in the Response of Nicotiana Tabacum to Triclosan Stress. Chemosphere 2021, 270, 129456.
- Xian, J.; Wang, Y.; Niu, K.; Ma, H.; Ma, X. Transcriptional Regulation and Expression Network Responding to Cadmium Stress in a Cd-Tolerant Perennial Grass Poa Pratensis. Chemosphere 2020, 250, 126158.
- Valivand, M.; Amooaghaie, R.; Ahadi, A. Seed Priming with H2S and Ca2+ Trigger Signal Memory That Induces Cross-Adaptation against Nickel Stress in Zucchini Seedlings. Plant Physiol. Biochem. 2019, 143, 286–298.
- Xu, Z.; Dong, M.; Peng, X.; Ku, W.; Zhao, Y.; Yang, G. New Insight into the Molecular Basis of Cadmium Stress Responses of Wild Paper Mulberry Plant by Transcriptome Analysis. Ecotoxicol. Environ. Saf. 2019, 171, 301–312.
- Corpas, F.J.; Gupta, D.K.; Palma, J.M. (Eds.) Reactive Oxygen Species and Oxidative Damage in Plants Under Stress, 1st ed.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-20421-5.
- Trchounian, A.; Petrosyan, M.; Sahakyan, N. Plant Cell Redox Homeostasis and Reactive Oxygen Species. In Redox State as a Central Regulator of Plant-Cell Stress Responses; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–50. ISBN 978-3-319-44080-4.
- Hasanuzzaman, M.; Fujita, M.; Oku, H.; Islam, M.T. (Eds.) Plant Tolerance to Environmental Stress: Role of Phytoprotectants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-203-70531-5.
- Qadir, S.U.; Raja, V.; Siddiqi, W.A.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Heavy Metal Bioaccumulation by Selected Plants from Fly Ash–Contaminated Soils in Suburban Area. Arab. J. Geosci. 2021, 14, 116.
- Blokhina, O. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194.
- Maleki, M.; Ghorbanpour, M.; Kariman, K. Physiological and Antioxidative Responses of Medicinal Plants Exposed to Heavy Metals Stress. Plant Gene 2017, 11, 247–254.
- Banerjee, A.; Roychoudhury, A. Role of Glutathione in Plant Abiotic Stress Tolerance. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: New York, NY, USA, 2019; pp. 159–172. ISBN 978-1-119-46869-1.
- Khullar, S.; Sudhakara Reddy, M. Cadmium and Arsenic Responses in the Ectomycorrhizal Fungus Laccaria Bicolor: Glutathione Metabolism and Its Role in Metal(Loid) Homeostasis. Environ. Microbiol. Rep. 2019, 11, 53–61.
- Maheshwari, R.; Dubey, R.S. Nickel-Induced Oxidative Stress and the Role of Antioxidant Defence in Rice Seedlings. Plant Growth Regul. 2009, 59, 37–49.
- Gardner, P.R.; Fridovich, I. Superoxide Sensitivity of the Escherichia coli 6-Phosphogluconate Dehydratase. J. Biol. Chem. 1991, 266, 1478–1483.
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42.
- Dubey, S.; Shri, M.; Gupta, A.; Rani, V.; Chakrabarty, D. Toxicity and Detoxification of Heavy Metals during Plant Growth and Metabolism. Environ. Chem. Lett. 2018, 16, 1169–1192.
- Fryzova, R.; Pohanka, M.; Martinkova, P.; Cihlarova, H.; Brtnicky, M.; Hladky, J.; Kynicky, J. Oxidative Stress and Heavy Metals in Plants. In Reviews of Environmental Contamination and Toxicology; De Voogt, P., Ed.; Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2017; Volume 245, pp. 129–156. ISBN 978-3-319-75036-1.
- Gjorgieva Ackova, D. Heavy Metals and Their General Toxicity for Plants. Plant Sci. Today 2018, 5, 14–18.
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. ISBN 978-3-7643-8339-8.
- White, P.J.; Pongrac, P. Heavy-Metal Toxicity in Plants. In Plant Stress Physiology; Shabala, S., Ed.; CABI: Wallingford, UK, 2017; pp. 300–331. ISBN 978-1-78064-729-6.
- Zhao, Q.; Kaluarachchi, J.J. Risk Assessment at Hazardous Waste-Contaminated Sites with Variability of Population Characteristics. Environ. Int. 2002, 28, 41–53.
- Evanko, C.R.; Dzombak, D.A. Remediation of Metals-Contaminated Soils and Groundwater; Tech. Rep. TE-976-01, Pittsburgh, GWRTAC Series; Ground-water remediation technologies analysis center: Pittsburgh, PA, USA, 1997.
- Koźmińska, A.; Wiszniewska, A.; Hanus-Fajerska, E.; Muszyńska, E. Recent Strategies of Increasing Metal Tolerance and Phytoremediation Potential Using Genetic Transformation of Plants. Plant Biotechnol. Rep. 2018, 12, 1–14.
- Mansoor, S.; Khan, N.F.; Farooq, I.; Kaur, N.; Manhas, S.; Raina, S.; Khan, I.F. Phytoremediation at Molecular Level. In Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 65–90. ISBN 978-0-323-89874-4.
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359.
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a Tool for Effective Management of Drought and Heavy Metal Toxicity. Chemosphere 2021, 271, 129458.
- Wang, H.; Lin, K.; Hou, Z.; Richardson, B.; Gan, J. Sorption of the Herbicide Terbuthylazine in Two New Zealand Forest Soils Amended with Biosolids and Biochars. J. Soils Sediments 2010, 10, 283–289.
- Beesley, L.; Marmiroli, M. The Immobilisation and Retention of Soluble Arsenic, Cadmium and Zinc by Biochar. Environ. Pollut. 2011, 159, 474–480.
- Xu, X.; Zhao, Y.; Sima, J.; Zhao, L.; Mašek, O.; Cao, X. Indispensable Role of Biochar-Inherent Mineral Constituents in Its Environmental Applications: A Review. Bioresour. Technol. 2017, 241, 887–899.
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647.
- Köber, R.; Daus, B.; Ebert, M.; Mattusch, J.; Welter, E.; Dahmke, A. Compost-Based Permeable Reactive Barriers for the Source Treatment of Arsenic Contaminations in Aquifers: Column Studies and Solid-Phase Investigations. Environ. Sci. Technol. 2005, 39, 7650–7655.
- Rzymski, P.; Tomczyk, K.; Rzymski, P.; Poniedziałek, B.; Opala, T.; Wilczak, M. Impact of Heavy Metals on the Female Reproductive System. Ann. Agric. Environ. Med. 2015, 22, 259–264.
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K.S.M. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery From Various Environments-A Review. Front. Environ. Sci. 2019, 7, 66.
- Das, N.; Bhattacharya, S.; Maiti, M.K. Enhanced Cadmium Accumulation and Tolerance in Transgenic Tobacco Overexpressing Rice Metal Tolerance Protein Gene OsMTP1 Is Promising for Phytoremediation. Plant Physiol. Biochem. 2016, 105, 297–309.
- Wu, G.; Kang, H.; Zhang, X.; Shao, H.; Chu, L.; Ruan, C. A Critical Review on the Bio-Removal of Hazardous Heavy Metals from Contaminated Soils: Issues, Progress, Eco-Environmental Concerns and Opportunities. J. Hazard. Mater. 2010, 174, 1–8.
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A Review on Drought Stress in Plants: Implications, Mitigation and the Role of Plant Growth Promoting Rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032.
- Farooq Khan, N.; Rasool, A.; Mansoor, S.; Saleem, S.; Rehman Baba, T.; Maurifatul Haq, S.; Aafreen Rehman, S.; Oluwaseun Adetunji, C.; Mariana Popescu, S. Potential Applications of Rhizobacteria as Eco-Friendly Biological Control, Plant Growth Promotion and Soil Metal Bioremediation. In Sustainable Crop Production—Recent Advances; Singh Meena, V., Choudhary, M., Prakash Yadav, R., Kumari Meena, S., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-80355-696-3.
- Fasani, E.; Manara, A.; Martini, F.; Furini, A.; DalCorso, G. The Potential of Genetic Engineering of Plants for the Remediation of Soils Contaminated with Heavy Metals: Transgenic Plants for Phytoremediation. Plant Cell Environ. 2018, 41, 1201–1232.
- Gupta, D.K.; Huang, H.G.; Corpas, F.J. Lead Tolerance in Plants: Strategies for Phytoremediation. Environ. Sci. Pollut. Res. 2013, 20, 2150–2161.
More
