You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Eleonora Cella and Version 2 by Rita Xu.
Antibiotic resistance is a significant global health concern that affects both human and animal populations. The
One Health
approach acknowledges the interconnectedness of human health, animal health, and the environment. It emphasizes the importance of collaboration and coordination across these sectors to tackle complex health challenges such as antibiotic resistance.
- antimicrobial resistance
- antibiotic resistance
- One Health
1. Examining the Global Impact of Antibiotic Resistance
Antimicrobial resistance (AMR) in pathogenic bacteria can be defined in two ways: microbiologically and clinically. Microbiological resistance is determined by the presence of a genetically acquired or mutated resistance mechanism in the pathogen, which is classified as resistant, intermediate, or susceptible based on specific cut-off values in laboratory tests [1]. Clinical resistance is defined by the level of antimicrobial activity that is associated with a high likelihood of treatment failure. Specifically, it means that using a drug against which the pathogen was tested and proved susceptible is more effective than using a drug against which the pathogen was tested and proved resistant. The cut-off values for clinical resistance may vary depending on factors such as the site of infection or the dosage of the drug [2].
AMR in bacterial pathogens is a significant global concern associated with high rates of illness and mortality [3][4][3,4]. Several factors increase the demands for antibiotic prescriptions, as well as situations that create financial incentives from medication distribution, such as unregulated access and usage of over-the-counter drugs, can lead to inappropriate prescribing of antimicrobials. Both Gram-positive and Gram-negative bacteria have developed multidrug resistant patterns, leading to challenging and sometimes infections that are untreatable with conventional antimicrobials. In many healthcare settings, there is a lack of early identification of causative microorganisms and their susceptibility to antimicrobials in patients with serious infections, leading to the excessive and unnecessary use of broad-spectrum antibiotics [3][4][3,4]. Consequently, there has been a notable increase in emerging resistance. Coupled with poor infection control practices, resistant bacteria can easily be disseminated to other patients and the environment [3][4][3,4].
The introduction of antibiotics as clinical agents radically changed the evolution and spread of resistance by providing considerable selective pressures, especially on members of the microbiota of humans and domestic animals but also in environments heavily polluted with antibiotics. This selective pressure has promoted the mobilization and horizontal transfer of a large range of antibiotic resistance genes (ARGs) among many bacterial species, particularly to those causing disease. The downstream consequences of such accumulating evolutionary events are gradually increasing the difficulty in the prevention and treatment of bacterial infections. As bacteria and mobile genetic elements often cross environments and species boundaries, it is critical to understand and acknowledge the connections between the human, animal, and environmental microbiota (the One Health Concept [5][6][5,6]) (https://www.cdc.gov/onehealth/ accessed on 1 July 2023) to manage this global health challenge [7][8][9][10][11][12][13][14][15][16][17][7,8,9,10,11,12,13,14,15,16,17] (Figure 1).
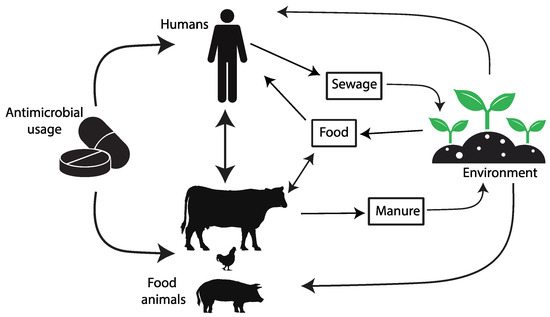
Figure 1. The diagram depicts the pathways of transmission for antimicrobial resistance between farm animals, the surrounding environment, and human populations.
The threats posed by AMR are of increasing concern, even in low- and middle-income countries (LMICs), as the rates of antibiotic use increase. Assessing the extent of AMR in LMICs is complicated due to inadequate surveillance programs, though efforts like the WHO’s GLASS project (2015) have been initiated [18]. The misuse of antibiotics in humans, animals, and crops, along with the mismanagement of pharmaceutical waste during production, drives AMR in LMICs. Factors like poor sanitation, limited healthcare access, and lax regulations contribute to AMR spread. The COVID-19 pandemic compounded these challenges, increasing antibiotic misuse in LMICs and diverting attention from the AMR threat [19]. AMR hotspots are seen in parts of India, China, Pakistan, and other regions. Emerging resistance is noted in Kenya, Morocco, and Uruguay. Survey coverage did not always match hotspots. Common antimicrobial classes in animal production had high resistance rates [20]. Through the active participation of various countries in the WHO’s GLASS project, valuable insights have been gleaned regarding the total consumption of antibiotic, measured in terms of Defined Daily Doses (DDD) per 1000 inhabitants per day [18] (Figure 2). However, it is important to acknowledge that these findings are limited due to the lack of universal public engagement in the specific country data collection process.
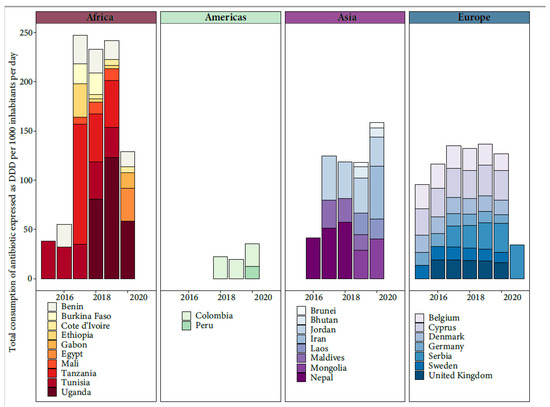
Figure 2. Total consumption of antibiotic expressed as Defined Daily Doses (DDD) per 1000 inhabitants per day from the available data of WHO’s GLASS project.
Treating antibiotic resistant infections usually requires the prolonged use of last-line antibiotics, and these approaches often fail, contributing to infection persistence [21]. Several factors contribute to the development and spread of antibiotic resistance [22], and among these are:
-
Incomplete Treatment: When individuals do not complete their prescribed antibiotic course, it creates an environment for the survival of the resistant bacteria, leading to the proliferation of resistant strains.
-
Use in Agriculture: Antibiotics are extensively used in livestock and agriculture to promote growth and prevent infections in animals. This widespread use contributes to the development of resistant bacteria that can be transmitted to humans through the food chain. In addition, mobile elements conferring antibiotic resistance can move from livestock-associated lineages to human lineages [28][29][28,29].
-
Global Travel and Trade: Resistant bacteria can spread quickly across borders through international travel and trade, making antibiotic resistance a global issue [30].
-
Lack of New Antibiotics: The development of new antibiotics has slowed significantly in recent decades, reducing the arsenal of drugs available to combat resistant infections [31].
