1. Sweeteners and the Gut Microbiome
1.1. Steviol Glycoside
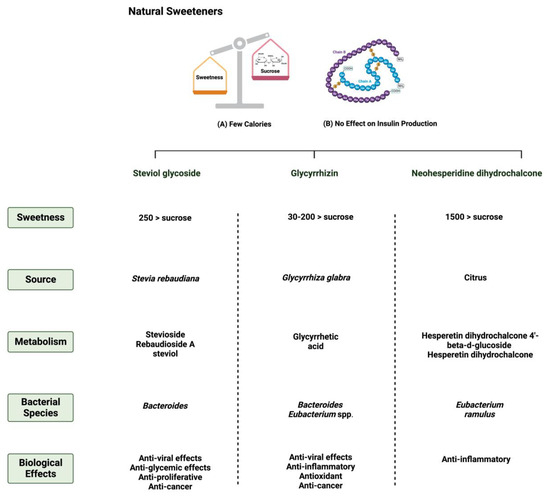
Stevia rebaudiana is a shrub found mainly in South America, specifically in Brazil and Paraguay
[1][26]. It belongs to the family
Asteraceae, and it is used as a natural and non-caloric sweetener because of its high sweetness intensity, which is caused by steviol glycosides
[2][27]. Purified steviol glycoside extracts have been used in the food industry as sweeteners in many regions
[3][28]. The European Food Safety Authority (EFSA) thus reported the acceptable daily intake of steviol glycosides to be 4 mg/kg/day
[4][5][29,30]. The leaves of
Stevia rebaudiana contain several diterpene glycosides, such as rubusoside and steviolbioside
[6][31]. Multiple in vitro studies have supported the metabolization of stevia extracts by the gut microbiome
[7][32].
Bacteroides species in the gut play an important role in metabolizing two of the main components of
Stevia rebaudiana by hydrolyzing rebaudioside A and stevioside to steviol in the gut
[8][33]. This suggests that neither component is absorbed in the upper gastrointestinal tract
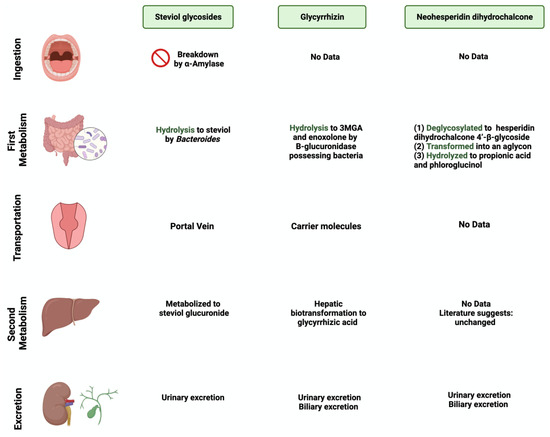
[9][34]. Using the portal vein, the absorbed steviol reaches the liver for further metabolism to steviol glucuronide and is excreted in the urine
[10][35].
1.2. Glycyrrhizin
One of the 300 active licorice compounds is glycyrrhizin, a triterpene saponin glycoside
[11][36]. It is used as an herbal product in medicine due to its anticancer and anti-inflammatory activities
[12][37]. It has a high sweetness intensity (up to 200 times sweeter than sucrose)
[13][38]. Ingestion of less than 100 mg/day of glycyrrhizin is considered safe
[14][39]. Due to its poor oral bioavailability, glycyrrhizin is metabolized by the gut microbiome
[15][40]. Both
Eubacterium and
Bacteroides species are involved in the de-glycosylation of glycyrrhizin to a major product, glycyrrhizic acid, and a minor product, 18β-glycyrrhetic acid 3-O-monoglucuronide
[16][41]. After that, both products reach the liver for further conjugation and reduction
[17][42]. Both biliary and urinary excretions occur to the major parts of the products, respectively
[18][43].
1.3. Neohesperidin Dihydrochalcone
Neohesperidin dihydrochalcone (NHDC) is a natural sweetener found mainly in the skin of citrus fruits; it possesses high stability and solubility
[19][44]. It is obtained and processed from its parent flavanone, neohesperidin, and has a sweetness intensity 250–1800 times higher than sucrose
[20][45]. Despite that, the usage of NHDC as a replacement for sucrose is limited in the food industry due to its flavor formulation, texture, and size
[21][46]. Although not widely known, the metabolism of NHDC by the gut microbiome has been discussed in the literature
[22][47]. The metabolism starts with NHDC being deglycosylated to hesperidin dihydrochalcone 4′-β-glycoside, transforming into an aglycone. The final step of NHDC metabolism is the hydrolysis of the aglycone to propionic acid and phloroglucinol
[23][48]. The products are then excreted either through urine or bile
[24][49].
Figure 1 and
Figure 2 summarize and provide an overview of the three natural sweeteners and their metabolism by the gut microbiome.
Figure 1. Overview illustration of natural sweeteners. The figure describes two of the main features of natural sweeteners. It also shows the sweetness intensity, the source of natural sweeteners, their metabolism by the gut microbiome, and their main biological effects. Created with
BioRender.com (accessed on 15 July 2023).
Figure 2. Overview illustration of natural sweetener consumption and metabolism. The figure is divided into different sites of metabolism for each of the natural sweeteners. Created with
BioRender.com (accessed on 15 July 2023).
1.4. Saccharin
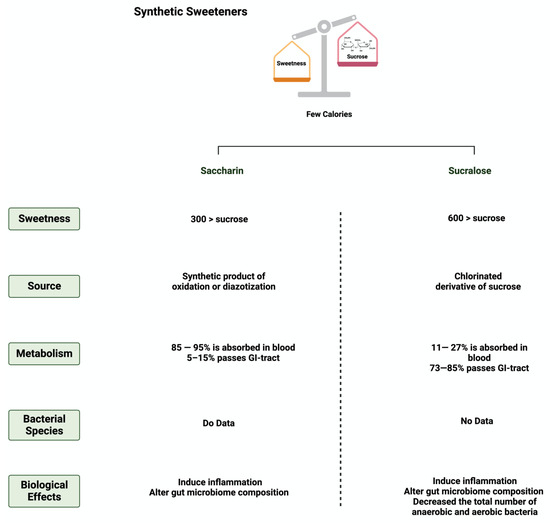
Saccharin (1,1-dioxo-1,2-benzothiazol-3-one), also known as E954, is a non-caloric sweetener used widely in the food industry
[25][50]. It is found either in an acid form or bound to calcium or sodium (higher stability and solubility)
[26][51]. Saccharin’s sweetness intensity is 300 times higher than sucrose
[27][52]. The FDA considers saccharin consumption to be safe due to its inability to be metabolized by the body
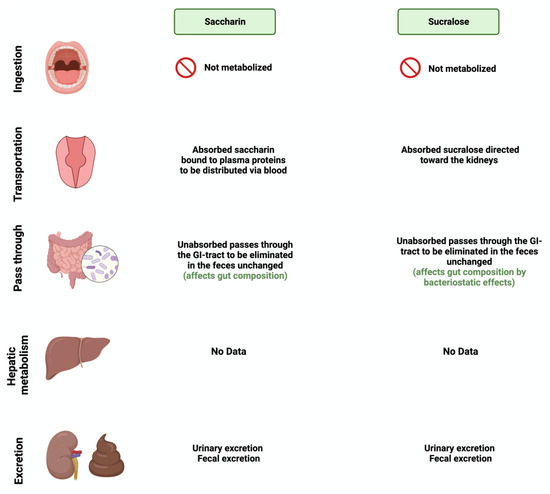
[28][53]. Once consumed, most of the ingested saccharin (85–95%) is absorbed and bound reversibly to plasma proteins when excreted in the urine. The rest passes through the GI tract to be eliminated, unchanged, in the feces
[9][34]. Due to this, studies have investigated the influence of saccharin on gut microbiome composition. The administration of 90 mg of saccharin in rats did not alter the total number of anaerobic bacteria, but eliminated specific anaerobic groups in the cecal contents
[29][54].
Additionally, rats receiving a 2.5% dose of saccharin inhibited the growth of three
Escherichia coli strains and three
Lactobacillus species
[30][55]. These studies may suggest that even if the body does not metabolize the sweeteners, their consumption impacts the gut microbiome’s composition and function, which might alter the host’s health status. However, recent studies using advanced technologies are required in order to assess saccharin’s safety and effectiveness and to address the controversial results in the literature.
1.5. Sucralose
Sucralose, or E-955, is a low-caloric, non-nutritive synthetic sweetener and is very similar in structure to sucrose
[31][56]. However, sucralose is formed when the three hydroxyl groups attached to the sucrose molecule are replaced by chlorine atoms
[32][57]. It is 600 times sweeter than sucrose
[33][58]. Like saccharin, sucralose is not metabolized by the body; however, unlike saccharin, most ingested sucralose passes through the GI tract to be eliminated in the feces. The rest reaches the kidneys for urinary excretion
[34][59]. The administration of sucralose influences its abundance in the gut microbiome. The relative abundance of
Clostridium cluster XIVa was affected in mice given 15 mg of sucralose/kg
[35][9].
Additionally, sucralose administration for six months influenced the abundance of 14 different taxonomic levels, as well as the regulation of amino acids and chronic inflammation, in C57BL/6 mice
[36][60]. This shows the urgent need for further research to investigate the observed effects on humans.
Figure 3 and
Figure 4 summarize and provide an overview of the two synthetic sweeteners and their metabolism by the gut microbiome.
Figure 3. Overview illustration of synthetic sweeteners. The figure describes the main features of synthetic sweeteners. It also shows the sweetness intensities, the sources of the synthetic sweeteners, their metabolism by the gut microbiome, and their main biological effects. Created with
BioRender.com (accessed on 15 July 2023).
Figure 4. Overview illustration of synthetic sweetener consumption and metabolism. The figure is divided into different sites of metabolism for each of the synthetic sweeteners. Created with
BioRender.com (accessed on 15 July 2023).
2. Sweeteners’ Role in Gastrointestinal Cancers
The effect of natural and synthetic sweeteners on the development of organ-specific cancer has been discussed for years
[37][61]. With the continued rise in the consumption rate of sweeteners worldwide, several reports have supported the positive influence of sweeteners on the development and progression of GI cancer
[38][62].
2.1. Apoptosis
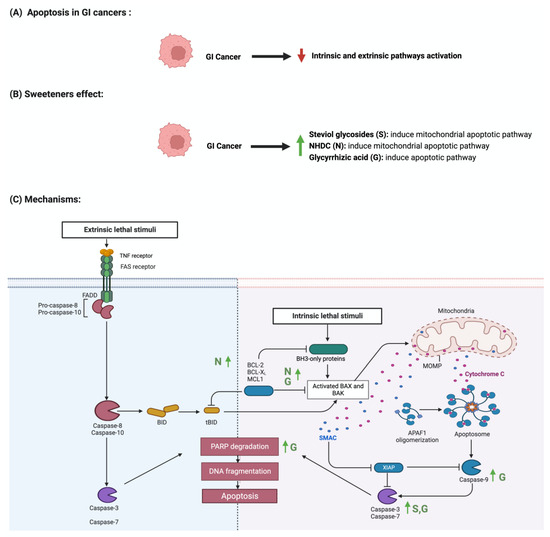
Apoptosis is programmed cell death characterized by morphological and biochemical changes
[39][63]. Its involvement in various processes, such as immune system development, makes it an essential physiological process
[40][64]. When unregulated, it plays a role in the development of several diseases, such as autoimmune diseases, neurodegenerative disorders, and cancers
[41][65]. Sweeteners have been reported to influence the process of apoptosis in cancers
[42][66]. Steviol, a colonic metabolite, inhibits apoptosis in GI cancer cells as effectively as 5-fluorouracil (100 ug/mL) through the mitochondrial apoptotic pathway
[43][67]. Additionally, in one study, steviol administration at a 1000 ug/mL concentration effectively reduced cell viability and induced apoptosis in colon cancer cells
[44][45][68,69]. The results of a study that investigated the effect of 17 steviol derivatives on different cancer cell lines showed a potent cytotoxic effect of those derivatives on the cell lines
[46][70]. Glycyrrhizin is also reported to possess apoptotic activities on GI cancers
[47][71]. The administration of glycyrrhizin on SW48 colorectal cancer cells induced apoptosis as the levels of regulator proteins such as Bax expression increased and Bcl-2 levels decreased
[48][72]. HT-29 colon cancer cells treated with different concentrations of
glycyrrhiza glabra L. reported the induction of apoptosis at a concentration of 200 μg/mL
[49][50][73,74]. Additionally, Wister rats administered 15 mg/kg of glycyrrhizic acid were reported to induce apoptosis, suppress precancerous lesion development, and reduce inflammation
[51][75]. In a different study, the oral administration of glycyrrhizic acid (15 mg/kg) in Wister rats once a week for 15 weeks induced apoptosis by enhancing the expression of cleaved caspase 3
[52][76]. The induction of apoptosis through pro-caspases 3, 8, and 9 was reported in gastric cells treated with glycyrrhizic acid
[53][77]. The sweetener neohesperidin dihydrochalcone, administered to an APC min/+ transgenic mouse model, inhibited colorectal tumorigenesis and induced apoptosis
[54][78]. Phloroglucinol (PG), a metabolite of NHDC, induced apoptosis in HT-29 cells via overexpressed caspase-3 and caspase-8, modified Bcl-2 family proteins, and cytochrome c release
[55][79]. In another study, PG protected mice’s intestinal damage from ionizing radiation by increasing apoptosis by affecting the p53, Bax, Bak, Bcl-2, and Bcl-X
S/L proteins
[56][80] The literature still lacks the evidence to show the underlying mechanism of the observed effect of sweeteners on GI cancers.
Figure 5 summarizes the effect of sweeteners on the apoptotic pathway.
Figure 5. Illustrations of the influence of sweeteners on the apoptotic pathway in GI cancers. The figure highlights the pathological changes in apoptosis due to GI cancer, the sweeteners’ effects, and the mechanisms through which the sweeteners target the pathway. Created with
BioRender.com (accessed on 15 July 2023).
2.2. The Nuclear Factor-κB Pathway
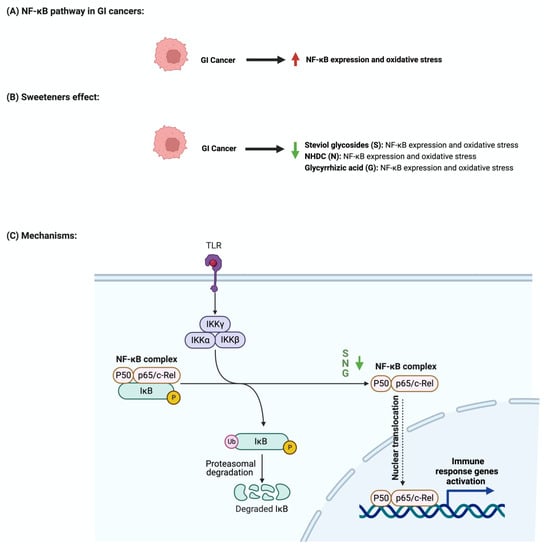
The nuclear factor-κB (NF-κB) pathway regulates genes that regulate inflammatory and immune responses
[57][81]. In cancer, NF-κB promotes cellular proliferation and metastasis and suppresses apoptosis
[58][82]. Although not abundantly discussed in the literature, multiple reports support the role of sweeteners in NF-κB pathway regulation
[59][83]. Stevioside administration to a colon carcinoma cell line (Caco-2) suppressed the expression of inflammatory cytokines IL-6, TNF-a, and NF-κB
[60][84]. Additionally, the administration of glycyrrhizic acid inhibited NF-κB expression, which led to the deactivation of inflammatory mediators in colon cells
[50][61][74,85]. In Wister rats, the administration of 15 mg/kg of oral glycyrrhizic acid reduced the expression of NF-κB, nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2)
[52][76]. Neohesperidin dihydrochalcone, along with the two other sweeteners, influenced NF-κB expression. Oral administration of neohesperidin dihydrochalcone in mice for six days attenuated the expression of NF-κB
[62][86]. Neohesperidin dihydrochalcone inhibited the induced NF-κB expression in paraquat-induced acute liver injury
[63][87]. More efforts and standardized steps are required in order to conduct more research in this field and to understand the underlying mechanism of this effect.
Figure 6 summarizes the effects of sweeteners on NF-κB expression.
Figure 6. Illustrations of the influence of sweeteners on NF-κB in GI cancers. The figure highlights the pathological changes in NF-κB due to GI cancer, the sweeteners’ effects, and the mechanisms through which the sweeteners target the pathway. Created with
BioRender.com (accessed on 15 July 2023).
2.3. Cellular Cycle Arrest
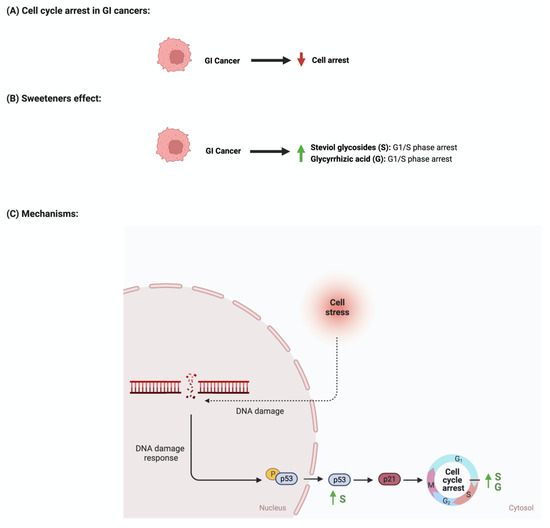
The development and function of every tissue depend on the cellular decision to transition from a proliferative to an arrested state
[64][88]. Cancerous cells dysregulate cell cycle arrest and continue to undergo uncontrolled cellular growth
[65][89]. The effect of sweeteners on cellular cycle arrest is scarcely reported in the literature. In a study that investigated the effect of steviol on gastric (HGC-27) and colorectal (Caco-2) cancer cells, it was reported that an increase in the expression of p53 and a decrease in the level of cyclin D occurred. Additionally, the researcher reported that steviol treatment caused G1 arrest in both cell lines
[43][44][67,68]. Glycyrrhizic acid administration to different gastric cancer cell lines (e.g., MGC-803, BGC-823, SGC-7901) induces cell cycle arrest through the downregulation of G1 phase proteins such as cyclin D1, D2, D3, E1, and E2
[50][53][74,77]. In addition, 18β-glycyrrhetinic acid, another metabolite of glycyrrhizin, promoted gastric cancer cell autophagy and induced cell cycle arrest in the G0/G1 phase in a transplanted nude mouse model modulating the miR-328-3p/STAT3 signaling pathway
[66][90]. Similar results were also reported for other cancers, such as cervical cancer
[67][91]. Additional information regarding the observed effect was not reported for other sweeteners, which shows that more collaborative efforts are needed in order to pursue more research in this field.
Figure 7 summarizes the effects of sweeteners on cell cycle arrest.
Figure 7. Illustrations of the influence of sweeteners on cell cycle arrest in GI cancers. The figure highlights the pathological changes in cell cycle arrest due to GI cancer, the sweeteners’ effects, and the mechanisms through which the sweeteners target the pathway. Created with
BioRender.com (accessed on 15 July 2023).
2.4. Synthetic Sweeteners and GI Cancers
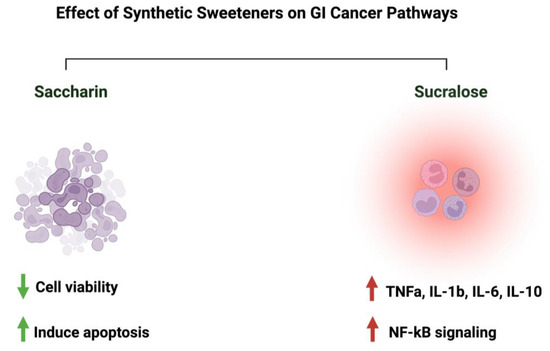
Discussing those two sweeteners raises many questions about their associated risk with gastrointestinal cancers. In an Italian cohort comprising 230 patients with histologically confirmed gastric cancer, after correcting for confounding factors, the researchers reported a lack of adverse effects of saccharin on the risk of developing neoplasms
[68][23]. Additionally, a review paper that discussed 22 cohorts and 46 case–control studies on the effects of sweeteners on different cancers concluded that there was a lack of evidence, but there was a link between saccharin, sucralose, and other sweeteners and cancer risks
[69][92]. Additionally, a study that used the intestinal epithelial cell line Caco-2 to investigate the effects of commonly used sweeteners reported that the administration of saccharin induced apoptosis at a lower concentration (100 uM), while at a higher concentration (1000 uM), it induced cellular death. The same effect was not observed for sucralose
[70][93]. However, other studies reported negative effects of sucralose on colorectal cancer. A murine model administered 1.5 mg/mL of sucralose for six weeks reported a significant increase in the number and size of colorectal tumors. Also, these researchers reported an effect on the gut microbiome and inflammatory markers (TNFa, IL-1b, IL-6, IL-10, and TLR4/Myd88/NF-kB signaling)
[71][94]. The list of studies discussing this effect is growing. However, more efforts from the research community are needed in order to address those differences in a systemic and mechanistic way, as well as to standardize the protocol to be followed and the appropriate dosage used, as it directly affects people’s health through food intake.
Figure 8 illustrates the effects of synthetic sweeteners on GI cancers.
Table 1 summarizes the available literature on the observed effects of all the sweeteners discussed herein.
Figure 8. Summary of the influence of synthetic sweeteners on GI cancers. The figure highlights the sweeteners’ positive or negative effects on GI-targeted pathways. Created with
BioRender.com (accessed on 15 July 2023).
3. Safety of Sweeteners and Challenges in the Field
3. Discussion
3.1. Safety of Sweeteners and Challenges in the Field
Recently, the discussion about the safety of one of the commonly used sweeteners in the food industry, “aspartame”, and its possible carcinogenic nature raised more questions about the safety of other sweeteners. Here, and in most of the reported articles, it has been shown that these natural and synthetic sweeteners lack genotoxicity and carcinogenicity and are safe when consumed in moderation
[72][73][74][75][96,97,98,99]. Throughout our research in the literature, most of the utilized concentrations/dosages of the sweeteners did not show adverse negative effects on the model which was used. However, some reports linked the consumption of specific sweeteners to cancer development
[71][94]. Those results show the urgent need to address the field’s main issues. First, protocol standardization, starting from the model used, mode of administration of the sweeteners, duration of the experiment, bioinformatics tools to interpret the results, and estimation of safety measures, is critical to ensure productivity and reproducibility. Second, “recommended dosage” determination, while considering other factors such as geographical location and age, might help us to understand those sweeteners’ consumption rates. Third, guidelines and regulatory process evaluation are crucial to ensure manufacturing safety. Fourth, the possible synergistic effects of sweeteners need further investigation, as these might occur when consuming different products that contain different sweetener types and dosages.
Currently, people are more aware of their health in terms of food and always search for “healthier” and low-caloric options as alternatives while maintaining a sweet taste. The controversy regarding the safety of sweeteners raises another important question: what would be the alternative to using sweeteners? Would
rwe
searchers go back to refined sugar, or move toward natural compounds such as flavonoids and phytochemicals? What are the safety and taste estimates of the consumption of those alternatives compared to sweeteners?
researchersWe have reported the positive effects of flavonoids on GI cancers and the gut microbiome for years. However, more efforts are required in order to evaluate whether they will be a “better” alternative, considering their bioavailability
[76][77][78][79][100,101,102,103]. Additionally, the effect of this “better” alternative on the gut microbiome needs more attention.
Although rwesearchers encourage more research to be conducted, there are limitations associated with this field. First, the misreporting of participants in terms of the amount/type/quantity of sweeteners consumed might affect the interpretation of the results. Second, selection bias involved in the conducted experiment/tested population would affect the generalizability of the results to the general population. Third, residual confounding shows the urgent need to develop bioinformatics tools that correct for those factors. Fourth, causality concerns are also prominent, along with how to correctly evaluate causality and differentiate it from correlation. Other limitations may include the experimental and interpretational challenges associated with linking specific bacterial species to the metabolism of sweeteners. Addressing those limitations in future studies could help us to improve the research outcomes.
4. What about Aspartame?
3.2. What about Aspartame?
More controversial discussions emerged when the World Health Organization (WHO) announced aspartame as a possible carcinogen. Aspartame is a sweetener used as a replacement for sucrose due to its high sweetness intensity
[80][105]. The effect of aspartame on the gut microbiome has been reported in limited studies. In mice (C57Bl/6) treated with different non-caloric artificial sweeteners, including aspartame, some effect on the gut microbiome abundance and metabolic pathways was reported
[81][16]. Additionally, the fasting glucose concentrations and the abundances of Enterobacteriaceae and
Clostridium leptum were increased in diet-induced obesity models treated with aspartame for eight weeks
[82][106]. With gastrointestinal cancers being the focus of this review, using aspartame (15 and 30 mM) for HT-29 human colorectal carcinoma proved to have a pro-angiogenic effect
[83][107]. However, consuming artificial sweeteners, including aspartame, was not associated with colorectal or stomach cancers
[84][108]. These data show the urgent need to address those controversial results, putting into perspective the model and the concentration of aspartame used.
4. Conclusions
Sweeteners are intense substances used in the food industry as alternatives to table sugar. Debates about the safety and the effect of using those sweeteners on the gut microbiome and the overall health status have gained attention recently. Throughout our study, we reported the relationships between three natural sweeteners (steviol glycoside, glycyrrhizin, neohesperidine dihydrochalcone) and two synthetic sweeteners (saccharin and sucralose) and the gut microbiome. Although relevant to the recent WHO statement, we did not include a detailed analysis of “aspartame” in our analysis, as, to our knowledge, there are limited data on the potential influences of aspartame on the human gut microbiome. We also discussed the effect of either the five sweeteners alone or, if supported by the literature, their metabolites in cancer-related pathways such as apoptosis and cell cycle arrest.
There are differences between countries regarding the various NNS types that are considered safe for human consumption; however, on the other hand, there is no proven linkage to cancer. In this review, we also addressed some of the challenges associated with the field, as well as the efforts required to improve such aspects, such as protocol standardization, systemic evaluation, and guideline regulations. Generally, the gut microbiome’s involvement in sweetener metabolism might be an interesting and promising field for futuristic cancer treatments, primarily when combined with the currently available therapeutics.