1. Introduction
Pancreatic cancer was responsible for 466,000 deaths worldwide in 2020; yet, the total number of cases diagnosed was only marginally greater (496,000)
[1]. It is the seventh leading cause of cancer death across the sexes
[1], though projections show it will rise to third place by 2025
[2]. This is in part due to the lack of biomarkers and imaging tools for early detection; it is also due to its aggressiveness
[3]. Pancreatic ductal adenocarcinoma (PDAC) accounts for 85–95% of all solid pancreatic tumours
[4] and usually originates in the exocrine cells of the pancreatic ducts. One of the most distinct features of PDAC is its high stromal content, which can constitute 90% of the tumour
[5]. The complex interplay between stromal components and cancer cells permits both disease initiation and progression, including metastasis to other organs
[6][7][6,7]. The greatest challenge in PDAC treatment is the continued production and deposition of a fibrous extracellular matrix (ECM), which is refractory to most interventions including chemo- and immunotherapies
[8]. The dense tissue gives rise to a hypovascular tumour with elevated interstitial fluid pressure (IFP), resulting in difficulties in drug delivery and distribution
[9]. Because of this, resistance to current therapies remains a major challenge.
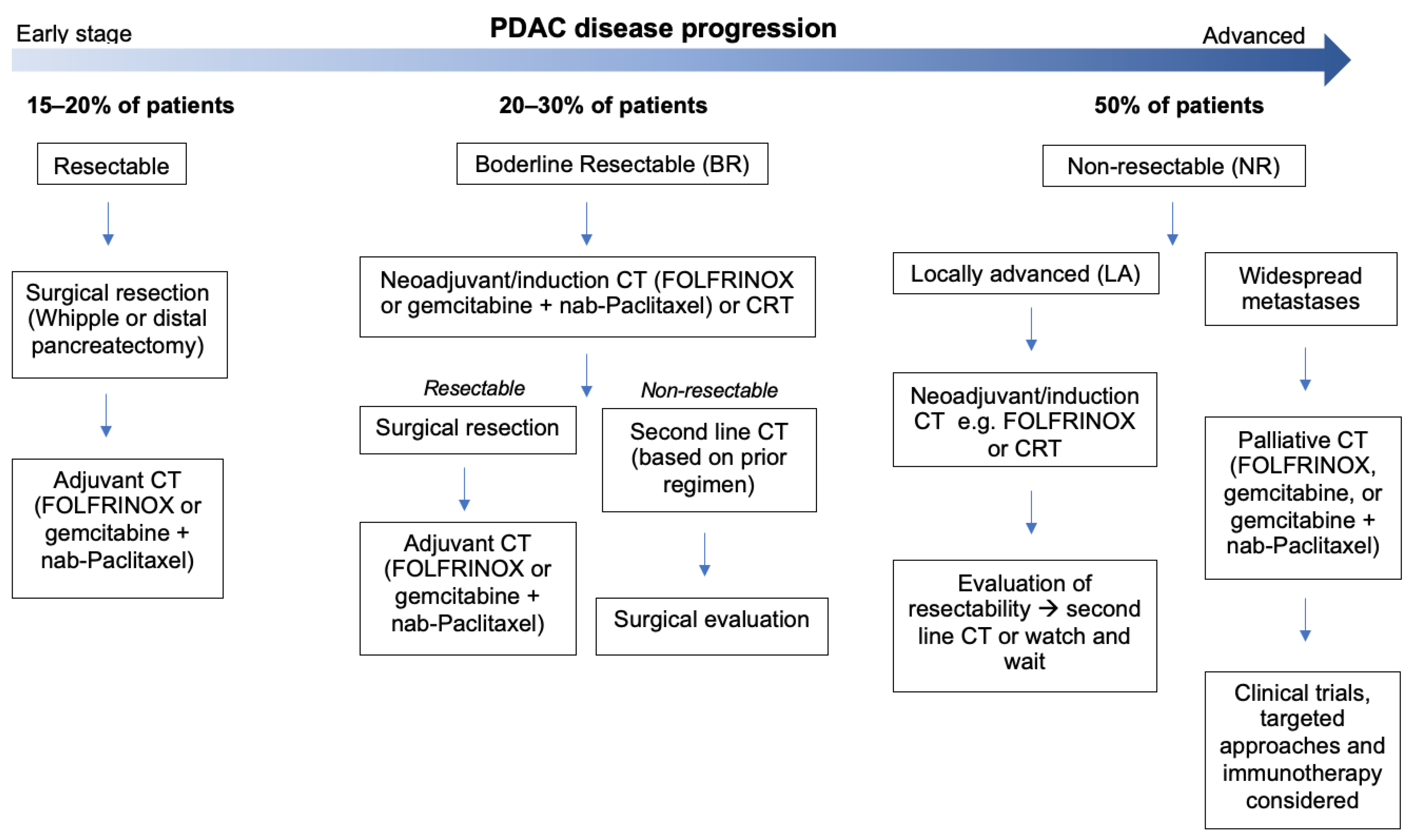
Given that surgical resection with radical intent remains the only curative approach in the clinical management of pancreatic cancer
[10], the state of disease at diagnosis is classified as resectable, borderline resectable (BR), or non-resectable (locally advanced or metastatic) according to strict criteria
[11][12][13][11,12,13]. Treatment commences accordingly (
Figure 1).
Figure 1. An outline of the standard treatment pathways for pancreatic ductal adenocarcinoma. At initial presentation, just 15–20% of patients are suitable for upfront resection. Adjuvant CT (AC) is the standard of care (SOC) following resection in PDAC. A greater proportion of patients present with borderline resectable (BR) disease (20–30%) and typically receive neoadjuvant CT (NACT) before surgical evaluation. Half of patients present with initially non-resectable (NR) disease, though if locally advanced (LA) surgical evaluation will be considered following NACT. As there is little evidence supporting surgical resection for metastatic PDAC, palliative CT is adopted. CT: chemotherapy.
A pancreaticoduodenectomy (Whipple) or distal pancreatectomy is the most commonly performed first-line treatment for PDAC
[14]. Whilst these surgeries can increase long-term survival, they infrequently achieve R0 resection and negatively impact on quality of life. Without any additional therapies, 60–80% of patients relapse within the first year
[15][16][17][15,16,17]. Combined with the fact that just 10–20% of patients are suitable for resection at diagnosis
[18], most cases require a multi-modal treatment strategy. Surgical resection followed by adjuvant CT is the standard approach for resectable PDAC following years of clinical trials suggesting its benefit to overall and disease-free survival (DFS)
[19][20][21][19,20,21]. The standardised regimens include gemcitabine (Gem), a nucleoside analogue, or FOLFIRINOX (FFX), a combination of leucovorin, fluorouracil, irinotecan, and oxaliplatin. The latter is more effective, with one study showing a 3-year DFS of 39.7% vs. 21.4% with Gem
[21]. However, 30–40% of patients fail to receive adjuvant chemotherapy due to postoperative complications
[22][23][24][22,23,24] or early disease progression
[25][26][25,26]. Of those that do begin postoperative chemotherapy, many fail to complete the course due to toxicity and decrease in performance status
[27][28][27,28]. Ultimately, 60–75% of patients receiving FFX and other chemotherapies soon relapse
[29][30][31][32][29,30,31,32], with the median overall survival (OS) following surgical resection and CT being ~20–40 months
[33][34][35][33,34,35].
Due to the inadequacy of this approach, neoadjuvant chemotherapy (NAT) has recently been implemented for PDAC, mostly in the BR and locally advanced (LA) settings
[36]. NAT can downstage advanced tumours to a resectable state, select patients with favourable tumour biology for surgery, improve margins, decrease node positivity, and control micrometastases
[37][38][39][37,38,39]. A significant survival benefit has been observed with NACT for BR, LA, and early (upfront resectable) PDAC
[40][41][42][40,41,42]. For many, the combination of adjuvant and neoadjuvant CT/RT has the greatest impact on OS
[43][44][45][46][47][43,44,45,46,47], though treatment success can be influenced by many variables, including patient age or the location of disease in the pancreas
[48][49][50][51][48,49,50,51]. Unfortunately, as ~50% of cases are metastatic at presentation, and many additional patients develop local recurrences or metastases during treatment
[52][53][54][52,53,54], most patients end up receiving palliative CT. In this setting, one study found that OS was 11.1 months for FFX and 6.8 months for Gem, with median response rates of 31.6% and 9.4%, respectively
[55]. Another study found that the response rate of metastatic PDAC to Gem was just 7%, with an OS of 6.7 months, whereas the combination of Gem plus nab-paclitaxel (GnP) gave rise to a better response of 23%, with an OS of 8.5 months
[56]. FFX and GnP remain the recommended palliative treatments for PDAC
[57].
As most cases of PDAC are highly refractory to systemic treatments, including chemo- and radiotherapies, and their benefit to survival is very low, various targeted agents have been investigated in combination alongside classical cytotoxics
[58][59][60][58,59,60]. These are designed around several key areas, including PDAC cell metabolism, deoxyribonucleic acid (DNA) repair, immune response, and the tumour microenvironment (TME). Whilst some have shown promising results in early studies, few have achieved more than a marginal benefit in DFS or OS in larger randomised trials
[61][62][63][64][61,62,63,64]. This lack of efficacy may stem from inter- and intra-tumoural heterogeneity, lack of specific biomarkers to predict effectiveness of target blocking, the use of specific CT regimens, adverse effects necessitating dose reduction, development of resistance, or, most notably, the dense, hypovascularised stroma
[9][65][66][9,65,66]. The PDAC stroma contributes to drug resistance by preventing the entry of therapeutic agents into tumour cells, restricting their anti-tumour effects
[67][68][69][67,68,69]. Destroying the stroma could alleviate this and improve patient outcomes. However, many initial anti-stromal therapies for PDAC have had toxic effects, lacked efficacy, and added little to DFS or OS
[70][71][72][70,71,72]. Some have even been associated with accelerated PDAC progression
[73][74][73,74]. An alternative solution is the use of PDT, which has not only proven to be feasible and safe for the destruction of PDAC cells
[75][76][77][75,76,77] but might also play a role in stromal depletion
[78][79][80][78,79,80]. Generally, PDT is well tolerated and does not depend on any specific molecular aberrations, making it applicable to more patients. One possibility is that by destroying the stroma, PDT could enhance the activity of targeted agents as well as traditional cytotoxics.
2. The PDAC Tumour Microenvironment and Its Regulation
The PDAC tumour microenvironment (TME) describes the confinement of a pancreatic tumour and the extensive constituents that surround and support it. The TME has two compartments: the stroma and the ECM. Whilst the ECM more specifically comprises non-cellular structural proteins, including collagens I, III, IV and V, integrins, proteoglycans, and glycoproteins
[81], the stroma contains an extensive mix of fibroblasts, bone marrow-derived stem cells, pancreatic stellate cells (PSCs), immune cells, neurons, and blood and lymphatic vessels, as well as the ECM
[82]. The most prominent immune cells are myeloid-derived suppressor cells (MDSCs), macrophages, mast, and effector T-cells
[83]. Together, these produce an immunosuppressive effect resulting in the reduction in the anti-tumour activity of the immune system
[84].
In PDAC, the desmoplastic reaction describes the initial and continued production of ECM in response to an invasive carcinoma
[85]. As the reaction proceeds, the area becomes stiffened and increasingly dense, and eventually, the TME adopts very different mechanical and biophysical traits compared to those of healthy pancreatic tissue
[86].
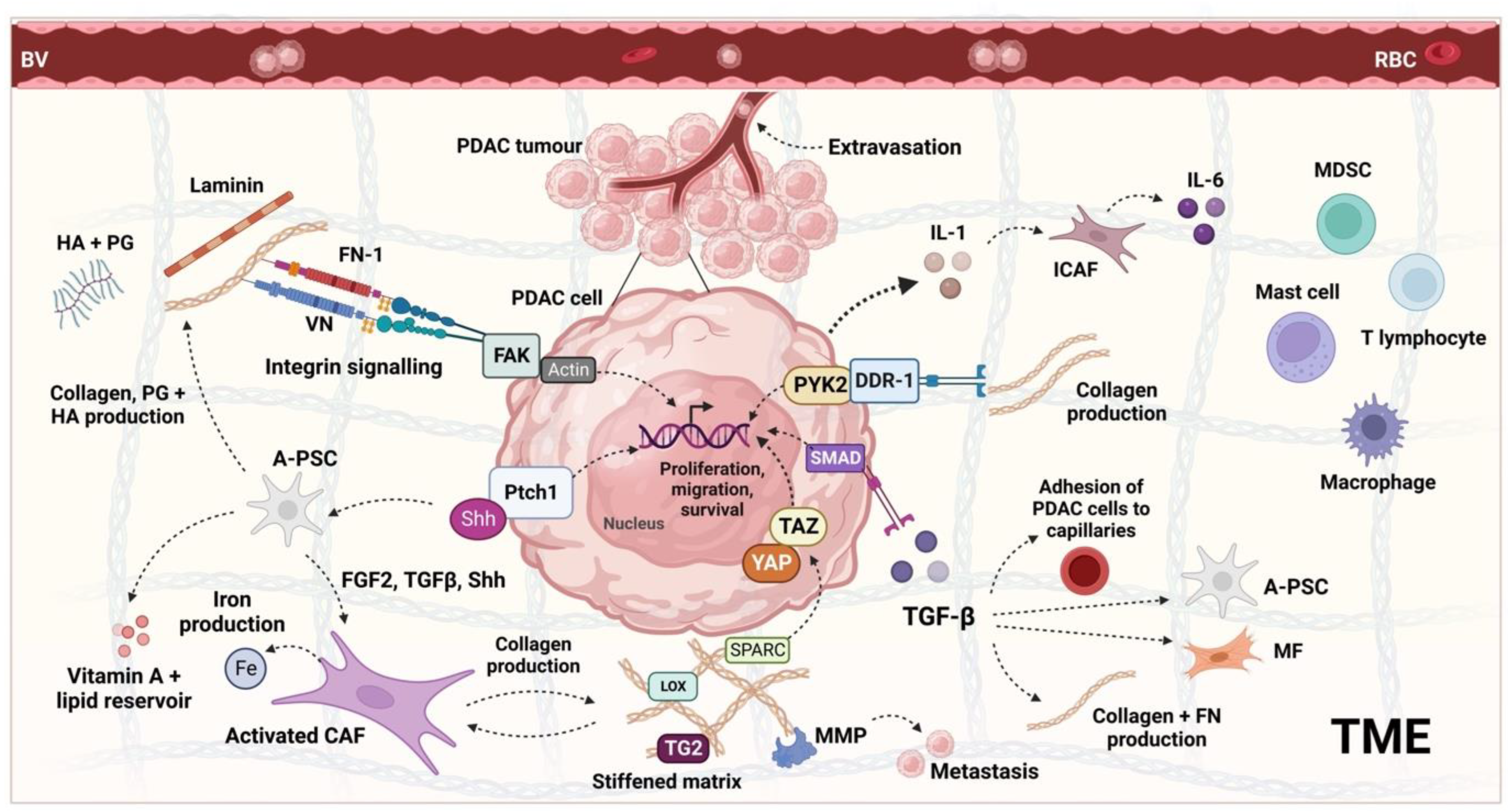
The close link between these TME components and PDAC progression (
Figure 2) has led to the development of various stromal depletion strategies, though most have failed. It is theorised that this is because the TME is highly heterogeneous between and within PDAC tumours
[87]. By reviewing 143 treatment-naïve resections and pre-treatment biopsies from over 200 cases of advanced PDAC, a 2021 study noted several recurrent histological patterns in the TME and named these ‘subTMEs’
[88]. There were three regions identified: ‘deserted’, characterised by a keloid or myxoid type ECM with thin, spindle-shaped fibroblasts; ‘reactive’, where the ECM comprises very few acellular components, and yet the fibroblasts are enlarged; or there are those with features of both. Comparing the TME and tumour cell samples, there were 5050 differentially expressed genes (DEGs) and 12,704 ribonucleic acid (RNA) DEGs, including known TME markers such as platelet-derived growth factor receptor alpha (PDGFRA). Tissue microarray (TMA) analyses of resections showed that deserted subTMEs had increased collagen content and B-cell marker (cluster of differentiation 20 or CD20) expression, whereas the reactive subTMEs had higher staining for cancer-associated fibroblasts (CAF), macrophages, T-cells, and endothelial cells. Of clinical interest, the co-occurrence of sub-TMEs in 53% of resections was a poor prognostic variable, as was the presence of a ‘deserted subTME’ due to its protective effect against Gem, GnP, and FFX. Though the effect of the TME subtype on the efficacy of anti-stromal agents has not yet been investigated, several other studies have highlighted variations in stromal composition and assigned a prognostic significance
[89][90][89,90]. Moffitt et al. (2015)
[89] used primary and metastatic TMA data to digitally separate the gene expression between PDAC stroma and normal tissue samples. The samples were clustered into three groups: those with ‘activated stroma’, ‘normal stroma’, or ‘absent stromal gene expression’. Kaplan–Meier analyses showed that the ‘activated stroma’ group had worse prognosis, with a median survival time of 15 months and a 1-year survival rate of 60% vs. 24 months and 82% in the ‘normal stroma’ group.
Figure 2. The main PDAC TME components and tumourigenic signalling pathways. The TME is a complex mixture of structural (ECM) and cellular components that play a reciprocal role in the progression of the stroma and the cancer itself. Facilitating many of the interactions between the tumour and stromal components are integrins. Via connecting proteins FN-1/VN-1, collagens interact with the integrins on the PDAC cell surface, which activates FAK and converts actin polymerisation into traction force, driving tumour cell invasion. Collagen also interacts with DDR-1, which binds and activates several signalling proteins, including PYK2, leading to the upregulation of pro-survival genes such as Hes1 and migratory pathways via p130Cas/JNK. The result is the upregulation of N-cad, which promotes the EMT and acquisition of a motile phenotype. Upon Shh pathway activation, PSCs form CAFs, which are the greatest producer of the ECM components, including collagen, PG, and HA. Secreted proteins SPARC, LOX, and TG2 mediate collagen crosslinking, which leads to tissue stiffness, the production of further collagen, and the activation of YAP/TAZ, which promotes cell proliferation. MMPs, which are upregulated by integrin signalling, also modulate the proteolytic activity of the matrices, leading to the release of individual PDAC cells from the TME. Cytokines such as TGF-β and IL-1 released from the PDAC secretome have many functions, including promotion of further collagen production, activation of CAFs, and the adhesion of PDAC cells to capillaries. Activated fibroblasts have several functions: MFs are crucial for the continued deposition of ECM components, whereas ICAFs release various tumour-promoting inflammatory molecules such as IL-6, which enhance the formation of the immunosuppressive environment by regulating immune cell activity. They also support the metabolism of PDAC cells, such as by providing them with labile iron for survival or acting as a cellular reservoir for vitamin A and lipids. BV, blood vessel; RBC, red blood cell; PSC, pancreatic stellate cell; CAF, cancer-associated fibroblast; MDSC, myeloid-derived suppressor cell; TGF-β, transforming growth factor beta; HA, hyaluronan; FN-1, fibronectin 1; VN, vitronectin; DDR-1/2, discoidin receptor ½; PYK2, FAK-related protein tyrosine kinase; FAK, focal adhesion kinase; MMP, matrix metalloproteinase; TG2, tissue transglutimase; LOX, lysyl oxidase; Shh, sonic hedgehog; FGF2, fibroblast growth factor 2; YAP, yes-associated protein; TAZ, transcriptional co-activator with PDZ-binding motif; ICAF, inflammatory cancer-associated fibroblast; MF, myofibroblast; A-PSC, activated pancreatic stellate cell; PG, proteoglycan; SPARC, secreted protein acidic and rich in cysteine; EMT, epithelial to mesenchymal transition; IL-6, interleukin 6; IL-1, interleukin 1; Fe, iron; PDAC, pancreatic ductal adenocarcinoma; JNK, c-Jun N-terminal kinase; N-cad, n-cadherin; TME, tumour microenvironment; ECM, extracellular matrix; SMAD, mothers against decapentaplegic; Ptch1, patched-1 receptor. The figure was created on Biorender.com (accessed on 5 January 2023).
It has since been suggested that pancreatic cancer stem cells (PCSCs) could be responsible for the stromal variation and subsequent effects on disease outcomes in PDAC
[91]. As cluster of differentiation-44 (CD44) and epithelial-specific antigen (ESA) are PCSC cell surface markers, one study analysed PDAC surgical resection tissue (n = 93) and found that a ‘loose’ stroma with fewer fibroblasts was highest in CD44
+/ESA
− (63%) and CD44
+/ESA
+ (50%) tumours and that a dense, fibroblast-rich stroma was highest in CD44
−/ESA
− tumours. In a 20-month follow-up study, no local recurrence was observed in patients with dense stroma, with the highest rate of recurrence seen in loose stroma (7% [95%CI: 1–19%] at 1 year and 18% [95%CI: 6–35%] at 3 years) (n = 31), but this was not statistically significant (
p = 0.230). Loose stroma was linked to shorter OS (median of 16.1 months [95%CI: 12.4–32.8] vs. 48.5 [95%CI: 16.0–NR]) as compared to dense stroma (
p = 0.025). After adjusting for nodal status, this was not significant (
p = 0.061). Despite this, the data suggest that stemness is linked to a loose stroma and worse outcomes in PDAC, suggesting that the stem cell compartment may dictate features of the stroma through differentiation of its components. Taken together, this suggests a need for patient stratification based on stromal heterogeneity, though in the context of PDT it is unclear what effect this would have.
3. PDT Mechanism and Clinical Progress
PDT is a form of photoactive therapy that elicits a cytotoxic effect via the release of reactive oxygen species (ROS) using a light-activated photosensitiser (PS). The treatment is somewhat specific owing to the partially selective accumulation of the drug within the target tissue and the targeting of the light to the tumour and its direct surroundings. The attractiveness of this approach has led to its use for several diseases, including oesophageal, mouth, and lung cancer
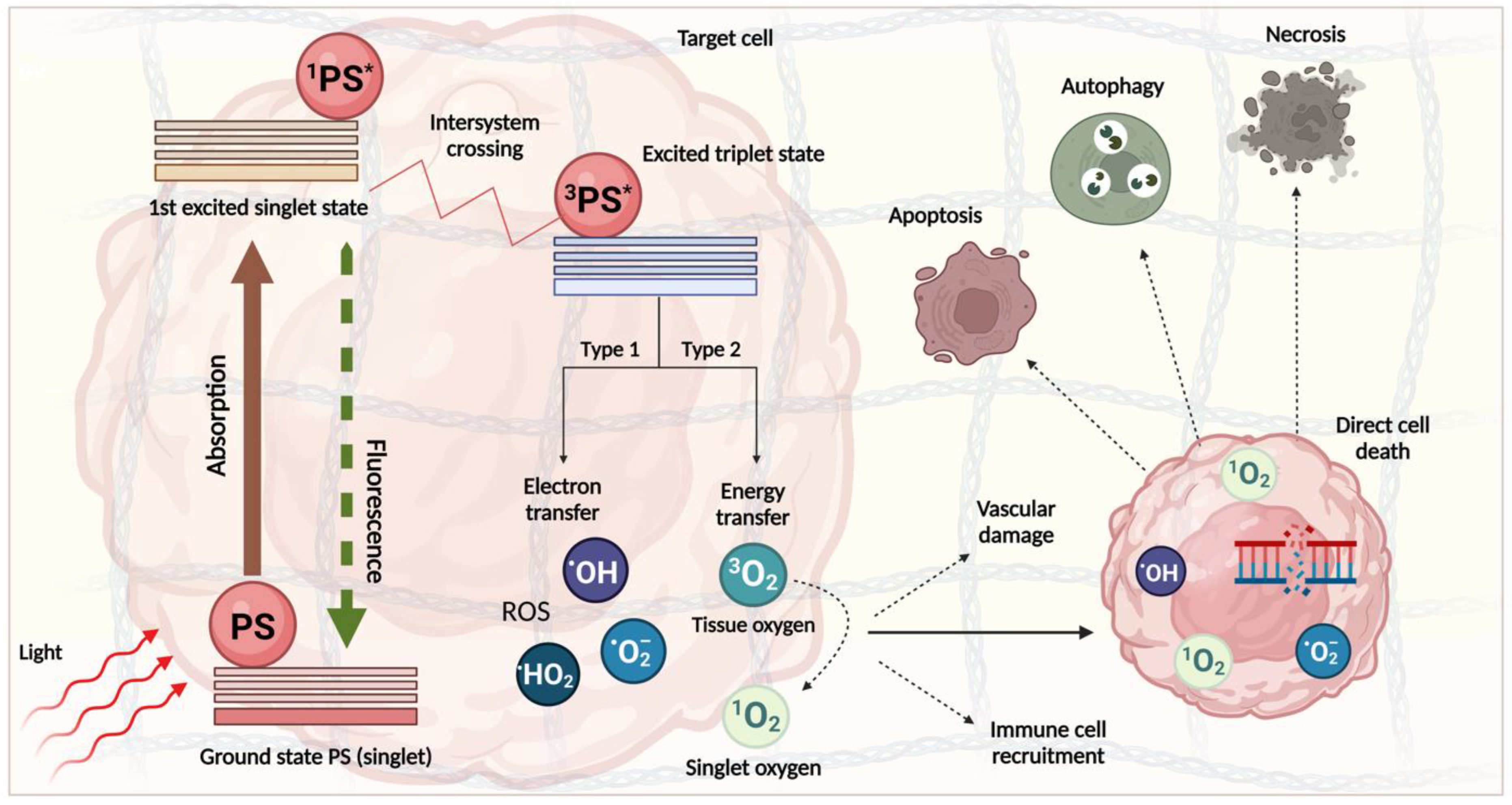
[92][93][94][92,93,94]. PDT has proved efficacious in destroying advanced PDAC tumours in preclinical and clinical trials, though investigation is ongoing. The mechanism of PDT is well understood. Once the PS molecule is irradiated with a light wavelength corresponding to its absorption spectrum, it undergoes excitation, and the reaction proceeds down one of two routes (
Figure 3). The type 2 reaction, where energy is transferred to a ground-state triplet oxygen molecule to form highly reactive singlet oxygen, is most common amongst currently approved PS molecules. This then exerts its effect on the target tissue via the introduction of oxidative stress and the subsequent destruction of various cellular components
[95]. The damage directly initiates cytotoxic death via several forms, including apoptosis, autophagy, and necrosis, though other less common modes of death have been noted
[96]. PDT also has two indirect effects: the instigation of a ROS-initiated inflammatory response causing an immune reaction and the injury of vascular endothelial cells leading to tumour vessel damage and the obstruction of blood flow. Importantly, the blocking of this flow is dependent on the timing between the drug and light applications (the drug–light interval or DLI). A shorter light application time following drug application favours this process. These responses play a significant role in light-mediated tumour destruction, with many protocols now being modified to enhance them further
[97][98][99][97,98,99]. However, for PDT-mediated stromal depletion, there are practical considerations when targeting vascular and immune cell components owing to the diverse roles and heterogeneity of the PDAC stroma.
Figure 3. The mechanism of action PDT. After the photosensitiser (PS) is taken up by the cancer cell, light is delivered to the treatment area and absorbed by the PS, which adopts an excited singlet state (1PS). The PS is transferred to an excited triplet state (3PS) through the process of intersystem crossing and transfers an electron to biological molecules to form free radicals, including the hydroxyl radical (OH), hydroperoxyl radical (HO2), or the superoxide anion radical (O2−) (Type 1 reaction), or transfers energy directly to tissue oxygen, generating unstable singlet oxygen (1O2) (Type 2 reaction). Inside the cell, these highly reactive molecules lead to oxidative stress and DNA damage, rendering the cell susceptible to several forms of death, including apoptosis, necrosis, or autophagy. This figure was created on Biorender.com (accessed on 5 January 2023).
The benefits of PDT include its ability to alter specific cellular targets depending on how the therapy is used. Factors such as the type of PS, light dose, light–dose interval, and drug dose–light dose interval all have an impact on the cytotoxic effect
[100][101][100,101]. For example, different photosensitisers tend to localise within different organelles; this determines the type of cell death due to the initiation of different signalling pathways
[102][103][102,103]. The PS can also be modified to increase targeting to the tumour vasculature, rather than the cancer cells
[99][104][99,104]. Whilst these data have previously only been used to improve the efficacy of PDT and decrease its off-target effects, this control could be leveraged in the context of stromal depletion to achieve a balance between its destruction and maintenance.
Clinical Application of PDT for PDAC
PDT using mTHPC (meta-tetra(hydroxyphenyl)chlorin) first entered clinical trials for PDAC in 2002
[105]. The first study established the efficacy and technical feasibility of percutaneous, computerised tomography (CT)-guided light delivery for PDT in unresectable PDAC. A median survival time of 9.5 months after PDT was recorded. The next trial used a shorter-acting PS, Verteporfin, which enabled better penetration of light to the tissue
[75]. A year later, the first phase I trial of the endoscopic ultrasound (EUS)-guided, flexible laser light catheter PDT was performed, and no negative effects were observed
[106]. A 2018 trial confirmed these results using Porfimer Sodium (Concordia Laboratories Inc, St Michael, Barbados)
[76]. Patients were given Gem/GnP following PDT, and the median OS was 11.5 months. The most recent 2021 study of EUS-guided Verteporfin-mediated PDT had a median OS of 6.8 months
[77]. It is difficult to draw conclusions regarding survival advantages from small, nonrandomised studies with few participants and a variable treatment history. In some instances, subsequent oncological treatments were given. Though larger, randomised studies are required to address this, the clinical trials so far have suggested a useful role for PDT as a (neo)adjuvant in PDAC, or even as a single therapy. In addition, all the studies were of patients with LA disease who either did not respond to previous CT or were unsuitable for surgery. It is only within this patient population that PDT has so far been examined, though its use for stromal depletion may be more useful for different stages of PDAC.
4. Role of PDAC Stroma and Implications for PDT
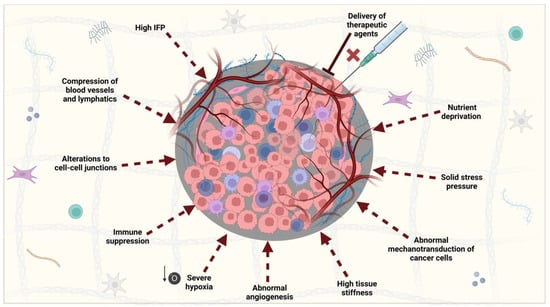
The dense peri-tumoral stroma in PDAC imparts an array of molecular and biophysical effects on the tumour and its constituents (
Figure 4). Together, these are responsible for the restriction of the delivery of treatments to the site, which is thought to be one of the reasons for poor survival. Whilst this cancer protective effect could be addressed using PDT-mediated stromal depletion, these challenges are extended to the successful clinical implementation of PDT for two reasons. Firstly, the desmoplastic reaction creates a dense environment with high compressive stress that causes blood and lymph vessels to be occluded; analysis of MMTV-M3C breast tumours showed that the fraction of perfused vessels decreased with tumour size and an associated higher level of stress
[107][127]. This is contributed to the hygroscopic nature of hyaluronic acid (HA), which causes the localised trapping of water
[108][128]. One study investigated orthotopic MiaPaCa2 PDAC tumours and found that the swelling stress was 16.01 ± 1.66 mmHg (2.13 ± 0.22 kPa), whereas in healthy tissues this was much lower, usually around zero
[109][129]. This was linearly proportional to the ratio of the HA/collagen area fraction (n = 5) and could be decreased experimentally by hyaluronidase, an enzyme that breaks down HA. Analysis of perfused blood vessels as a function of tumour swelling showed that there was an exponential decay relationship in vivo, suggesting their physical occlusion. Once the vessels are occluded, IFP, which is zero in normal tissues, rises and equilibrates with microvascular pressure as the extracellular fluid cannot drain
[110][130]. Analysis of PDAC tumour IFP levels in genetically engineered
KPC mice, which act as a clinically relevant model of PDAC, have been reported as being as high as 76 ± 4.2 mmHg vs. −0.73 ± 0.6 mmHg in the normal mouse pancreas
[111][131]. Whilst abnormal angiogenesis usually contributes to leaky vasculature in many solid tumour types, where drugs are able to exit the bloodstream more easily via the enhanced permeability and retention (EPR) effect, the occlusion and eventual collapse of blood vessels in PDAC as a result of the high pressure imposed by the stroma prevents this
[108][128]. As the movement of macromolecules into the tumour is prevented, drug delivery is restricted. A 2022 study found that serum concentrations of ‘free’ doxorubicin (Dox) in subcutaneous mouse PDAC tumours were just 0.8 µg/mL 15 min after administration, and the drug was cleared entirely by 4 h
[112][132]. The same challenges are faced with the entry of PS agents in PDAC
[113][133]. Secondly, the lack of oxygen transport makes the TME hypoxic, which restricts the efficacy of PDT by limiting light penetration as well as the actual cytotoxic effect (as this is dependent on the presence of molecular oxygen). The hypoxia also feeds into the desmoplastic reaction, which perpetuates the effect further
[114][134]. Careful depletion of the PDAC stroma by PDT might allow these obstacles to be overcome.
Figure 4. The overall physical and biophysical effects of the PDAC stroma on the tumour and its TME. The unique stroma in PDAC has a broad range of implications that prevent the delivery of therapeutic agents, mainly via their effects on blood vessels. This includes high levels of IFP, solid stress, and tissue stiffness. Adding to this effect is the unusual morphology of the vessels, owing to abnormal angiogenesis characteristic of the disease. The result of these features creates a harsh environment where PDAC cells are subject to nutrient deprivation, severe hypoxia, and immune suppression. This contributes to tumourigenesis and is further enhanced by the production of cytokines which interfere with PDAC cell–cell junctions and their mechanotransducive properties. As a result, PDAC cells gain a more migratory and invasive phenotype. IFP, interstitial fluid pressure. This figure was created on Biorender.com (accessed on 5 January 2023).