Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Muhammad Asad Farooq and Version 2 by Peter Tang.
Beta2-adrenergic receptors (β2-ARs) are an emerging class of receptors that are capable of modulating the functioning of immune cells. β2-AR is reported to activate regulatory immune cells and inhibit effector immune cells. Blocking β2-AR increases activation, proliferation, and cytokine release of T lymphocytes. Moreover, β2-AR deficiency during metabolic reprogramming of T cells increases mitochondrial membrane potential and biogenesis.
- β2-adrenergic receptor
- CAR-T therapy
1. Introduction
Neurotransmitters play a bridging role between the nervous system and the body. Catecholamines are important set of neurotransmitters released by supra-renal glands and comprise adrenaline/epinephrine (A/E) and nor-adrenaline/nor-epinephrine (NA/NE). Adrenergic receptors are activated in response to stimulation by adrenaline and nor-adrenaline to chemically coordinate the signals/messages from nervous system to the target tissues [1][2][1,2]. Adrenergic receptors (ARs) belong to the G-protein coupled, seven transmembrane receptor (GPCR) family and constitute alpha-adrenergic receptor (α-AR) and beta-adrenergic receptor (β-AR) subtypes, which further have been classified as Alpha-1 adrenergic receptors (α1-ARs), Alpha-2 adrenergic receptors (α2-ARs), Beta-1 adrenergic receptors (β1-ARs), Beta-2 adrenergic receptors (β2-ARs) and Beta-3 adrenergic receptors (β3-ARs). In short-term or acute stress conditions like fear or exercise, the body releases catecholamines via activation of the sympathetic nervous system (SNS) that bind with adrenergic receptors on various organs, resulting in increased heart rate, dilation of pupils, mobilization of energy, and diversion of the blood flow from other body organs to the skeletal muscles to cope with the aforementioned stress [3].
However, in chronic stress conditions, the body retains a persistently higher concentration of catecholamines and sustained activation of SNS, leading to the initiation and progression of cancer [4]. Many studies have demonstrated that this cancer progression is due to catecholamines triggering β-adrenergic receptors, more specifically, downstream signaling of β2-Adrenergic receptors in cancer cells [5]. Later on, it was demonstrated that psychological factors and depressive disorders can lead to cancer incidence. During stress, elevated levels of catecholamines can lead to cancer initiation by making the genome unstable, rendering them exposed to environmental carcinogens [6]. Because of this, more studies were carried out aiming to unearth the role of β2-AR in tumor initiation and progression. Recently, the role of β2-AR in immunity has been the focus of many studies. β2-Adrenergic receptors can modulate the functions of various immune cells as the corresponding receptors are displayed on the surface of T cells, natural killer (NK) cells, and dendritic cells (DCs) [7][8][7,8]. The ligand for β2-AR is released inside the tumor microenvironment (TME) and leads to inhibitory signaling in T lymphocytes [9][10][9,10].
The immune system plays a critical role in identifying and eliminating cells that undergo malignant transformation, in addition to its primary function of eradicating infectious agents. If the inflammatory response remains unresolved, the affected cells undergo transformation and become cancerous [11]. However, at this stage, the immune system identifies the tumor cells by recognizing the tumor-specific antigens displayed on their surfaces [12], followed by effector immune responses primarily mediated by CD8+ T cells and NK cells. The activation of these immune responses forms the basis for immunotherapy, as it halts tumorigenesis [13][14][13,14].
Due to the remarkable success of the initial clinical trials of chimeric antigen receptor (CAR)-T therapy, particularly in pediatric patients with hematological malignancies, the clinical response rates in leukemia patients have reached as high as 90% [15][16][15,16]. As a result, the number of successful clinical trials for CAR-T therapy targeting hematological malignancies which direct numerous antigens has upsurged significantly, yet the parallel success of CAR-T therapy in solid tumors is still being awaited [17][18][17,18]. The number of ongoing clinical trials in solid tumors is far less than in liquid tumors, courtesy of the toxic side effects and suboptimal therapeutic outcomes which are achieved almost every time. There can be several explanations for this, including the following. Hematological cancers commonly exhibit similar antigens, and their distribution across various types of hematological cancers is generally similar, with some exceptions [19]. On the contrary, antigens related to solid tumors vary greatly, not just from tumor to tumor, but also between different forms of a similar tumor, i.e., primary and metastatic forms [20]. Moreover, hematological cancers are widespread in the circulatory system, which makes them less dense and easily targetable as compared to solid tumors. The solid tumors are denser and are concentrated on a single site, creating a physical barrier for CAR-T cells by developing extensive vasculature to supply ample nutrients to the fast-growing tumor, thus inhibiting the chemo-attractive signals that are necessary for CAR-T cells to reach the tumor site [21]. The immune-suppressive characteristics of solid tumors, which arise due to the presentation of checkpoint inhibitory ligands and metabolites from diverse metabolic pathways collectively create a tumor microenvironment (TME). This microenvironment makes it exceedingly challenging for CAR-T cells to infiltrate and effectively eliminate solid tumors, leading to significant barriers to CAR-T cell therapy’s success [22]. Adrenergic stress is one of the culprits, among others, responsible for immunosuppression inside the TME. Previously, thwe researchers hhave attempted to target immunosuppressive factors inside the TME in an attempt to increase the efficacy of CAR-T therapy in the prostate and pancreatic tumor microenvironments [23][24][23,24].
2. Nor-Adrenaline in Tumor Microenvironment
The production of adrenaline and nor-adrenaline within the TME can arise from various sources, including the tumor cells themselves, the nerve fibers that innervate the tumors, and the surrounding stromal cells such as fibroblasts and immune cells. It seems that NA, mainly from the innervating nerves, plays a role in the incidence and initial progression of the cancer, as TME is absent initially. After TME is established, it is mainly the TME that pours sufficient amounts of NA into the tumor surroundings that carry its immunosuppressive roles in CD8+ T cells. This NA comes mainly from two sources. Firstly, the tumor itself contains all the necessary machinery to synthesize NA. Secondly, many tumor types are innervated by sympathetic nerve fibers [25][28]. Neuronal progenitor cells migrate towards the tumor tissue during the process of neurogenesis and adapt a sympathetic tone while innervating tumor tissue. Moreover, the sensory neurons innervating tumor tissues are also reprogrammed to sympathetic nerve phenotypes [26][29]. Altogether, this creates hyperactive SNS signaling inside the TME. Growing evidence suggest that an immunosuppressive role of the adrenergic system inside the TME could possibly hinder the antitumor functions of immunotherapy [6][27][6,27].3. Adrenergic Stress Endorses Immunosuppressive Tumor Milieu Development
The catecholamines bind to adrenergic receptors on target cells, including immune cells, and trigger a range of biological responses. In the context of the immune system, adrenergic stress can change the activities of different immune cell subsets due to its wide expression. One of the key mechanisms by which adrenergic stress promotes immunosuppression is through the recruitment and activation of myeloid-derived suppressor cells (MDSCs) [28][30]. MDSCs are regulatory immune cells that have the skill to inhibit T cells and promote tumor growth. Adrenergic stress has been shown to promote the expansion and activation of MDSCs in the tumor microenvironment through the activation of adrenergic receptors on MDSCs themselves, as well as on other cells that produce cytokines and chemokines which promote the recruitment and activation of MDSCs [29][31]. β2-AR activation on MDSCs has been shown to enhance the expression of arginase-1, an enzyme that can deplete arginine, an amino acid that is important for T cell function. Additionally, adrenergic stress inhibits T cell functions by upregulating interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β) [30][31][32,33]. The production of these anti-inflammatory cytokines, owing to the activation of β2-AR on the surfaces of tumor-associated macrophages (TAMS), also demonstrated as M2-phenotype. Adrenergic signaling in cancer plays a significant role in fostering the release of vascular endothelial growth factor (VEGF), a crucial mediator of angiogenesis, from both tumor cells and M2-macrophages residing in the tumor microenvironment [32][34]. VEGF facilitates the recruitment of endothelial cells, leading to the formation of new blood vessels, which in turn support the delivery of oxygen and nutrients to the tumor [27]. Additionally, the activation of β2-adrenergic receptors (β2-AR) can enhance the activity of regulatory T cells (Tregs), thereby amplifying the immunosuppressive environment around the tumor [33][34][35,36]. According to the literature, adrenergic stress has been shown to inhibit T cell activation and proliferation, and to promote apoptosis. Similarly, it can inhibit NK cell and B cell activation. The observed effects are facilitated through the stimulation of adrenergic receptors, leading to the secretion of cytokines and chemokines that contribute to the impairment of immune cell function [35][37]. In conclusion, adrenergic stress plays a key role in the development of the TME by promoting the recruitment and activation of immune-regulatory cells, inhibiting the function of pro-inflammatory immune cells (Figure 1), endorsing the production of immunosuppressive cytokines and chemokines, and promoting tumor angiogenesis [36][38]. Here,In the researchersis review, we discuss the role of adrenergic stress, specifically in T lymphocytes.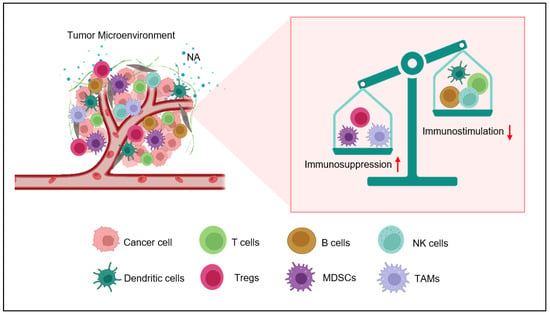
Figure 1. Nor-adrenaline (NA)-mediated immunosuppression inside the tumor microenvironment. Nor-adrenaline released in the tumor microenvironment activates β2-AR on the surfaces of various immune cells. The activation of these adrenergic receptors activates myeloid-derived suppresser cells (MDSCs) and recruits T-regulatory cells (Tregs) and M2 tumor-associated macrophages (TAMS). On the other hand, the activity of natural killer (NK) cells, T cells, and dendritic cells (DCs) is inhibited by β2-AR signaling.
