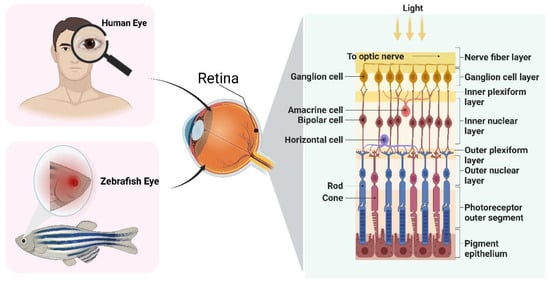
Zebrafish provide reliable endpoints for the study of retinal injury and the investigation of developmental toxicity in the ocular system as an excellent vertebrate model. One prominent method utilized in both rodent and zebrafish retina research is optical coherence tomography retinal imaging
[34][47]. This non-invasive interference technique provides cross-sectional and facial images of the rodent and fish retina, enabling precise identification of minimal lesions and significant changes in retinal structure, thus achieving high-resolution retinal imaging
[21][35][35,48]. Yet, it is worthwhile to emphasize that a variety of behavioral paradigms can be utilized to quickly, intuitively, and directly identify impairments in zebrafish visual function. Visually mediated behavioral changes have been widely utilized to evaluate the ocular toxicity of pollutants in the early stages of zebrafish life
[36][49]. In visual research, mice and rats are commonly used as primary animal models, whose retinas are mainly composed of rod cells, resulting in color vision defects or loss, relatively low visual acuity, and more reliance on olfactory, tactile, and auditory cues in bright environments
[21][35]. In contrast, the zebrafish serves as a valuable animal model for studying visually mediated behaviors, offering notable advantages. Firstly, zebrafish have a cone-dominated vision similar to humans, unlike mice, which have rod-dominated vision. Zebrafish possess cone photoreceptors that are sensitive to UV, blue, green, and red light, resulting in excellent color vision and visual acuity
[21][22][35,36]. Secondly, zebrafish exhibit strong reproductive capabilities, with externally developing embryos that are optically transparent, facilitating easy observation. Additionally, zebrafish have relatively large eyes in proportion to their body size, allowing for manipulation of the eye bud during early embryonic development
[25][37][39,50]. Thirdly, the visual system of zebrafish develops rapidly, with eye development completed by 72 h post-fertilization (hpf), enabling the direct observation of morphological defects within a short timeframe. Functional assessments can be conducted after 120 hpf, enabling faster identification of issues related to mature visual acuity compared to mice, which typically take around 15–20 days
[38][51]. Fourthly, the small size of zebrafish makes them well-suited for behavioral tests in multiwell plates, and their behavior can be effortlessly monitored using automated video tracking systems
[11][26]. Lastly, both adult zebrafish and larvae exhibit easily analyzable visual behaviors without the need for training, and there exist various robust behavioral paradigms for rapidly assessing the visual function of zebrafish and detecting visual impairments
[21][26][35,40].
3. Quick Approaches for Assessing Ocular Toxicity
Behavior integrates the responses of animals to internal and external stimuli and can be used as an indicator of the effects of environmental pollutants on animals at molecular, biochemical, and physiological levels
[39][52]. Such behavioral disorders arise from changes in neuronal structure and/or physiology. Particularly, zebrafish behavior exhibits high sensitivity to environmental pollutants, making it a particularly effective model for assessing the toxic effects of these pollutants
[39][52]. In recent times, Zebrafish behavior has been quantitatively assessed in a growing number of studies to evaluate the toxicity endpoints of pollutants in zebrafish
[40][41][53,54].
Zebrafish possess a visual processing circuitry that comprises the neural retina, optic nerve, optic tectum, and extraocular muscles. Their behavior heavily relies on a mature retina, which enables stable high-resolution imaging and eye movement reflexes that respond to fast-moving visual stimuli
[38][51]. The activity of innate visual reflexes supports a variety of visually mediated behaviors and effectively assesses the visual function of zebrafish
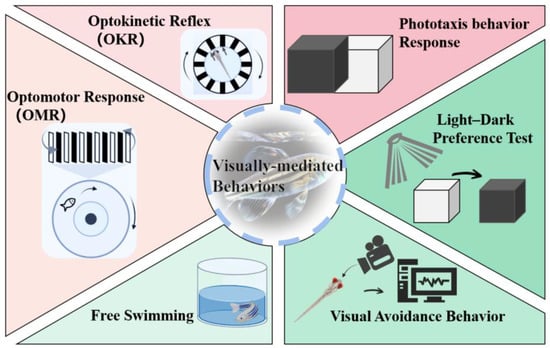
[32][11]. Several commonly used methods for studying zebrafish behavior include the optokinetic reflex (OKR), optomotor response (OMR), phototaxis, light–dark preference test, free-swimming activity, and visual avoidance behavior analysis (
Figure 2)
[21][32][42][11,35,55]. These behavioral responses have been extensively utilized in toxicology studies to identify different types of visual cues driving behavior, genes involved in vision and visual impairments, and the specific neurons and circuits responsible for perceiving visual stimuli and eliciting behavioral responses, often utilizing in vivo functional imaging techniques
[17][32].
Figure 2. Visually mediated behavioral paradigm in zebrafish.
3.1. OKR
OKR is a convenient method for evaluating visual function in awake animals, as it provides visual stimulation and relies on the functional retina without the need for training
[43][56]. The test typically involves immobilizing zebrafish in a transparent tank and stimulating them with gradually increasing or decreasing circular or vertical stripes, allowing only their eyes to move as they track the rotating stripes, followed by observing their eye movements
[38][44][51,57]. This behavior matures between 73 and 80 hpf and persists throughout adulthood
[45][58]. During OKR testing, fish with expected visual function deficiencies are anticipated to exhibit a reduced frequency of eye scanning or abnormal eye movements
[46][47][59,60]. However, there are variations in the experimental protocol for conducting the OKR test in practice. These differences include the growth stage of the zebrafish, fixation methods, rotation speed of the stripe pattern, and the parameters used for behavioral evaluation of the experimental zebrafish.
OKR is also frequently used in assessing visual function in larvae, and it has been employed in studies investigating the effects of environmental contaminants on zebrafish
[18][16]. These investigations have revealed that zebrafish larvae exposed to certain polybrominated diphenyl ether (PBDE) congeners, such as 2,2′,4,4′,5-pentabromodiphenyl ether (BDE99), exhibited decreased saccadic responses and responses to blue and green light stimuli in OKR tests assessing visual behavior
[18][16]. Similarly, exposure to polycyclic aromatic hydrocarbons (PAHs) present in crude oil led to diminished eye movements
[48][61], while exposure to the plasticizer bisphenol S (BPS) significantly impaired eye-tracking ability during visual behavior OKR testing
[43][56]. Furthermore, exposure to triphenyl phosphate (TPhP) and triclocarban (TCC) was found to reduce the OKR response, with TPhP demonstrating a dose-dependent decrease
[30][49][44,62]. In general, exposure to these environmental contaminants may have detrimental effects on the visual function of zebrafish. However, it is important to note that exposure to certain environmental contaminants can also cause zebrafish to exhibit a positive OKR. For instance, exposure to the commercial PBDE mixture, DE-71, significantly increased visual–motor response
[50][63].
3.2. OMR
The OMR is a rapid, straightforward, and efficient method for assessing visual function in zebrafish
[17][32], which involves visual stimulation of the fish with moving stripe patterns presented alternately from below or beside the fish, consisting of vertical stripes of different colors, such as black and white, gray with different contrasts, or other different colors
[17][51][32,64]. The OMR is an innate visuomotor reflex behavior in which fish swim in the same direction as high-contrast visual stimuli
[52][65]. These behaviors may be displayed both while swimming freely or while immobilized with the head of zebrafish larvae embedded in agarose while allowing the trunk and tail to move
[17][32].
Scoring the response of larvae is possible through video recordings of their swimming behavior, typically with fixed larvae, or by recording the distance moved relative to the direction of the stimulus
[17][32]. The latter approach can be performed in a high-throughput manner using 384-well plates
[17][32]. While OMR is similar to OKR, the stimulus in OMR drives the movement of the head and body rather than the eyes. These tests have different visual functional features, while OMR and OKR test results overlap. OMR emphasizes the visual motor ability of adult zebrafish, which depends on functional retinal projection
[43][56]. Additionally, the design of OMR experiments may vary depending on the developmental stage of the zebrafish, the experimental equipment used, and the parameters for behavioral evaluation.
Zebrafish larvae exposed to polychlorinated biphenyls, specifically PCB1254, exhibited abnormal behavior in OMR tests, with a significant reduction in the proportion of actively swimming fish compared with control larvae
[53][15]. Similarly, male zebrafish exposed to BPS exhibited a significant reduction in their tracking ability during OMR testing
[43][56]. However, no significant difference was observed in the OMR tests of zebrafish following acute exposure to the insecticides atrazine and diazauron, as all groups exhibited positive visuomotor responses
[54][66].
3.3. Phototaxis Behavior Response
Phototaxis is an innate behavior in which zebrafish larvae exhibit a response to light, whereby changes in the sensitivity of their eyes to light stimuli decrease the perceived difference between light and dark areas
[18][36][16,49]. The phototaxis behavioral response test serves as a rapid and straightforward screening tool used to assess eye defects induced by environmental contaminants
[30][55][56][44,67,68]. This test capitalizes on the natural inclination of zebrafish to move toward the lighted chamber, enabling the assessment of the coordination between motor movements and sensory perception in their visual system. It is widely employed to identify recessive visual defects in model biological systems
[18][21][16,35]. To prevent any discrepancies in individual larva movements, zebrafish larvae with normal swimming function are typically selected for investigations.
The phototaxis behavioral response test typically involves the utilization of a rectangular acrylic box with a sliding partition that separates compartments A and B. A specific number of zebrafish larvae are placed in darkened chamber B for a predetermined period. Once the larvae have acclimated to the dark environment, the partition is opened to expose chamber A to ambient light, while chamber B remains dark. The larvae in each chamber are counted, and the percentage of larvae that swim from the dark chamber to the light chamber after a designated time is used to assess their responsiveness to light. The test can be conducted using blue, green, and red light in the lighting room to evaluate the impact of color perception. To assess whether the larvae accidentally entered chamber A, a controlled experiment was performed in which the chamber remains darkened after the partition is raised
[49][62].
Environmental contaminants have the potential to induce functional impairments in the eye or visual processing centers, resulting in damage to photoreceptors, retinal morphological changes, and cell apoptosis
[33][46]. Photoreceptors and their accompanying opsins are particularly sensitive to exogenous substances among the numerous components of the visual system
[18][16]. Several studies have demonstrated that zebrafish larvae are less responsive to light after being exposed to environmental contaminants
[57][69].
The phototaxis behavior of zebrafish larvae was observed to be inhibited following a single exposure to TCC
[49][62], BDE99
[18][16], phenanthrene polycyclic aromatic hydrocarbon (Phe)
[33][46], TPhP
[30][44], and the agricultural chemical boscalid
[58][17]. Notably, zebrafish larvae exposed to TPhP exhibited a dose-dependent reduction in the phototaxis response
[30][44]. Various wavelengths of light signals (short, medium, and long) were employed to assess the ability of zebrafish larval cone cells to perceive different wavelength spectra. It was found that exposure to BPS inhibited the phototaxis of zebrafish larvae across all wavelength bands (short, medium, and long) in order to test the ability of larval cone cells to perceive varying wavelength spectra
[59][70]. In contrast, exposure to DE-71 resulted in an increase in light-seeking behavior, as indicated by the phototaxis test results of zebrafish larvae
[50][63].
3.4. Light-Dark Preference Test
The light–dark preference test is a well-established approach to assessing anxiety-like behavior in mice and investigating the mechanisms underlying drug-induced neurobehavioral changes
[60][71]. Recently, this test has also been recognized as an extremely valuable tool for evaluating the vision of zebrafish as well as behavioral alterations due to retinal damage and regeneration
[24][38]. The light–dark preference test is particularly advantageous for studying the behavioral neuroscience of zebrafish, as opposed to rodents, who typically prefer darkness
[61][72]. Research involving zebrafish commonly utilizes this test, including high-throughput analysis of neural phenotypes and drug screening
[7][22].
Typically, the light–dark preference test involves exposing zebrafish to alternate light and dark cycles
[62][73]. The experimental setup can vary in terms of the developmental stage of zebrafish larvae, the number of holes in the porous plate, and the duration of the experiment, including the duration of light and dark conditions. Several key behavioral parameters are measured during the light–dark preference test, including movement times, average speed, maximum speed, chemotaxis, and the total distance traveled
[63][74].
Exposure of zebrafish to agrochemicals, specifically the phenylurea herbicides linuron and pyrethroid esfenvalerate, has been demonstrated to exert inhibitory effects on locomotor activity
[64][65][75,76]. Notably, locomotion under dark conditions was also found to be reduced following exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47)
[66][77]. In contrast, exposure to BPS, retinoic acid, and the substitute for perfluorooctane sulfonic acid, F-53B, results in hyperactive swimming behavior, as indicated by the results of light-dark preference tests
[57][67][68][69,78,79]. Furthermore, the presence of pharmaceutical compounds in aquatic environments can exert an influence on the development of zebrafish eyes. For instance, exposure of zebrafish to amitriptyline (AMI), venlafaxine (VEN), and sertraline (SER) leads to significant alterations in their swimming distance under both dark and light conditions
[69][80]. Additionally, exposure to cyclophosphamide (CP) markedly inhibits the swimming speed of zebrafish larvae
[70][81]. Conversely, the locomotor activity of zebrafish larvae remains unaffected by exposure to the beta-blocker atenolol
[71][82]. However, under prednisolone exposure, the light–dark preference test results reveal that prednisolone alters the embryonic response to darkness, although these changes are not directly mediated by visual alterations
[36][49].
3.5. Free Swimming
Swimming ability is a critical behavioral parameter for fish as it plays a pivotal role in their survival, reproduction, and defense
[72][83]. Impairments in visual and sensory processes can lead to lower swimming capability in zebrafish, which can have a negative impact on feeding, growth, and predator avoidance, eventually jeopardizing their ability to flourish in natural settings
[56][68]. The free-swimming activity test assesses the visual function of zebrafish by monitoring their movements in an unrestricted state. This test involves placing zebrafish in a transparent tank, observing their swimming behavior without any interference from shock or noise, and recording their swimming trajectory under continuous visible light using a high-resolution camera. One of the primary parameters assessed in this test is swimming speed
[56][68]. The specific protocols for conducting free swimming tests can vary, including the developmental stage of zebrafish larvae and the experimental design.
Nanoplastics (NPs) are emerging contaminants that have adverse effects on the environment and can serve as carriers for other coexisting pollutants
[73][74][84,85]. For instance, the sunscreen butyl methoxydibenzoylmethane (Avobenzone, AVO) can adsorb onto the surface of nanoplastic particles, hindering their degradation and preventing their entry into the aquatic environment via conventional wastewater treatment methods 52. Exposure to NPs has been found to significantly decrease the swimming activity of zebrafish larvae. Further, zebrafish larvae showed a significant reduction in swimming activity when exposed to the combined exposure of NPs and AVO
[75][76][86,87]. Nevertheless, research has demonstrated that retinoic acid at a dose of 2 nM improves the free swimming speed of zebrafish under both light and dark conditions
[67][78].
3.6. Visual Avoidance Behavior
Zebrafish embryos exhibit unique avoidance behavior, which makes them a valuable model for assessing their motion detection ability. Simple animations composed of basic shapes are employed to elicit avoidance responses in zebrafish during the early developmental stages. The perception of motion necessitates image formation and tracking of positional changes over time, encompassing intricate visual processes that interact with the nervous system
[36][49]. The specific implementation of this test varies depending on the growth stage of the zebrafish, the patterns employed for visual stimulation, and the individual experiment plan.
Previous research has demonstrated that zebrafish larvae exposed to MPs, Cu, prednisolone, PCB-95, and combined exposures of Cu and MPs exhibit abnormal behavior during early developmental stages. These abnormalities manifest as either a lack of significant avoidance response towards visual stimuli or reduced avoidance behavior
[36][77][78][49,88,89].