Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Zivan Gojkovic and Version 3 by Jessie Wu.
The biogeochemical cycling of mercury in aquatic environments is a complex process driven by various factors, such as ambient temperature, seasonal variations, methylating bacteria activity, dissolved oxygen levels, and Hg interaction with dissolved organic matter (DOM). As a consequence, part of the Hg contamination from anthropogenic activity that was buried in sediments is reinserted into water columns mainly in highly toxic organic Hg forms (methylmercury, dimethylmercury, etc.). This is especially prominent in the coastal shallow waters of industrial regions worldwide.
- mercury cycling
- phytoplankton
- Hg toxicity
- aquatic environments
1. Biogeochemical Cycling of Mercury and Methylmercury
Anthropogenic emissions have increased atmospheric concentrations of mercury (Hg) by at least a factor of three over the last century [1][12]. Hg naturally occurs in different minerals, in which it remains relatively stable and does not present significant risks [2][3][23,24]. The problem comes when these minerals are used for different human activities. The extraction of these minerals results in the emission of large amounts of Hg into the environment. [2][23]. Based on recent findings, anthropogenic sources for mercury emissions include fossil fuel combustion, production of non-ferrous metals, iron and steel production, waste burning, production of cement, and some other industrial activities [4][25]. Certain sources state that 24% of anthropogenic mercury emissions are from coal combustion and thermal conversion [5][26]. Additionally, the evidence suggests that prior to the rapid industrialization in the last century, the utilization of Hg in precious metal mining further contributed to the inputs of Hg into the atmosphere and, thus, enlarged inputs of Hg into the ocean [1][12]. The total annual emissions of Hg into the atmosphere are estimated to be between 6000 and 9000 tons, mainly as elemental Hg0 and sometimes as divalent HgII [6][27]. According to recent studies, around 800 tons of atmospheric Hg is generated by natural processes, which makes up approximately 18% of the total atmospheric Hg pool [7][28].
The main sources of Hg inputs into open ocean regions include flow from rivers and estuaries, groundwater, releases from benthic sediments, hydrothermal vents, and direct atmospheric deposition [1][12]. Models and measurements suggest that the dominant source of Hg deposits to oceans is direct atmospheric deposition into surface waters, with global inputs ranging from 2800 to 5800 t over the past decade [1][12]. Another important source of Hg for the marine ecosystem is that of fluvial origin, which originates from industrial discharges that contaminate rivers with a wide variety of pollutants [8][29]. Furthermore, Hg vapors in the atmosphere may come into contact with suspended particles, creating bonds and adhering to them in such a way that leads to their deposition into sediments of the seabed. This way, Hg can later pass into the aquatic environment by effects of sea currents and the action of microorganisms [9][30]. These effects have caused current Hg levels to be five times higher in the atmosphere and two times higher in the oceans than natural levels [3][24].
In the environment, the formation of MeHg is mostly mediated by mercury-methylating bacteria, which mediate the conversion of inorganic divalent mercury (HgII) into MeHg under oxygen-deficient conditions (see Figure 1) [10][31]. Such mediators include certain sulfate-reducing bacteria, iron-reducing bacteria, methanogens, and fermenters [10][11][12][13][14][15][1,31,32,33,34,35]. However, oxygenated ocean surface waters should not be neglected, as certain studies have demonstrated that approximately 20–40% of the MeHg measured below the surface mixed layer originates from the surface and then enters deeper ocean waters [10][31]. This methylation takes place mainly in the sediments, water columns, and periphyton [16][36].
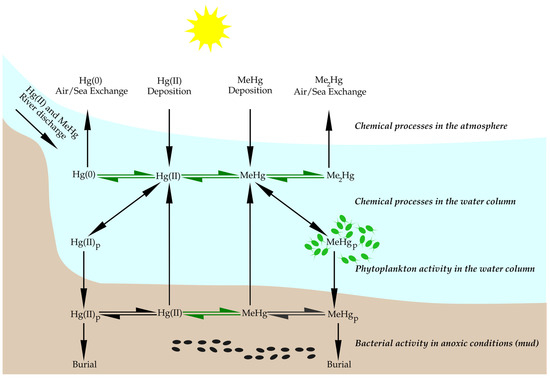
Figure 1. Biogeochemical cycling of Hg in coastal areas. Legend: Hg(0)—elemental mercury; Hg(II)—divalent mercury; Me2Hg—dimethylmercury; MeHg—methylmercury. Index p indicates that any Hg form with index p is bound to the particulate organic matter. Black arrows represent chemical processes while green arrows indicate biologically mediated processes. Green and black dots represent phytoplankton and sulfate-reducing bacteria, respectively. Sulfate-reducing bacteria thrive in environmental conditions where Hg methylation occurs with pH in the 5 to 10 range. Redox potential from slightly negative (−0.4 mV) to zero, and dissolved oxygen of less than 0.2 mg/L. Adapted from [17][18][19][20][37,38,39,40].
Oxygen-deficient conditions of seafloor sediments (also called “dead zones”) that are rich in dissolved sulfates create ideal conditions for methylating sulfate-reducing bacteria [12][32]. The formation of such dead zones is accelerating due to anthropogenic eutrophication of multiple water bodies and global warming [10][12][31,32]. Various other environmental factors are also determining factors in the divalent Hg methylation process, such as temperature, pH, and the composition of media [21][16].
2. Mercury Bioaccumulation in Aquatic Food Chains
Once mercury enters the water system, it is converted by microorganisms into organic forms, such as methylmercury and dimethylmercury. Highly toxic organic forms of mercury with bioavailable properties are ingested by all kinds of organisms, thus being transferred through all the links in the food chain [9][30]. Ingested Hg persists in the body and bioaccumulates, so larger organisms tend to accumulate higher amounts of this element. This effect happens because their diet is based on an intake of a large number of smaller organisms, which have previously ingested Hg. For this reason, the consumption of large marine organisms, such as tuna or swordfish, can lead to health problems in the human population and in different animal species because they tend to accumulate greater amounts of Hg [9][30]. The consumption of marine organisms is the primary source of human MeHg exposure [22][8]. The bioconcentration of MeHg in phytoplankton and zooplankton can be as high as 105 and 106 times compared to MeHg concentrations in seawater, respectively [22][8]. Intracellular MeHg is later bound to proteins of phytoplankton cells and further bioaccumulated in marine food webs. Thus, as a primary entry point of Hg into aquatic food webs, algae play an important role in the intake and transformation of Hg species in aquatic ecosystems [23][83].
When MeHg enters the human body, the enterohepatic cycle is unable to expel it, so it is retained and substantially increases its half-life in the body [21][16]. The hydrophobic properties of MeHg allow it to pass the blood–brain barrier and even enter the placenta. MeHg interacts directly with both cellular and nuclear components, causing neurotoxic effects in the brain and nervous system, damaging the kidneys, and causing irreparable damage to fetuses [21][24][16,84].
Legislation regarding Hg limits in the environment and food varies by state and the environmental matrix considered. The Minamata Convention on mercury does not establish specific environmental limits, but it obliges to control and reduce Hg emissions and release globally [25][85]. The WHO (World Health Organization) raises awareness of Hg toxicity and exposure risks for the general population and gives an example of documented central nervous system damage in subjects exposed to 20 µg/m3 Hg in air for several years [26][86]. European Commission Directive 2008/105/EC (of the European Union) establishes environmental quality standards for water to protect aquatic organisms and ecosystems and limits Hg content to 20 ng/L in surface water [27][87]. The EU also has several additional regulations related to Hg, the most recent being European Commission Directive 2023/915/EC, establishing maximum levels of Hg at 1 mg/kg for fish [28][88].
The MeHg ion (CH3Hg+) has a great affinity for organic and inorganic sulfuric compounds, such as sulfides and thiols, the presence of which causes MeHg speciation, giving it hydrophobic properties and increasing its bioavailability [24][29][68,84]. For example, it has been observed that the MeHg complex with cysteine behaves as a mobile nutrient that is actively transported to the endosperm of rice grains and that the concentration of thiols can both promote and inhibit the methylation of IHg by anaerobic bacteria [24][29][68,84]. Generally, the methylation rate may be affected by a specific strain of bacteria and chemical structure and concentration of organic ligand and thiol compounds [30][89].
3. Effects of Mercury Exposure on Phytoplankton
Photosynthetic marine microorganisms (phytoplankton) carry out half of the global CO2 sequestration while generating half of the O2, which is equivalent to 1% of the global biomass of plants [31][90]. For this reason, they play a key role both in regulating the planet’s biogeochemical cycles (especially carbon cycles), as well as in the global ecosystem and climate change [31][32][90,91]. The great capacity of phytoplankton to fix CO2 can be very useful in the future, enabling the design of CO2 capture systems based on microalgae, as they need much less space and resources, in addition to fixing CO2 with an efficiency between 10 and 50 times higher than other photosynthetic organisms [32][33][91,92]. Furthermore, the possibility of the utilization of microalgae as a food source is becoming of greater interest since they do not compete with terrestrial crops for agricultural land [34][93].
Phytoplankton encompasses the free-floating photosynthetic microorganisms present in the top layer of natural waters, namely, eukaryotic algae and cyanobacteria [35][94]. By photosynthetic biomass production, microalgae influence the composition and productivity of communities of all higher organisms [35][94]. To perform photosynthesis, microalgae take up nutrients from their environment, including trace metals [35][94]. This greatly influences the biogeochemical cycling of these elements, as metals accumulated by phytoplankton will be further transferred to other microbial communities and grazers [35][94]. Microalgae can be affected by various pollutants present in aquatic ecosystems [36][95]. Heavy metals constitute important environmental pollutants because of their potent metabolic toxicity for organisms [37][96]. Heavy metals like mercury may accumulate in primary producers, such as microalgae, and, ultimately, be transferred to other trophic levels [38][22].
There is substantial evidence that exposure to both IHg and MeHg induces general toxic effects in primary producers, including a reduction in growth and photosynthesis, as well as oxidative stress [35][36][39][10,94,95]. In turn, these negative effects inhibit their development and reproduction by causing physiological and metabolic irregularities [40][97]. Fortunately, it is established that the concentrations of Hg typically found in water are far below the amounts that significantly affect the photosynthesis and growth of microalgae [41][98].
However, mercury is distinguished from other heavy metals due to its tendency to bioaccumulate along entire aquatic food webs [42][99]. Mercury has a specific interaction with sulfhydryl groups in enzymes, and coupled with oxidative stress caused by its exposure, mercury can exert toxicity at all trophic levels [42][99]. Once inside algal cells, Hg may bind to cytosolic ligands and be distributed into organelles. The principle of Hg toxicity is blocking functional groups of enzymes by either displacing the ions from these sites or by modifying their conformation [35][94].
HgII was proven to be highly toxic to the photosynthetic system of microalgae by affecting the electron transport chain, changing the photochemistry of photosystem II, and, ultimately, lowering the quantum yield of photosynthesis [36][95]. Moreover, excessive reactive oxygen species (ROS) caused by HgII exposure can cause detrimental effects on gene expression and, all in all, cellular damage [36][95]. Some studies have shown that at low concentrations, MeHg may not have a significant effect on the electron transport chain but rather affects the metabolism of organelles in the cytoplasm and, consequently, membrane integrity, while IHg directly affects plasma membrane integrity [39][10]. Certain studies have also found that genes involved in cell motility, nutrition, and amino acid metabolism of the alga Chlamydomonas reindhartii were downregulated even under environmental concentrations of Hg (10−11–10−8 M) [36][95].
The intake of metals in phytoplankton cells results from passive (diffusion and adsorption) and active uptake mechanisms (complexation of dissolved metals) and is driven by bioavailability conditioned by metal speciation and abundance [43][100]. HgII and MeHg are present in the environment in different forms, which fundamentally affect their bioavailability and toxicity for microalgae [39][10]. The impact of dissolved organic matter (DOM) is hard to predict, as in the previous studies, both increased and decreased Hg uptake by microalgae was detected. The key factors influencing this process were the concentration and composition of DOM, as well as microalgae species [44][73]. Higher HgII exposure concentrations further lead to higher cell uptake [36][95].
Both plants and animals have developed defense mechanisms to fight against mercury exposure, including phytoplankton [42][99]. Microalgae alleviate mercury toxicity by employing at least three intracellular or extracellular strategies [42][45][76,99] and by the increased production of antioxidants [46][101]. The first strategy is metal exclusion by reducing the metal-reactive cell surface with fewer ligands to limit metal accumulation [35][45][76,94]. The immobilization of Hg on the cell surface can significantly reduce metal toxicity. Some sources state that up to 56% of total accumulated cellular mercury can be stored in cellular debris fractions [42][99]. The second strategy is cellular mercury vaporization by reduction to dissolved gaseous Hg0, which is a less bioavailable form [42][45][76,99]. However, this strategy takes place only in some algae species and the detailed mechanism still seems to be unknown. The reduction of Hg has a very rapid onset and generally depends on the duration of the exposure [35][94]. The third strategy is to employ intracellular sulfur-rich complexes to sequester present Hg and, thus, to control its intracellular speciation and to allow separation into vacuoles [35][45][46][76,94,101]. The sequestration of mercury by the production of metal-binding thiol peptides is important to resist high plasmatic Hg concentrations and to restore the function of enzymes inactivated by Hg [42][99]. The primary species of such thiol-rich peptides found in phytoplankton are phytochelatins. Phytochelatins are produced as a response to the presence of various metals, like Cd, Cu, Pb, Ag, Zn, or Hg, in plants, algae, or yeast with a general structure of (γ-Glu-Cys)n-Gly (n = 2–11) [42][99]. Phytochelatins are synthesized by the enzyme phytochelatin synthase, with glutathione as the main precursor; however, the contribution of phytochelatins to metal detoxification is specific to each metal and algal species. The differences may further include the enzymatic synthesis of phytochelatins and the stoichiometry of binding to metals [42][99]. The precursor of phytochelatins, glutathione, is the main non-protein thiol, the pool of which is involved in metal sequestration as well as in the mitigation of oxidative damage in cells [42][99]. In the event of mercury exposure, the glutathione concentration in the cell is increased and phytochelatin synthesis is induced [35][94]. Both glutathione and phytochelatins are able to bind cytosolic Hg and, thus, minimize its nonspecific binding to physiologically important biomolecules; however, phytochelatins have a higher capacity to bind Hg species than glutathione [47][102]. Besides its role in the detoxification of some xenobiotics and metals, glutathione is employed in various metabolic processes, such as the transfer and storage of reduced sulfur and the control of oxidative stress.
