You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Ling Yang and Version 3 by Jessie Wu.
The roles of gut microbiota are highly regarded in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). The intestinal bacteria regulate the metabolism of bile acids depending on bile salt hydrolase (BSH), 7-dehydroxylation, hydroxysteroid dehydrogenase (HSDH), or amide conjugation reaction, thus exerting effects on NAFLD development through bile acid receptors such as farnesoid X receptor (FXR), Takeda G-protein-coupled bile acid protein 5 (TGR5), and vitamin D receptor (VDR), which modulate nutrient metabolism and insulin sensitivity via interacting with downstream molecules. Reversely, the composition of gut microbiota is also affected by the level of bile acids in turn.
- bile acid
- bile acid receptor
- gut microbiota
- NAFLD
- NAFLD treatment
1. The Mechanism of How Gut Microbiota Influences the Progression of Nonalcoholic AFatty Liver Disease LD through Bile Acids
1.1. Farnesoid X Receptor
1.1. FXR
1.1.1. Gut Microbiota Have an Impact on Nonalcoholic AFatty Liver DiseaseLD through Bile Acid–Farnesoid X ReceptorXR Pathway
Farnesoid X receptor (FXR)R is highly expressed in the liver and ileum. In the liver, bile acid-activated FXR inhibits the expression of cholesterol 7-alpha- hydroxylase (CYP7A1) via the induction of small heterodimer partner (SHP; NR0B2). CYP7A1 is generally considered the rate-limiting enzyme that initiates bile acid synthesis. The activation of FXR in the distal ileum induces the expression of Fgf15 (FGF19 in humans), which binds to the FGF receptor 4 (FGFR4)/β-klotho heterodimer complex when it reaches the liver by portal blood to inhibit CYP7A1 expression [1][38]. FGF15/19 could also inhibit bile acid generation by increasing the stability of SHP [2][39]. Bile acid affinities for FXR are as follows: CDCA > LCA = DCA > CA [3][40]. Phe-chol and Tyr-chol are also strong human FXR agonists. It is proven that Phe-chol is an agonist twice as strong as CDCA. These bile acids could increase the expression of the FXR effector genes Fgf15 in the intestine and Shp in both the intestine and liver [4][12].
Research about cholesterol gallstone disease finds that the bacterium Desulfovibrionales is associated with enhanced cecal secondary bile acid production and is enriched in patients with gallstone disease. H2S is the product of Desulfovibrionales that could induce hepatic FXR and inhibit CYP7A1 expression, which results in the accumulation of cholesterol in the liver [5][25]. Activating hepatic FXR could ameliorate NASH according to research about the application of disulfiram in the treatment of NASH. Disulfiram inhibits the growth of Clostridium and reduces Clostridium-mediated 7α-dehydroxylation activity to suppress secondary bile acid biosynthesis, which in turn activates hepatic FXR signaling to ameliorate NASH [6][26]. Hepatocyte MyD88 ablation exhibits an increase in Ruminococcus and Oscillospira and a decrease in Sutterella and Allobaculum, resulting in the decrease in the main FXR agonist CA and the increase in FXR antagonist T-βMCA, thereby contributing to glucose intolerance, inflammation, and hepatic insulin resistance in mice [7][27].
Interestingly, activation of the FXR in the intestine seems to have the opposite effect on nonalcoholic fatty liver disease (NAFLD). Intestine-specific Fxr disruption ameliorates hepatic triglyceride accumulation in mice under a high-fat diet [8][41]. Theabrownin reduces BSH-enriched bacteria and increases the levels of ileal conjugated bile acids to inhibit the intestinal FXR-FGF15 signaling pathway, resulting in increased hepatic production and fecal excretion of bile acids, which in turn reduces hepatic cholesterol and decreases lipogenesis [9][28]. Type 2 diabetes (T2D) patients treated with metformin revealed a decrease in Bacteroides fragilis and an increase in FXR antagonist glycoursodeoxycholic acid (GUDCA), which supports the beneficial effect of intestinal FXR antagonist to metabolic dysfunction [10][29]. The Western diet induces an increase in the abundance of Firmicutes and a relative reduction in the abundance of Bacteroides, which mediates significantly increased DCA and LCA. DCA-mediated FXR activation in the myeloid cells in the intestine can lead to the production and activation of type I IFN resulting in the dysfunction of intestinal Paneth cells and intensifying gut inflammation in mice [11][30]. Another study has proven that hepatic thyroid hormone signaling modulates glucose homeostasis through repressing intestinal FXR signaling [12][42].
However, there are opposing conclusions concerning the effect of intestinal FXR agonists.
Lactobacillus rhamnosus GG-treated mice showed a reduction in taurine-β-muricholic acid (T-βMCA), an FXR antagonist, and the amelioration of liver inflammation and fibrosis, while these changes are reversed by intestine-specific FXR inhibitors. It proves that increasing the intestinal FXR signaling pathway leads to the suppression of bile acid de novo synthesis and prevents excessive bile acid-induced liver injury and fibrosis in mice [13][31]. And both liver and intestine FXR agonists could reduce bacterial translocation via the portal-venous route to the liver in cirrhosis [14][43]. Application of the intestine-restricted FXR agonist fexaramine (FEX) markedly increases taurolithocholic acid (TLCA) and improves metabolism indicators in db/db mice. FEX increases the transformation of LCA from CDCA by amplifying the amount of Acetatifactor and Bacteroides, which have high BSH and 7α- and 7β-dehydroxylase activity. LCA then activates TGR5 signaling to stimulate GLP-1 secretion from L cells [15][44], resulting in promoting adipose tissue browning, improving hepatic insulin signaling and glucose metabolism [16][32]. Another research study found increased levels of total and taurine-conjugated bile acid pool sizes and intestinal FXR signaling after Roux-en-Y gastric bypass (RYGB) surgery, which is linked to increased TGR5 expression and stimulates adaptive thermogenesis in rats [17][45]. As there is a frequent substance exchange between the gut and brain, it is intriguing to find that inhibition of FXR in the dorsal vagal complex enhances insulin action in HFD-fed rats [18][46].
In conclusion, gut microbiota transforms bile acid composition to regulate FXR distributed in the different locations and influences the metabolism status in NAFLD as shown in Figure 1.
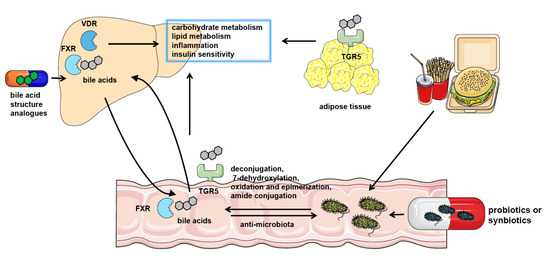
Figure 1. The crosstalk between gut microbiota and bile acids in NAFLD. NAFLD patients have significantly altered gut microbiota composition and bile acid pool. Gut microbiota catalyzes bile acid metabolism via deconjugation, 7-dehydroxylation, oxidation, and epimerization or amide conjugation. The bile acids could also regulate the constituents of the gut microbiome by the detergent actions. Bile acids play a role in regulating nutrient metabolism in NAFLD by binding with bile acid receptors such as FXR, VDR, and TGR5 in the liver, intestine, and adipocytes or other tissues. And pharmacological treatment using probiotics or synbiotics or applying analogs of bile acids are effective approaches to the therapy of NAFLD.
1.1.2. The Molecular Mechanism of How Farnesoid X Receptor R Influences Nonalcoholic Fatty Liver AFLDisease
The expression of FXR is suppressed by iron [19][47]. FXR transcription activation could also be regulated by glucose via FXR own protein O-GlcNAcylation [20][48]. The histone deacetylase Sirtuin (SIRT) overexpression causes deacetylation of FXR with low FXR protein expression. And it could also regulate FXR target gene transcription by influencing histone methylation [21][49].
In NAFLD, FXR activation induces the expression of transporters that provide outflow routes for bile acid efflux to avoid toxic bile acid overload, such as bile acid export pump (BSEP) [22][50] and organic solute transporter-alpha and -beta (OSTα/β). The inhibition of ASBT by FXR in the enterocytes also prevents the uptake of bile acids [23][51]. It also upregulates the expression of ATP-binding cassette sub-family G member 5/8 (ABCG5/8) that is in charge of cholesterol efflux [24][52]. Accordingly, intestinal FXR activation renders the bile acid pool more hydrophylic and less efficient in emulsifying lipids, resulting in cholesterol fecal elimination via ABCG5/8 [25][53].
FXR activation results in a reduction in sterol regulatory binding protein-1c (SREBP-1c) expression by the FXR-SHP axis and represses hepatic de novo lipogenesis [26][54]. The increased expression of SHP after FXR activation suppresses hepatocyte nuclear factor 4α (HNF4α) activities. HNF-4 promotes the expression of microsomal triglyceride transfer protein (MTP), which is involved in transferring triglycerides, cholesterol esters, and phospholipids to newly synthesized apolipoprotein (apo) B [27][55]. FXR could also decrease apolipoprotein CIII (apo CIII) promoter activity [28][56] and induce the gene expression of apoC-II [29][57]. It is known that apo CIII inhibits triglyceride lipolysis whereas apo CII is responsible for triglyceride hydrolysis in chylomicrons and very low-density lipoprotein (VLDL). FXR also regulates glucose metabolism via the upregulation of SHP and represses gluconeogenic gene glucose-6-phosphatase (G6Pase) expression [30][23]. On the other hand, the activation of FXR also represses the expression of the liver-type pyruvate kinase gene (L-PK) through interacting with carbohydrate-responsive element binding protein (ChREBP) and HNF4α to result in the release of these transcription factors from the promoter of L-PK and repressing hepatic glycolysis [31][58]. In addition, FXR activation protects NAFLD by blunting hepatic inflammation. FXR interacts with NLRP3 and caspase 1, thereafter preventing NLRP3 inflammasome assembly and activation in macrophages [32][59], which is contradicted with the increased inflammation caused by the elevation of type I IFN after FXR activation in the myeloid cells in the intestine [11][30] (Figure 2). Moreover, FXR activation in hepatic stellate cells (HSCs) is reported to alleviate liver fibrosis via the inhibition of TGF-β/SMAD3 in a SHP-dependent way [33][60] or triggering the expression of anti-fibrotic genes, like peroxisomal proliferator-activated receptor γ (PPAR γ) [34][61].
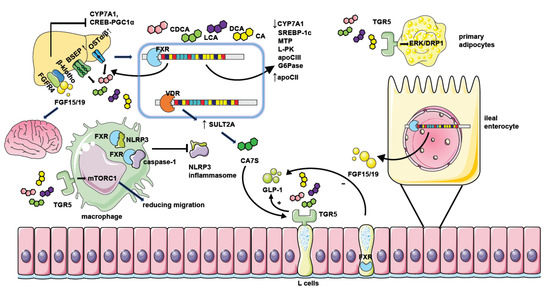
Figure 2. The mechanism of NAFLD regulation by bile acid receptors. Hepatic FXR activation suppresses bile acid synthesis by inhibiting CYP7A1 expression. Intestinal FXR activation could also inhibit CYP7A1 by inducing the generation of FGF15/19 and modulating gene transcription of CYP7A1 through binding with FGFR4/β-klotho receptors in the liver. Hepatic FXR activation promotes bile acid efflux by increasing the expression of bile acid transporters such as BSEP and OSTα/β thus ameliorating the toxic effect of excessive bile acids. FXR activation when binding with bile acids leads to decreased expression of genes involved in lipid or glucose metabolism including SREBP-1c, MTP, L-PK, apoCIII, and G6Pase. It could also repress the CREB-PGC1α pathway by inducing FGF15/19. Together, these factors result in reduced hepatic triglyceride accumulation and improved insulin sensitivity. Moreover, FGF15/19 could also facilitate weight loss by combining with nerve cell receptors. FXR activation in macrophages inhibits the assembling of the NLRP3 inflammasome and ameliorates hepatic inflammation in NAFLD. The activation of TGR5 in L cells causes the secretion of GLP-1 to potentiate insulin secretion, and the activation of TGR5 in primary adipocytes enhances ERK-DRP1 to increase energy expenditure. However, the activation of FXR in L cells inhibits GLP-1 secretion. TGR5 activation in macrophages also suppresses inflammation response by manipulating mTORC1. Hepatic VDR activation increases the generation of CA7S by promoting SULT2A, which stimulates TGR5 in L cells to promote GLP-1 secretion.
FGF15/19, the effector of FXR activation, could also regulate metabolism directly. It could inhibit hepatic gluconeogenesis by inhibiting the cAMP regulatory element-binding protein (CREB)-peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) pathway [35][62]. FGF19 treatment prevents the accumulation of lipid droplets in Fxr-null mice [36][63] and inhibits lipogenic enzyme expression by elevation in SHP expression [37][64]. Moreover, FGF15/19 could reach the brain and mediate beneficial weight loss [38][39][65,66]. But knockdown of the obligate coreceptor mediating FGF15/19 signaling named β-klotho shows resistance to diet-induced obesity in mice [40][67].
1.2. Takeda G-protein-Coupled Bile Acid Protein 5
1.2. TGR5
1.2.1. Gut Microbiota Have an Impact on Nonalcoholic AFatty Liver DiseaseLD through Bile Acid-G-Protein-Coupled Bile Acid Protein TGR5 Pathway
TGR5 is a cell surface receptor belonging to the GPCR, which is expressed in enteroendocrine L cells, white and brown adipose tissue, skeletal muscle, gallbladder, non-parenchymal liver cells, and the brain [41][6]. The order of activation of TGR5 by bile acids is LCA > DCA > CDCA > CA [41][6]. In addition to bile acids, some steroid hormone intermediates, such as pregnandiol, are also the ligands of TGR5 [42][68].
Oligofructose could reduce body weight gain and improve glucose metabolism in mice fed a Western-style diet. It enriches bacteria belonging to Lachnospiraceae and Eggerthellaceae families, which are strongly correlated with cecal HDCA levels. HDCA is elevated after oligofructose administration and it activates TGR5 to stimulate GLP-1 secretion to relieve metabolism disorder [43][33]. Research shows that intestinal hypoxia-inducible factor 2α (HIF-2α) ablation in mice leads to lower lactate levels by controlling the expression of intestinal Ldha, which in turn results in less Bacteroides vulgatus and greater Ruminococcus torques abundance. Together, these changes elevate taurine-conjugated cholic acid (TCA) and DCA levels and activate TGR5, contributing to the elevation of white adipose tissue thermogenesis [44][34]. Bloom of Akkermansia muciniphila and a reduction in Lactobacillus in high fat/high sucrose (HFHS)-fed mice result in an increase in α/β-murocholic acid (MCA), DCA, and UDCA, which activate TGR5 and increase energy expenditure [45][35]. Akkermansia muciniphila is significantly decreased in lean individuals with T2D than their counterparts, which also has a negative correlation with serum 3β-CDCA levels and a positive correlation with insulin secretion and FGF15/19 concentrations [46][36].
1.2.2. The Molecular Mechanism of How Takeda G-Protein-Coupled Bile Acid Protein R5 Influences Nonalcoholic Fatty Liver AFLDisease
GLP-1 is secreted by enteroendocrine L cells and subsequently potentiates postprandial insulin secretion by pancreatic β cells. TGR5-mediated GLP-1 secretion is dependent on the cAMP mechanism [15][44]. TGR5 is found to activate mTORC1 signaling in several studies [47][48][69,70] and TGR5-mTORC1 signaling in the ileum is also considered to regulate the secretion of GLP-1 [47][69]. However, FXR activation in L cells decreases the secretion of GLP-1 by interfering with the glucose-responsive factor ChREBP [49][71] and inhibiting SCFAs induced GLP-1 secretion by decreasing free fatty acid receptor 2 (FFAR2) expression [50][72]. TGR5 activation in primary adipocytes is necessary for the respiration increase induced by mitochondrial fission through activation of extracellular signal-regulated kinase (ERK)/dynamin-1-like protein (DRP1) signaling [51][73]. It is reported that TGR5 activation in macrophages reduces macrophage migration depending on mTORC1 [48][70], thus ameliorating liver inflammation in NAFLD. TGR5 activation in hepatocytes also negatively regulates the hepatic inflammatory response by suppressing the NF-κB pathway by the mediation of the interaction between IκBα and β-arrestin2 [52][74].
