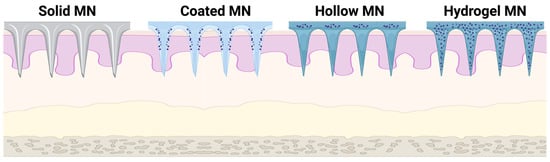
2. Materials Used for Hydrogel-Forming Microneedles: Fabrication and Characteristics
Since MNs act by penetrating the protective human skin/mucosal layer, the material must be biocompatible, i.e., not immunogenic nor foreign body reaction prone. In most cases, hydrogels are biocompatible and can be applied without causing any harm/discomfort to the host. Many of the polymers used for HFMs were previously researched and extensively trialed for use in medicine—particularly with the essential properties of biocompatibility and biodegradability (
Table 1).
Table 1.
Materials used for hydrogel-forming microneedles.
2.1. Natural Polymer Fabricated Hydrogel-Forming Microneedles
Biocompatible materials are used to fabricate MNs made of natural polymers. This type of drug delivery system uses MNs that form hydrogels. In carbohydrate-based MNs, natural polysaccharides such as chitosan, cellulose, and starch are used to create the hydrogel matrix. Protein-based MNs natural polymers could be composed of gelatin or silk fibroin as the matrix-forming component. These types of MNs are biocompatible, biodegradable, and non-toxic. Biodegradability, biocompatibility, and swelling capacity are among the properties of these MNs. In the field of drug delivery, wound healing, and transdermal vaccination, natural polymer-fabricated hydrogel-forming MNs have shown promising results.
Natural and synthetic polymer hydrogels differ in their characteristics and applications. Natural polymer hydrogels, derived from biogenic sources such as proteins or polysaccharides, exhibit inherent biocompatibility and biodegradability. These hydrogels typically possess a high water content and can faithfully replicate the extracellular matrix, providing an appropriate microenvironment for cellular growth and tissue regeneration
[16][15]. Synthetic polymer hydrogels offer adjustable and stable matrices for drug delivery, tissue engineering, and biomedical applications. However, synthetic polymer hydrogels may necessitate additional modifications to enhance biocompatibility
[17][16]. In summary, while natural polymer hydrogels excel in biocompatibility and mimicking the native tissue environment, synthetic polymer hydrogels provide multifunctionality and precise control over material properties, making them widely applicable in biomaterials
[18][17]. The choice between natural and synthetic polymer hydrogels depends on the specific requirements of the desired application, balancing factors such as biocompatibility, mechanical performance, and control over functionality.
2.1.1. Carbohydrate-Based Microneedles
Carbohydrates are among the oldest MN base materials
[19][18]. Carbohydrates provide a range of functional groups that allow tunable properties and designated performance to be designed and developed. In living beings, polysaccharides support structural components, energy storage, lubrication, and inter-cellular signal transmission. The use of natural polysaccharides for pharmaceutical applications has become commonplace due to recent discoveries regarding the novel role that biopolymers play in medicine and pharmacy. One crucial aspect of polysaccharides is their biocompatibility, making them suitable for various medical applications without causing harmful effects on the human body.
In addition to biocompatibility, specific polysaccharides have been found to possess bioactive properties, such as the ability to inhibit cancer cells
[20][19]. For example, polysaccharide-based pectin inhibits cancer cells by inducing apoptosis
[21][20], as do extracts from
Grateloupia longifolia,
Gracilaria lemaneiformis, and other plants
[22][21]. Therefore, natural polysaccharides with bioactive properties are critical biomaterials for healthcare management.
Chitosan or Chitosan Derivatives
The alkaline glucosamine and
N-acetyl glucosamine copolymer chitosan (CS) is derived from chitin, which originates from lower life forms such as certain algae, fungi, invertebrates, insects, or crustaceans. This material’s superior biocompatibility, biodegradability, and antimicrobial properties make it an essential component of food and biomedical engineering
[23][22]. CS has also been widely used in medicine, cosmetics, and water treatment applications due to its enhanced water solubility and antibacterial activity, which are attributed to the superior interaction between carboxymethyl groups and water and its inherent biocompatibility.
Chitosan hydrogel MNs have also been explored for transmucosal vaccine delivery, e.g., via the mouth or nose portal
[24][23]. Bobbala and Hook delivered an antigen in chitosan hydrogel MNs to the oral mucosa in a rat model and elicited a robust immune response, suggesting the feasibility of HFM oral vaccine delivery
[25][24].
Concerning oral/dental applications, the use of dental pulp stem cell-derived exosomes (DPSC-Exos) was explored
[26][25], targeting the treatment of periodontitis, which is an oral disease condition characterized by a high proportion of proinflammatory macrophages as a result of immune responses to periodontopathogenic bacteria. DPSC-Exos was capable of suppressing periodontal inflammation and modulating immune response due to miR-1246 present in DPSC-Exos, a therapeutic potential in treating periodontitis based on a Gene Ontology term enrichment analysis. The research team demonstrated that incorporating DPSC-Exos into chitosan hydrogel (DPSC-Exos/CS) accelerates healing in mice periodontitis models, which changed the proinflammatory macrophage phenotypes to anti-inflammatory. Further research is on the way for the therapeutic agent carried by chitosan hydrogel
[26][25].
Hyaluronic Acid
Hyaluronic acid (HA) is a glycosaminoglycan found as a natural constituent in epithelia, dermis, and connective tissues, including dental pulp and periodontal connective tissue. The skin contains approximately 50% of the total body HA. The human body degrades about one-third of its total HA daily and replaces the same amount with newly synthesized HA. During physiological conditions, HA is converted to its more hydrophilic sodium salt. It can hold 1000 times its own water weight because HA contains numerous hydroxyl groups. HA water retention ability enables many homeostatic functions as well as support and maintenance of diverse physiological processes in the human body. HA MNs are highly biocompatible, resistant to deformation, and are a common ingredient in skin care products
[27][26].
Moreover, HA MNs are strong enough to penetrate the skin, readily dissolve, and release drug/active ingredients within a predetermined short period. Furthermore, no requirement of heating or organic solvents during HA MN fabrication enhances the preservation and stability of heat- and/or chemical-liable agents/drugs, such as insulin. Gill and Prausnitz
[28][27], and Liu et al.
[29][28] were among the first groups to pioneer the fabrication of insulin-loaded HA MNs. Liu et al. demonstrated that the novel insulin-loaded HA MNs exhibited self-dissolving properties and appeared safe. Hygroscopy, stability, drug release profiles, and dissolution characteristics of the insulin-loaded HA MNs were also characterized via a diabetic rat model by the same group
[29][28].
Oral anesthetic injections without surface anesthesia can increase pain and anxiety during dental therapy. An HA MN patch with MN tips containing fast-dissolving lidocaine hydrochloride (LDC) was developed to address this problem. In the isolated porcine oral mucosa, LDC-containing HA MN patches were able to puncture the stratum corneum at a depth of approximately 0.28 mm. The fast-dissolving LDC on the HA MN patch enables anesthesia within 3 min at the tentative local anesthesia injection site. The adhesive MN patch was proposed to process the potential to aid transmucosal delivery of anesthetics, further enhancing the delivery of pain-free dental treatments.
[30][29].
Sodium Alginate
Sodium alginate (SA) is a linear polysaccharide derived from brown algae. It is composed of poly-(1,4-diaminobenzoic acid) and nanoparticles of 1,4-L-glucuronide in different proportions, and its aqueous solution is highly viscous. As a result of the high number of carboxyl groups and hydroxyl groups in the molecular structure, SA has a high degree of chemical activity and can rapidly form a hydrogel containing a three-dimensional mesh structure, and it is non-toxic and odorless and exhibits outstanding biocompatibility and environmental friendliness. Safe dental application of alginate, e.g., dental impression materials, could be dated back to the 1950s
[31][30]. SA and its derivatives are also readily available, inexpensive, simple, and renewable
[32][31].
Blood loss is a common complication following trauma and surgery that can cause serious harm to the body
[33][32]. Alginate is an excellent hemostatic polymer-based biomaterial as it is biocompatible, biodegradable, non-toxic, quickly gelled, and widely available
[34][33]. In the medical field, SA hydrogels have been widely used, including injectable hydrogels
[35][34], hemostatic needles
[36][35], medical dressings
[37][36], etc.
A North American group developed an injectable, biodegradable scaffold based on alginate microbeads for in vitro bone tissue engineering using periodontal ligament and gingival mesenchymal stem cells
[38,39][37][38]. The stem cells remained viable in the laboratory and could differentiate into osteogenic and adipogenic tissues. Furthermore, the degradation behavior and swelling kinetics of the scaffold were characterized. Therefore, alginate has proven to be a promising non-toxic scaffold for stem cells, providing a good strategy for engineering bone tissue
[40][39]. Additionally, a microneedle-mediated drug delivery system containing sodium alginate for immunochemotherapy has been developed. In glioma-bearing mice, microneedles loaded with lipopolysaccharide (LPS) and doxorubicin (DOX) demonstrated excellent efficacy in promoting immune response and inhibiting tumor growth
[41][40].
Pullulan
Pullulan (PL) is an α-(1→6)-linked (1→4)-α-d-tri-glucosides polysaccharide/glucan or maltotriose, which is a carbohydrate biopolymer produced by
Aureobasidium pullulans. The application of pullulan-based MN has been advocated since 2020
[42,43][41][42]. The authors present the first dissolving microneedle (DMN) system using PL. A variety of concentrations of PL gels were tested for viscosity and film formation, and then MNs were created using the appropriate concentration of PL gels. PL DMN was loaded with model molecules and proteins/peptides, and their stability was assessed using circular dichroism. Ex vivo studies were conducted using Franz diffusion cells to determine the permeation of Flu-Na and FITC-BSA-loaded PL-DMN into porcine skin. The findings suggest that PL DMNs can be effective for transdermal drug delivery
[44][43].
In another study, transdermal insulin delivery was achieved using pullulan MN patches. MNs penetrated skin up to 0.38 mm depth and dissolved within two hours, releasing up to 87% of insulin. Storing insulin-loaded MNs at 4–40 °C for four weeks is possible without losing their structure. Additionally, PL MNs were non-cytotoxic, indicating they are suitable for skin application. A non-invasive treatment option for insulin could be made possible by PL MNs based on these findings
[45][44].
Cellulose
In recent years, cellulose, a versatile and biocompatible material, has been garnering attention for its potential use in manufacturing microneedles for transdermal drug delivery
[46][45]. Two studies have examined the use of cellulose-based microneedles and their unique properties. The one-step created semi-dissolving microneedles comprised a water-soluble needle layer and a backing layer containing 2,2,6,6-tetramethylpiperidine-1-oxyl-oxidized bacterial cellulose nanofibers. That material was used as drug reservoirs for delivering more significant quantities of drugs to the skin
[47][46]. Another study examined the inclusion of cellulose nanofibers in dissolving microneedle arrays. Including cellulose nanofibers increased needle stiffness and decreased dissolving and transdermal delivery rates
[48][47].
The cellulose-based microneedle is capable of delivering drugs transdermally. By leveraging the unique properties of cellulose, these microneedles improve drug loading capacity, enhance mechanical properties, and provide controlled drug release, which opens up new possibilities for advancing transdermal drug delivery systems in diverse applications, including oral disease management.
Combined Carbohydrate-Based Microneedles
Wei et al.
[49][48] used CS and PL as raw materials to prepare HFMs. The chitosan-based MNs they developed showed good swelling and water retention properties and biocompatibility. The application of these hydrogel-based MNs as vehicles for drug delivery was then evaluated through a skin insertion study and a drug loading/release study. The mechanical properties of the MNs allowed easy insertion into newborn porcine skin with observed rapid release of drugs
[49][48]. Combining these two polymers may produce synergistic effects, such as improved mechanical strength, swelling behavior, and drug loading capacity
[50][49]. Due to the swelling and water retention properties of CS and the film-forming properties of pullulan, it may be possible to deliver and release medications more effectively. Moreover, the biocompatibility of these materials may reduce the possibility of adverse reactions or tissue damage.
2.1.2. Protein-Based Microneedles
HFMs made from protein-based natural polymers are promising drug delivery systems that use biocompatible materials
[51][50]. Proteins such as gelatin and silk fibroin are matrix-forming materials to manufacture MNs. They demonstrated significant potential in delivering transdermal drugs, vaccinations, and tissue engineering.
Water-Soluble Silk Fibroin
Silk fibroin (SF) is derived from silkworm silk after sericin degradation or degumming as a natural polymer. It comprises 18 amino acids forming a natural structural protein without physiological activity. SF is widely used in preparing sustained-release drug systems because of its biocompatibility, controllable degradation, and adjustable drug release
[52,53][51][52]. The SF-based drug-sustained release systems can encapsulate and stabilize various small molecules, proteins, and large biomolecules such as DNA, facilitating controlled and prolonged content release
[54][53]. In recent years, much attention has been given to using SF hydrogels in tissue engineering and drug delivery. It was reported that SF hydrogels prepared by various physical or chemical treatments, coupled with other biomaterials, provide a variety of drug release patterns, hence showing promise in the fields of cartilage tissue regeneration, wound repair, anti-cancer, and anti-infection therapies
[55][54].
In an ectopic root canal transplantation model, silk fibroin scaffolds, not in MN format, though containing basic fibroblast growth factor (bFGF), were evaluated for pulp regeneration with DPSCs
[56][55]. It was found that DPSCs seeded in the scaffold survived and displayed cytoplasmic elongation for at least four weeks in culture. Incorporating bFGF into tooth fragments and scaffolds increased the viability of DPSCs. This bFGF-incorporated scaffold generated pulp-like tissue consisting of transplanted and host-derived cells and displayed good vascularity, matrix deposition, and the formation of dentin-like tissue. The results of this study indicate that silk fibroin scaffolds incorporating bFGF are a promising candidate for future treatments in regenerative endodontics
[57][56]. While some studies have explored the use of SF MNs for delivering other types of stem cells for tissue regeneration and wound healing applications, the specific combination of DPSCs and SF MNs in dentistry is yet to be explored.
Gelatin
Gelatin is a jelly-like substance derived from animals and composed of peptides and proteins released by partial hydrolysis of collagen, which are typically obtained from skin, bones, and connective tissues. The hydrolysis process breaks some bonds between and within the component proteins. Several gelatin chemical characteristics are similar to those of its parent collagen. In general, pig skin and cattle bones are used to manufacture photographic and pharmaceutical-grade gelatin. In terms of its composition, gelatin falls under the category of hydrogels. Various products use gelatin, including capsules, cosmetics, ointments, and foods. Gelatin MNs can be traced to as early as 2013
[58][57].
Gelatin has been shown to have osteogenic potential, which is often used as a tissue engineering scaffold for bone regeneration
[59][58]. The efficacy of bFGF-gelatin hydrogel complex, not in MN presentation, was evaluated on bone regeneration around dental implants. A total of 24 titanium implants were placed into the mandibles of 4 beagle dogs, and different amounts of bFGF were applied to fill the bone defect sites. A minimum amount of bone regeneration was observed in the groups with 0 and 0.1 mg of bFGF after eight weeks, whereas new bone formation was observed in the groups with 1, 10, and 100 mg of bFGF and autogenous bone. The results suggest that bFGF-gelatin hydrogel complexes with an optimal amount of bFGF can be used to augment bone around implants
[60][59]. Again, the specific combination of bFGF in gelatin hydrogel MNs aiding oral bone tissue healing is yet to be explored further.
2.1.3. Mixed Carbohydrate-Protein Microneedles
One of the first gelatin MNs was a transdermal insulin delivery patch made from starch and gelatin developed by Ling and coworkers (2013). The MNs readily penetrate the test animals’ skin upon application and dissolve in five minutes. The effects of insulin-loaded MNs on diabetic rats were similar to those produced by subcutaneous injections, and the hormone-loaded MNs were stable after a month of storage
[58][57].
In a study by Jana and coworkers
[61][60], modified sodium carboxymethyl cellulose (CMC) and gelatin were used to fabricate DMN patches. The authors took advantage of the CMC-gelatin mechanical strength while the MN vehicle itself appeared able to prevent insulin degradation, thus potentially maintaining the shelf-life of this essential hormone.
2.2. Synthetic Polymer Fabricated Hydrogel-Forming Microneedles
Hydrogel MNs made from synthetic polymers can be designed to dissolve or swell in response to moisture or heat, allowing for the controlled release of drugs or other substances into the body. The mechanical properties of synthetic polymer fabricated MNs could be more substantial and usually more consistent than that of natural polymer fabricated MNs, thus improving their effectiveness and reliability. Additionally, synthetic polymers can be customized to acquire specific properties, including tunable degradation rates enabling more versatility for drug delivery.
2.2.1. Gelatin Methacryloyl
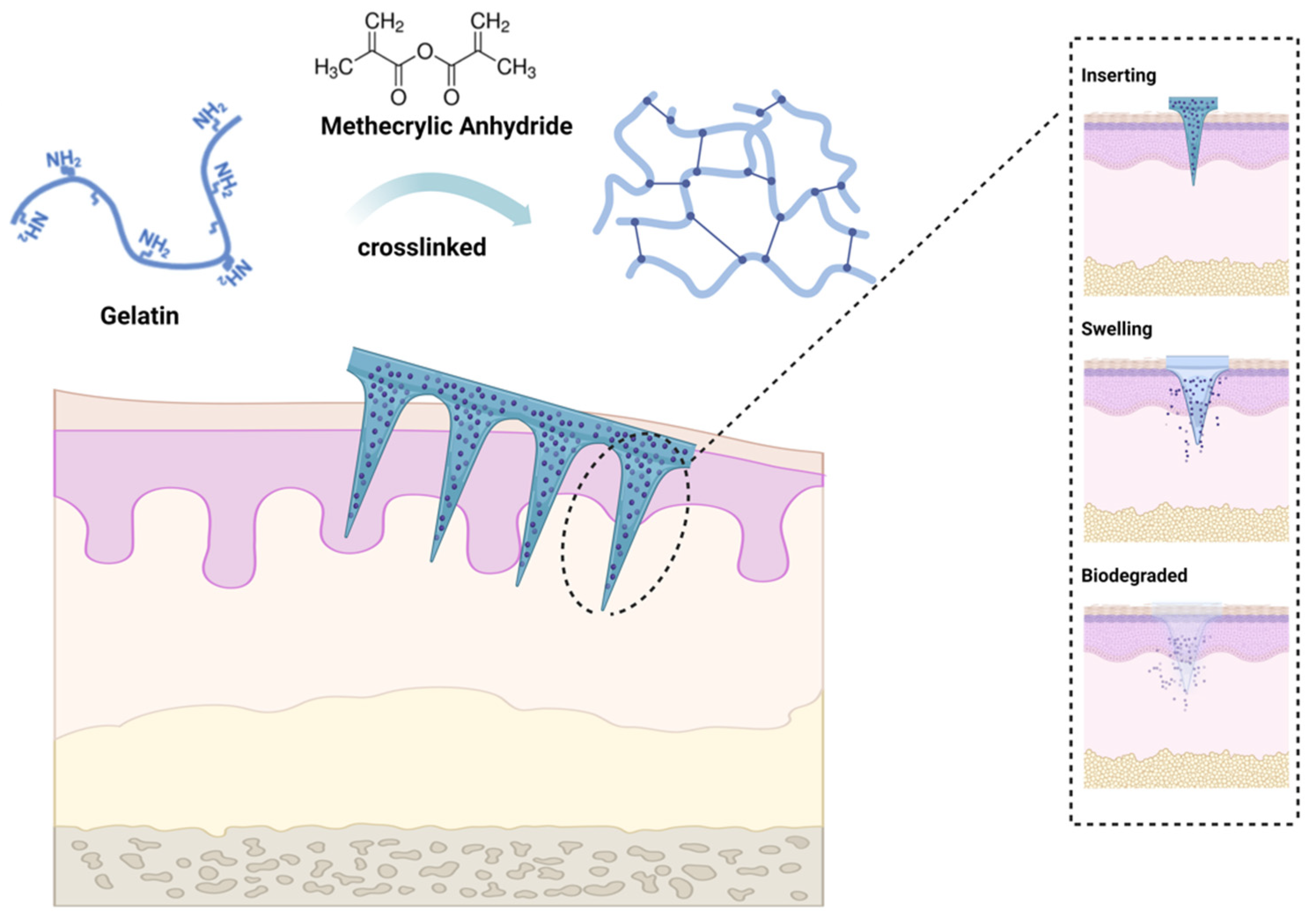
Gelatin methacryloyl (GelMA) is a light-cure hydrogel developed by Van den Bulcke and coworkers
[62][61]. The biocompatibility and controlled molding of GelMA led to the extensive application of the agent in the biomedical field shortly after its development and commercialization. By reacting methacrylic anhydride (MA) with amino groups, the double-bond amide groups would be grafted onto gelatin chains, then cross-linking of the latter would be facilitated through amide chemical reaction induced by UV activation of photoinitiators
[63][62] (
Figure 2). Since GelMA also exhibits good biodegradability and moldability, it has attracted considerable research interest. The unique characteristics of GelMA hydrogel and its simplicity of preparation made it a good MN candidate for wound healing dressings, drug delivery, biosensing, and tissue regeneration in a wide range of biomedical applications
[64][63]. It was reported that GelMA could be used to prepare MN arrays with acceptable release profiles suitable for the delivery of water-soluble drugs
[65][64]. GelMA hydrogel MNs have numerous applications in the medical field, such as transdermal insulin delivery, wound healing promotion, tissue regeneration, and biosensing. GelMA-based microneedles have been explored in wound healing, which possess adhesive properties and can be loaded with growth factors or other therapeutic agents or cells to promote wound healing processes. Incorporating GelMA microneedles into a wound site allows a controlled release of bioactive molecules, leading to accelerated tissue regeneration and improved healing outcomes
[56,66,67][55][65][66]. Despite this, GelMA hydrogel MNs hold great potential for dental and oral applications due to their biocompatibility, biodegradability, and tunable mechanical properties, particularly local anesthesia, periodontal disease treatment, and/or controlled drug delivery.
Figure 2. Gelatin methacryloyl (GelMA) microneedle application. GelMA is a cross-linked hydrogel material prepared by grafting methacrylic anhydride (MA) onto gelatin. GelMA MN exhibits sufficient mechanical toughness to penetrate the skin upon insertion, followed by swelling, degradation, and drug release, leaving no residue within the skin tissue. The figure was created with
BioRender.com.
2.2.2. Methacrylate-Based Hyaluronic Acid
Methacrylate-based hyaluronic acid (HAMA) is obtained by chemically modifying the natural polysaccharide HA with MA
[67][66]. This composite hydrogel has a continuous three-dimensional network structure, good swelling properties, mechanical properties, and drug loading capacity and is highly stable in a simulated human physiological microenvironment (pH 7.4)
[68][67]. Xu et al.
[69][68] developed a HAMA MN patch that carries platelet-derived growth factor D (PDGF-D) and human adipose-derived stem cells (ADSCs) for the management of diabetic ulcers. According to their diabetic mouse model, the MN patch delivered ADSCs and PDGF-D appeared to promote angiogenesis and wound healing.
At the dental front, a small molecule from the thiadiazolidinone family, NP928, was designed to be directly delivered into damaged teeth via a MAHA injection. Upon being cured by dental blue light securing in situ delivery, NP928 was observed to release from MAHA, which promoted the production of reparative dentine through upregulating Wnt/β-catenin activity in pulp stem cells
[68][67]. Along such lines, the HAMA hydrogel-based MNs format may enhance another therapeutic agent’s delivery pathway in repairing damaged dental tissues.
2.2.3. Polyvinyl Alcohol
Polyvinyl alcohol (PVA) is a water-soluble polar polymer with excellent biocompatibility, biodegradability, inherent, non-toxic, and mechanical properties. It can be chemically or physically cross-linked to form a hydrogel, and due to its wound-healing properties via transforming growth factor beta (TGF-β) upregulation
[70][69], PVA hydrogels are also widely used as a matrix material for wound dressings
[71][70].
Stratum corneum poses a formidable barrier to effectively delivering large and/or charged macromolecules such as small interfering RNA (siRNA). The latter would be helpful in skin disorders management
[72][71]. Despite the effectiveness of intradermal siRNA injections, the procedure is painful. MN arrays may be an alternate and effective way for nucleic acid delivery, including siRNAs in a less painful manner. To enable penetration of the skin barrier, a loadable, PVA-based dissolvable protrusion array device (PAD) was developed. A PAD-mediated siRNA delivery effectively silenced a transgenic reporter mouse model in the skin
[73][72].
2.3. Combined Natural and Synthetic Polymer-Fabricated Hydrogel-Forming Microneedles
A study reported that PVA and chitosan (PVA/CS) nanofiber scaffolds are excellent regenerative endodontics models
[74][73]. Using these nanofibers and ciprofloxacin and IDR-1002, a multifunctional scaffold with anti-biofilm and anti-inflammatory properties was created. It was demonstrated that tooth fragments filled with these nanofibers produced pulp-like tissue in vivo. In dentistry, this type of scaffold, in MN/MN patch presentation, could aid regeneration beyond the pulp revascularization
[75][74].
3. Fabrication of Hydrogel-Forming Microneedles
Hydrogel MNs can be prepared in a variety of ways, varying with the hydrogel material(s) and cross-linking mechanism
[76,77][75][76] (
Table 2). As mentioned in the previous section, the materials used for the preparation include natural polymers and synthetic polymers, which can be physically cross-linked, electrostatically interacted, or chemically cross-linked to form a hydrogel with the needle patch formation, as described in this section.
Table 2.
Fabrication methods for hydrogel-forming microneedles as an example of polymeric microneedle (MN) production.
In detail, the fabrication of hydrogel microneedles involves an intricate process that includes mold fabrication, hydrogel formulation, casting, and post-fabrication treatments. Initially, a microneedle-shaped mold is meticulously crafted using advanced photolithography
[78][77] or micro-molding techniques. Additionally, biocompatible polymers are meticulously blended with a suitable crosslinking agent to create a tailored hydrogel formulation. The hydrogel formulation is carefully poured or injected into the meticulously fabricated mold cavities without air bubbles by judiciously applying vacuum or centrifugation. Next, the hydrogel is crosslinked chemically or physically to ensure it is uniform and structurally robust. In addition to dehydration, sterilization and surface modifications to enhance drug loading and release properties are some post-fabrication treatments that are meticulously handled after fabrication to enhance mechanical strength
[79][78].
Currently, very few oral/dental MNs are available on the market. Many methods have been explored to produce MNs for dental use, including micro-molding, casting, electrospinning, and 3D-printed hydrogel-filled MN arrays. In recent years, these methods have proven to be promising for developing MNs for dental applications, such as localized delivery of drugs and vaccines into the oral cavity. However, more research is needed to explore the full potential of MNs in dentistry.
3.1. Micro-Molding Method
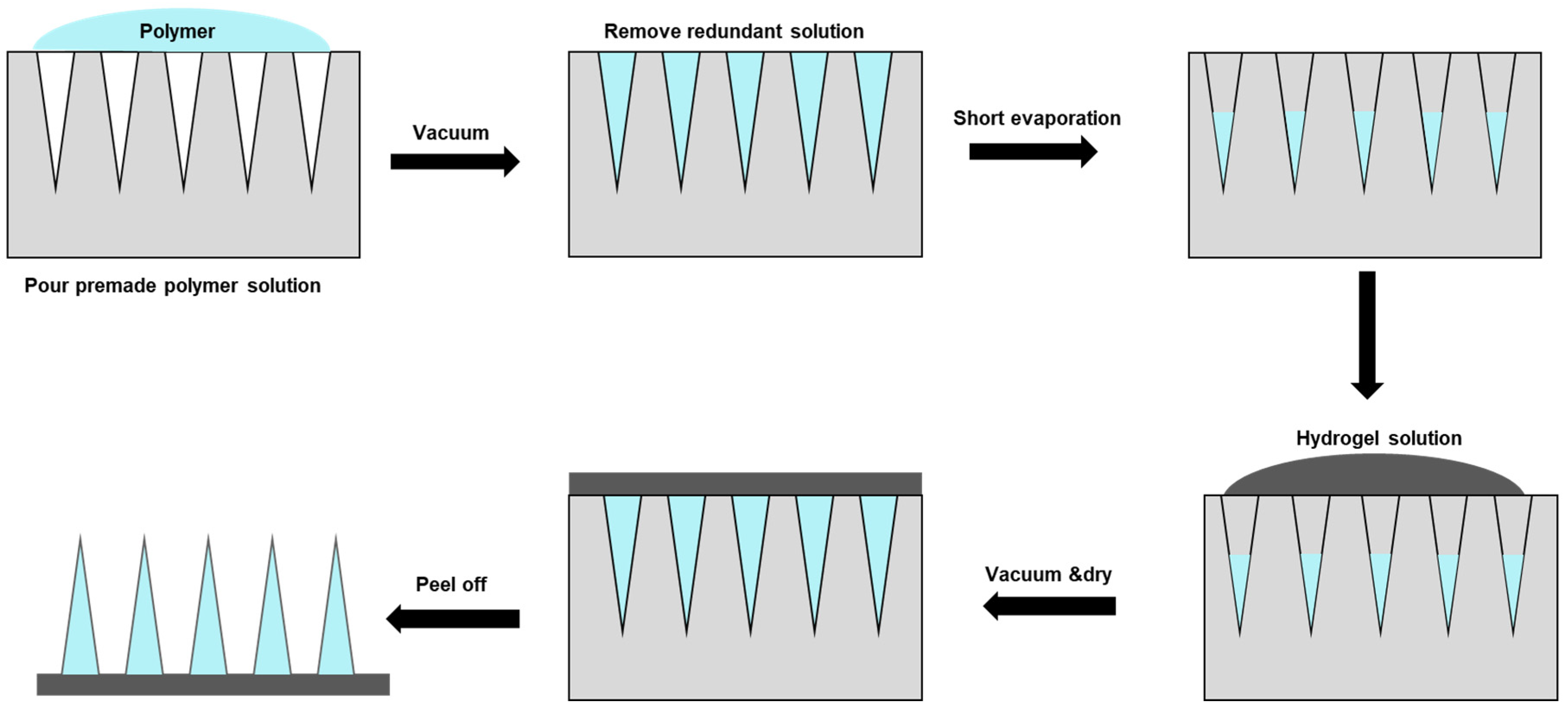
Micro-molding hydrogel MNs preparation is one standard method applied
[80][79]. Typically, polydimethylsiloxane (PDMS) casts are used, which are cast around a solid master template and then cured at 70 °C for two hours. A negative micro-mold can be produced from the master template for HFM fabrication. Due to the reusable nature of the micro-mold, multiple HFM arrays could be produced easily and quickly, which is particularly advantageous for optimizing parameters. A two-step fabrication is a common technique for fabricating MNs for drug delivery, with active ingredients concentrated at the MN tips (
Figure 3).
Figure 3. Hydrogel microneedle patch fabrication through a two-step method. First, the polymer solution with active ingredients was covered and filled in the polydimethylsiloxane (PDMS) female mold under a vacuum. Next, the redundant solution was removed to ensure each cavity was filled with the same volume of drug solution. The solution was then let to evaporate. Hydrogel solution was then poured into the mold under a vacuum, and the sample was allowed to cure/solidify and dry in a sealed desiccator overnight, followed by demolding, packaging, and storing.
3.2. Casting
Casting is another standard method of fabricating HFM. By using photolithography or a microfabrication technique, a master mold with the desired MN shape is created. Hydrogel material solution is then poured into the mold, typically composed of a mixture of water-soluble monomers/catalysts and the active ingredient(s). To enable solidification, the aqueous mixture is then allowed to crosslink, typically through UV exposure, heat, and/or a chemical initiator. The MNs can then be removed from the mold and stored until use
[81][80].
The casting method enables the manufacture of MNs with complex shapes and dimensions that can be tailored to specific applications
[82][81]. Additionally, this method allows for incorporating bioactive agents such as drugs, peptides, or growth factors into the hydrogel solution, which can be released upon MN insertion into the skin
[81][80]. The casting method is relatively simple, cost-effective, and scalable, making it an attractive option for producing hydrogel MNs for various biomedical applications.
3.3. Electrospinning
Electrospinning involves drawing a biodegradable polymer solution, e.g., PVA, into a fine jet using a high-voltage electric field. The solvent evaporates as the jet travels toward a collector, and the polymer solidifies into a fibrous mat. HFM can be created by coating fibers with a hydrogel material, such as polyvinylpyrrolidone
[83][82] or HA. Parameters for electrospinning, e.g., voltage, flow rate, and distance between the needles and the collector, can be controlled to produce MNs of various shapes and sizes
[84][83].
MNs produced by electrospinning have a high aspect ratio, which means their length exceeds their width, allowing them to penetrate the skin more efficiently and effectively
[85][84]. Moreover, the electrospun polymer, by its nature, is capable of releasing the hydrogel-loaded drugs or active agents in a controlled manner because of the ability of manufacturers to control the diameter and length of the MNs and porous structure by adjusting the electrospinning parameters
[86][85]. However, electrospinning is among the more complex processes and requires specialized equipment.
3.4. Three-Dimensional-Printed Hydrogel-Filled Microneedle Array
It remains uncommon that existing hydrogel-filled MN arrays are designed to deliver different therapeutic agents to diverse tissue compartments at particular vicinity and to reach different depths of the targeted tissue mass. To fulfill this, Barnum and coworkers
[87][86] developed an MN array system composed of a 3D-printed resin-based rigid outer layer attached to a drug-washed hydrogel. By fabricating MNs of varying compositions and lengths in a single patch, the same or different drug(s) can be delivered to various depths within the target tissue mass, perhaps with varying dosing protocols. The composition of the hydrogel and the shape of the needles can be adjusted in addition to the spatial distribution of the drug(s) involved. The delivery of vascular endothelial growth factor using a hydrogel-filled MN array was pioneered as a proof-of-concept approach
[87][86].
Informed by various imaging techniques, the hydrogel-filled, 3D-printed, UV-polymerized resin-based, custom-made MN array of different needle densities, shapes, and lengths can be designed to acquire various forms and mechanical properties to ensure successful drug and biologics delivery. In line with the abovementioned concept, polyethylene glycol diacrylate hydrogel was developed, which retains properties for therapeutic drug delivery and skin penetration efficacy. In that study, an array of 100 MNs was successfully printed that release drugs in response to delivery site stimuli/characteristics such as temperature and pH
[88][87].