Medullary thyroid carcinoma (MTC) is a malignant tumor that arises from parafollicular C cells, which are responsible for producing calcitonin. The majority (75%) of MTC cases are sporadic forms, while the remaining (25%) have a hereditary component. In these hereditary cases, MTC can occur in conjunction with other endocrine disorders (i.e., pheochromocytoma) or as an isolated condition known as familial medullary thyroid carcinoma. The primary genetic mutation associated with the development of MTC, regardless of its hereditary or sporadic nature, is a point mutation in the RET gene. Evaluation of serum calcitonin levels represents the most reliable and sensitive marker for both the initial diagnosis and the postsurgical monitoring of MTC. Unfortunately, most patients do not achieve normalization of postsurgical serum calcitonin (CT) levels after surgery. Therefore, there is a need to find new biomarkers to be used with serum CT in order to increase test sensitivity and specificity.
- medullary thyroid cancer
- circulating tumor cells
- miRNA
- cell-free DNA
1. Introduction
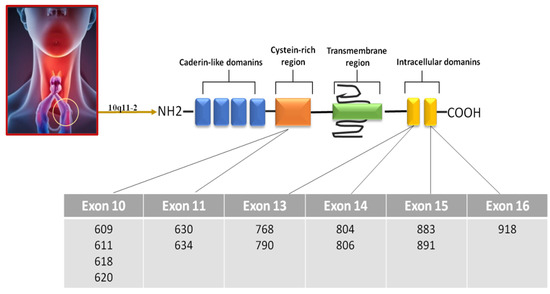
2. Circulating Tumor Cells
Circulating tumor cells (CTCs) are rare and heterogeneous (epithelial, mesenchymal CTC (MCTC), and both mixed types) cells that are shed from primary or metastatic tumor lesions into the bloodstream [20]. They are an important biomarker for cancer, as their presence in the bloodstream indicates that cancer has spread beyond its original site and has the potential to metastasize to other parts of the body [20] (Figure 2).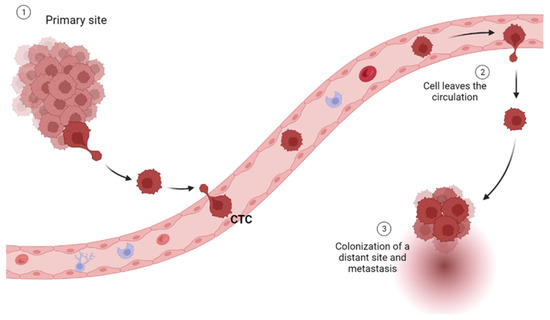
3. Cell-Free DNA
Cell-free DNA (cfDNA) derived from tumors is a promising biomarker for cancer diagnosis and monitoring. It consists of small fragments of DNA released into the bloodstream by tumor cells. Analyzing cfDNA can provide valuable information about tumor genetic alterations, such as mutations, copy number variations, and epigenetic modifications. cfDNA testing is minimally invasive as it can be obtained with a simple blood draw. The detection and analysis of tumor-derived cfDNA can help in early cancer detection, treatment selection, and monitoring of the treatment response. However, cfDNA testing faces challenges, including the low abundance of tumor-derived cfDNA, the presence of normal DNA, and technical limitations in detecting rare mutations. Ongoing research aims to improve the sensitivity and specificity of cfDNA-based tests to maximize their clinical utility in various cancer types including MTC. Most of the studies concentrate on the mutational profile of the cfDNA. By analyzing 29 MTC patients, Ciampi and colleagues revealed that 4/26 (15.4%) cases showed positive pre-operative cfDNA with a significantly higher presence of RET p.M918T mutation (p = 0.0468) and a higher frequency of persistent disease [26][28]. Three patients had positive post-operative cfDNA (but not pre-operative), and seven cases with persistent disease harbored either pre- or post-operative positive cfDNA [29]. In all cases, free DNA correlated with serum CT [26][28], but pre-operative cfDNA was not useful for diagnostic purposes, although it was for outcome prediction, while post-operative cfDNA showed significant results in response to therapy monitoring. In a series composed of both MTC and differentiated thyroid cancer, Allin et al. [30] found that most patients (67%) with advanced thyroid cancer had earlier detectable cfDNA. The detection rate was higher in MTC compared with PTC and FTC, and the detection rate was higher in patients with metastatic disease (79%) compared with those with local recurrence (33%) and no macroscopic disease (0%), indicating that cfDNA correlates with disease aggressiveness. In their series, early disease progression was found in 3/15 (20%) MTC cases. Again, cfDNA correlated with serum CT [30]. When compared to healthy control subjects (n = 19), cfDNA showed a high ability to diagnose MTC (n = 58) [31]. In that paper, positive values of Ct corresponded to a lower amount of cf-DNA, and the same was found for RET mutational status. These findings might suggest that cfDNA represents a potential substitute marker for MTC in the case that the classic markers, such as CT and RET, are negative [31]. But might cfDNA predict the overall survival of MTC patients with better reliability than calcitonin? Cote et al. [32] tried to answer this question. They analyzed 75 patients with confirmed sporadic MTC diagnosis, a serum calcitonin measurement > 100 pg/mL, and a tumor tissue harboring the RET p.M918T mutation (50/75). In 16/50 (32%) patients, cfDNA showed the same mutation, which effectively correlated with worse overall survival [32]. In addition, the detection of RET p.M918T cfDNA was strongly associated with a worse outcome than CT doubling time [32]. The diagnostic role of cfDNA was also evaluated in 21 MTC cases in a study by Higazi [33], in which the authors measured the cfDNA integrity using specific markers (Alu244/Alu83 ratio) and found that cfDNA integrity was more elevated in MTC compared to other thyroid malignancies [33]. Taken together, these studies seem to indicate that cell-free DNA from tumor origin can be used either for diagnostic or prognostic purposes, but its efficacy is increased when used for predicting disease outcome and response to therapy, especially in cases that are negative for CT and/or RET mutational status. Again, a major issue that researchers have to address is the existence (or not) of a cutoff able to predict disease recurrence with high accuracy. Cell-free DNA seems to better correlate with serum CT, so the use of cfDNA in combination with CTCs might be useful for CT-negative cases.4. MicroRNAs (miRNAs)
In the last years, miRNAs have raised great interest as potential biomarkers, particularly in cancer research. miRNAs are short non-coding RNA molecules (about 22 nucleotides in length) that act as endogenous regulators of gene expression at the post-transcriptional level. Mature miRNAs derive from primary miRNAs, which are transcribed in the nucleus and subsequently processed into pre-miRNAs by Drosha. They are then exported to the cytoplasm, where are finally cleaved by the Dicer complex, resulting in mature miRNAs [34]. miRNAs have a tissue-specific expression and play important roles in many physiological and pathological processes, including tumorigenesis [35][36][35,36]. miRNAs that are released from the cells in the bloodstream are referred to as circulating miRNAs. Circulating miRNAs can be achieved with minimally invasive approaches and have several intrinsic features, making them interesting as biomarkers. miRNAs have been shown to have sensitivity, specificity, and predictive power [37][38][37,38]. In addition, in contrast to other RNA classes, they are very stable and can be reliably quantified in the blood [39]. Moreover, miRNAs detected in biological fluids can “mirror” changes in the cells of origin, and their levels may be associated with specific clinical features, responses to treatments, and patient outcomes [40].4.1. miRNA Detection
Despite the characteristics that make miRNAs suitable biomarkers, detecting these molecules may be challenging due to their intrinsic characteristics, such as small size, low levels of expression, sequence similarity among different members, and tissue- or developmental stage-specific expression. Two general approaches are commonly used in the research of miRNAs:- (1)
-
The use of profiling methods, such as microarray, quantitative real-time polymerase chain reaction (qRT-PCR)-based array, quantitative nCounter, or next-generation sequencing (NGS), to quantify hundreds of miRNAs in samples obtained from patients with a pathology of interest in comparison to control subjects. This approach is usually followed by the validation of identified miRNAs using qRT-PCR or other techniques.
- (2)
-
The selection of a subset of specific miRNAs related to specific tissues, cells, gene expression pathways, and diseases. In this case, the number of miRNAs to study is limited, and other experimental approaches can be used. These include individual qRT-PCR, which allows increasing sensitivity and reproducibility of the analysis, and droplet digital PCR technology, which provides miRNA absolute quantification without the requirement of standard curves, efficiency correction methods, or technical replicates [41].
4.2. Circulating miRNAs in MTC
To date, six studies have been published exploring miRNA expression levels in plasma and serum samples obtained from MTC patients. Romeo et al. identified increased levels of plasma miR-375 in MTC in comparison to healthy controls and subjects in remission. Circulating miR-375 levels were higher in male compared to female patients, while this difference was not present in matched healthy controls. Importantly, high levels were associated with distant metastases and reduced overall survival and were a strong prognostic marker of poor prognosis in MTC patients. In addition, although miR-375 plasma levels did not reach statistical significance as a predictor of the vandetanib response, the trend observed suggested that miR-375 could be considered as a possible biomarker for the response to treatments in future studies [44]. Zhang et al. showed that the serum levels of miR-222-3p and miR-17-5p were significantly augmented in MTC patients compared to patients with benign nodules and healthy control subjects. Receiver operating characteristic (ROC) curve analyses confirmed the high diagnostic accuracy of the two miRNAs, suggesting they can represent potential diagnostic biomarkers in MTC, especially when used in combination [45]. Shabani et al. examined plasma levels of miR-144 and miR-34a, in MTC patients with or without RET mutations and in healthy controls. Both miRNAs were expressed at higher levels in patients with MTC than in controls and in patients carrying RET mutation than in RET wild-type patients. However, a ROC curve analysis showed that specificity and sensitivity are not suitable for using miR-144 and miR-34a as circulating MTC biomarkers [46]. Censi et al. further assessed the diagnostic and prognostic value of circulating miR-375 levels in MTC, showing that the levels of this miRNA were 101 times higher in the serum of MTC patients than in all the other patients and controls included in the study, without overlap. However, no correlation between serum and tissue miR-375 levels was observed. In addition, serum miR-375 and CT levels showed a negative correlation in MTC patients. The ROC curve analysis showed that serum miR-375 levels can be used as a biomarker in MTC diagnosis, with a notable specificity [47]. Melone et al. applied miRNome profiling in the context of a wider multiomics approach, aimed at identifying common molecular and functional signatures of different neuroendocrine neoplasms (NENs), including MTCs. They identified a set of 13 miRNAs that could be evaluated as possible circulating biomarkers. Overall, these miRNAs were significantly overexpressed in NEN patients compared to healthy subjects. Among them, miR144-3p, miR7-5p, and miR335-5p were significantly upregulated in MTC. Furthermore, miR-375 was significantly upregulated in all the different NEN subgroups [48]. Finally, Besharat et al. focused on miRNAs obtained from plasma extracellular vesicle samples. Using miRNA arrays in the first cohort of MTC patients, they identified a set of circulating miRNAs as diagnostic biomarkers. Among them, miR-26b-5p and miR-451a showed a higher expression, which decreased during follow-up in disease-free patients. The diagnostic performance of the two miRNAs was validated in a second independent cohort of MTC patients and control subjects using digital PCR. ROC curve analyses confirmed the high diagnostic role of miR-26b-5p and miR-451a [49]. Despite a low overlapping among the miRNAs identified, some circulating miRNAs seem to be particularly promising as potential biomarkers in MTC patients. miR-375 is one of the most interesting miRNAs since its upregulation has been reported in three different studies [44][47][48][44,47,48] both in plasma and serum of MTC patients. It has shown high diagnostic and prognostic accuracy. In addition, miR-375 was significantly upregulated in different subgroups of NENs, suggesting that its overexpression may be a common trait of neuroendocrine cancers. miR-144 levels were also reported as being increased both in plasma and serum [46][48][46,48]. However, in this case, specificity and sensitivity were too low to propose this miRNA as a new circulating biomarker for MTC. Worth noting, only one study has been performed to date on plasma extracellular vesicles (pEVs) [49], which reported an upregulation in miR-26b-5p and miR-451a in MTC patients with significant diagnostic performance. These miRNAs had not been previously identified, probably due to the different biological samples used in other studies. In addition, the use of pEVs can avoid the effect of hemolysis, which is known to affect the expression levels of these miRNAs [50].
