Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 3 by Dean Liu.
Although microglia exist as a minor glial cell type in the normal state of the brain, they increase in number in response to various disorders and insults. However, it remains unclear whether microglia proliferate in the affected area, and the mechanism of the proliferation has long attracted the attention of researchers.
- microglia
- proliferation
- facial nerve
- axotomy
- M-CSF
- cFms
1. Introduction
The central nervous system (CNS) is constructed from heterogenous cell types, including neurons and glial cells. Glial cells are grouped into macroglia (astrocytes and oligodendrocytes) and microglia [1][2]. In the early 1900s, the CNS was thought to be made up exclusively of neurons (the first element) and astrocytes (the second element), but in 1913, Cajal recognized that there was also a distinct “third element” of the CNS [3][4]. Subsequently, a pupil of his, del Rio-Hortega, selectively dyed microglia as part of his analysis of the third element [5]. For this reason, it is considered that microglia were discovered by Rio-Hortega. Like his teacher, Rio-Hortega believed that microglia originated from mesodermal cells. However, Rio-Hortega expanded this concept, suggesting that microglia originated from mesodermal precursor cells (extracerebral cells) that enter the brain and adopt an ameboid morphology, followed by a ramified morphology in the developing brain [5].
Soon after their discovery, however, microglia began to engender confusion in regard to their cell origin (cell lineage). In the 1980s, two groups put forward the idea that microglia were derived from monocytes, and this theory became widely popular [6][7]. According to this theory, monocytes produced in the bone marrow infiltrate the brain parenchyma from the bloodstream during the early developmental stage of the brain and then gradually transform into microglia in the parenchyma. This bone-marrow-derived monocytes theory was readily accepted by many researchers worldwide. However, subsequent analyses of radiation bone marrow chimeras left the theory open to question [8][9], since they revealed that bone-marrow-derived monocytes do not usually transform to ramified microglia in the adult parenchyma.
At the same time, some research groups advocated that microglia originate from neuroectodermal cells. One group described that glioblasts differentiate into astrocytes, oligodendrocytes, and microglia in the developing brain, and thus, resting microglia should be considered neuroglia, which are derived from neuroepithelia [10]. Another group insisted that microglia originate from the floor plate glial cells arising from the neuroepithelium, and thus, they concluded that microglia are of neuroectodermal origin [11]. Both these groups argued that microglia differentiate from neuroepithelia just as the other glial cell types do.
However, it is now clarified that microglia originate from yolk-sac-derived fetal macrophages that develop into the microglial precursors observed in early-stage murine embryos [12]. In rodents, these precursor cells exist mixed in the developing neuroectodermal cells as early as embryonic day 8 [13][14], when neovascularization is not yet completed. In the perinatal stages, two kinds of morphologically distinct microglia, ameboid microglia and ramified microglia, can be seen in the brain. The ameboid microglia have short or no processes growing from a relatively large cell body [15][16] and are observed particularly in the supraventricular corpus callosum and around ventricles. The ramified microglia include a few long prolongations extended from a small cell body and are seen in the cerebral cortex at the same stage. The ameboid microglia were thought to be derived from monocytes that entered from the blood vessel [15][17], and they have been shown to stay in place over the two weeks after birth. However, by three weeks postnatally, the ameboid microglia disappear and only the ramified microglia are seen throughout the CNS [18]. In the adult brain, microglia mostly take on the shape of ramified microglia and distribute to the CNS with regular spacing.
What is the relationship between ameboid microglia and ramified microglia? It is generally speculated that ameboid microglia change into ramified microglia during brain development [19][20][21]. On the other hand, some researchers have argued that ameboid microglia do not transform into ramified microglia, because a smooth transition from ameboid to ramified microglia is not observed during brain development. Thus, the transformation from ameboid microglia to ramified microglia has not been sufficiently explained.
2. The Proliferative Feature of Microglia
As described above, microglia in the normal adult CNS are present as ramified microglia accounting for a minor population among glial cells. Their cell density is kept very low in the normal CNS. Microglia are estimated to make up 5–12% of total cells in mouse brain [22]. Recent quantification revealed that microglia constitute approximately 7% of non-neuronal cells in the mammalian brain [23]. Courtney et al. estimated that microglia account for 4.5–5.5% of total cells in the mouse brain [24]. In addition, a meta-analysis of studies quantifying the cell number of glial cells in humans reported that human microglia are generally considered to make up around 10% of total glial cells [25]. Although microglia have characteristic biological features such as a competence for morphological change, migratory capacity, and phagocytic ability, they also have the notable ability to increase their number in response to various pathological events, including trauma [26][27], or diseases/disorders [28][29]. Such microglial features have caught the eye of researchers because the phenomenon of microglia increasing their cell numbers stands out in the CNS. In the human brain, increases in microglial cell number have been recognized in neurodegenerative diseases, including Alzheimer’s disease [30][31], Parkinson’s disease [32][33][34], multiple sclerosis [35][36], Huntington’s disease [37], stroke [38][39], ischemia [40], amyotrophic lateral sclerosis (ALS) [41][42], and prion diseases [43][44]. Increases in microglial cell number were also observed in lesioned sites including the injured spinal cord [45][46], axotomized hypoglossal nucleus [47][48], and the spinal cord undergoing rhizotomy or sciatic nerve transection [49] in rodents. In this context, researchers should carefully consider the statement “microglia increase their number in response to various pathological events”. An increase in cell number in vivo usually occurs due to a migration from around tissue or cell proliferation at the site of insult or injury. Which event contributed to the increase in microglial cell number is an important question. In the usual lesion models, peripheral macrophages enter the injured parenchyma, and thus, it is hardly possible to distinguish the resident microglia-derived activated microglia from macrophages infiltrated from the periphery. Even if microglia-like cells are increased at the injured sites, researchers cannot say that they all came from resident microglia because they may contain the infiltrated macrophages in addition to activated microglia. Researchers have sometimes experienced such ambiguous results in the experiments, and inevitably the following questions arise. Did microglia alone proliferate at the site of trauma? Do macrophages also infiltrate into the injured place? How can you distinguish the resident microglia and infiltrated macrophages? These questions are related to the matter that the activated microglia and the peripheral macrophages express some common surface antigens and thereby they are not easily distinguished from each other. Thus, it remained to be determined whether the increase in microglial cells in lesioned sites was ascribable to the mitosis of microglia themselves or the infiltration of peripheral macrophages. A peripheral nerve axotomy model was found to be appropriate for analyzing the proliferation of microglia. For example, axotomy of the rat facial nerve does not cause the infiltration of blood-derived cells in the ipsilateral facial nucleus [50][51][52], enabling us to examine the response of microglia alone in the parenchyma (Figure 1A). That being said, researchers need to take care when mice are used for this purpose. In the mouse facial nerve axotomy model, unlike in rats, significant amounts of blood-derived cells are known to infiltrate the parenchyma [53][54]. From this point of view, a rat model is suitable for analyzing the reaction of microglia alone in parenchyma. In fact, notable results have been achieved by using a rat facial nerve transection model.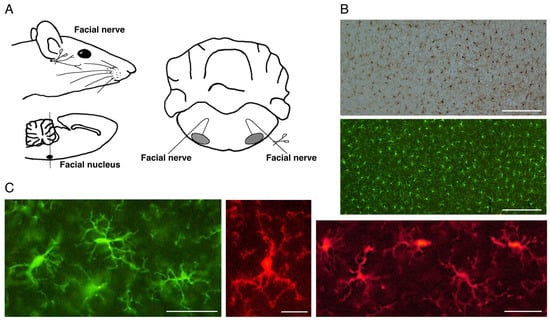
Figure 1. The rat motoneuron injury model and ramified microglia in the normal state. (A). Schema of rat facial nerve axotomy. Motoneurons in the facial nucleus of the brainstem extend their nerves to facial expression muscles. In the axotomy model, the facial nerve was unilaterally cut at the stylomastoid foramen, and subsequently both facial nuclei were analyzed. (B). Distribution of ramified microglia in the normal adult rat cerebral cortex. Ramified microglia were stained by the avidin biotin complex (ABC) method (upper panel) and fluorescence method (lower panel). Scale bar = 200 μm. (C). Morphology of ramified microglia. Typical morphology of ramified microglia is shown. Scale bar = 50 μm (left and right) or 20 μm (center).
3. M-CSF: A Trigger of Microglial Proliferation
As described above, microglia were anticipated to proliferate in response to M-CSF in the axotFN. Thus, Yamamoto et al. analyzed what happens in the M-CSF-stimulated microglia using an in vitro system [55] in which microglia were prepared from a newborn rat brain-derived mother culture and used as neonatal microglia (neoMicroglia) [56].
The neoMicroglia were found to incorporate bromo-deoxy uridine (BrdU), indicating that they were mitotic cells. However, they entered a quiescent state when maintained with new medium. Such non-proliferative microglia were found to change to proliferative cells by stimulation with M-CSF. The M-CSF-stimulated microglia begin to increase their cell number after a 2-day delay (Figure 23B), and over the 3-day culture the cell number became approximately threefold the baseline value, depending on the concentration of M-CSF (0–40 ng/mL). The neoMicroglia increased their cell number in response to not only M-CSF but also GM-CSF and IL-3 (Figure 23C), as has been previously reported [57].
When the microglia of an adult animal received an interest from a viewpoint of cellular characteristics, Rieske et al. succeeded in culturing adult axotFN-derived microglia [58]. This study first established an explant culture to prepare the adult microglia. Owing to the method, axotFN-derived microglia (axotMicroglia) can be obtained with high purity [59]. These axotMicroglia were intrinsically proliferative, and thus, they increase in number in culture without addition of any growth factors (Figure 23D). Over the 10-day culture, their number increased sevenfold or more (Figure 23E). The cultured microglia vigorously incorporated BrdU in their nuclei, suggesting that they undergo mitosis. Analysis of axotMicroglia in vitro clarified that the M-CSF protein was present in both the cells and the conditioned medium. The receptor of M-CSF, cFms, was also found to be expressed in the microglia [59]. The axotMicroglia appeared to autonomously proliferate, suggesting that they proliferate in such a manner that they produce/secrete M-CSF and respond to it—that is, in an autocrine fashion.
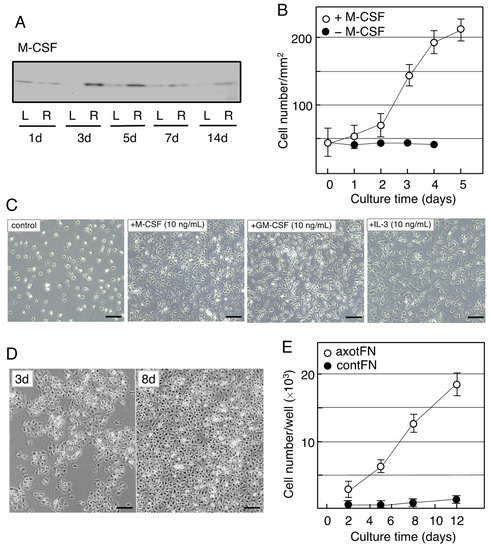

Figure 23. Induction of M-CSF in the axotomized facial nucleus and the effects of M-CSF on microglia. (A). Changes in M-CSF levels in the axotFN. The right facial nerve of adult rats was transected, and the contFN (L) and axotFN (R) of each rat were analyzed for M-CSF in immunoblotting at 1, 3, 5, 7, and 14 days post-insult. (B). Effects of M-CSF on microglial proliferation. M-CSF (20 ng/mL) was added to the microglial culture (2 × 104/well) and the cell number within a 1 mm2 area (○) was counted [60]. The cell number in the absence of M-CSF is shown as (●). The results are expressed as means ± SDs of three separate experiments. (C). Effects of M-CSF, GM-CSF, and IL-3 (multi-CSF). M-CSF (10 ng/mL), GM-CSF (10 ng/mL), and IL-3 (10 ng/mL) were added to microglial culture and maintained for 3 days. Scale bar = 50 μm. (D). Increase in the cell number of axotFN-microglia. AxotFN recovered at 3 days post-insult were subjected to explant culture [59]. The microglia attached to the dish were photographed at 3 and 8 days after-culture. Scale bar = 50 μm. (E). Proliferation of axotFN-microglia. The axotFN transected 3 days previously (axotFN; ○) and contFN (contFN; ●) were subjected to explant culture [59]. The emerged cells were counted at 2, 5, 8, and 12 days following culture. The results are expressed as means ± SDs of three separate experiments.
References
- Kaur, C.; Ling, E.A.; Wong, W.C. Development of the Various Glial Cell Types in the Cerebral Cortex of Postnatal Rats. Acta Anat. 1989, 136, 204–210.
- Werkman, I.L.; Lentferink, D.H.; Baron, W. Macroglial Diversity: White and Grey Areas and Relevance to Remyelination. Cell. Mol. Life Sci. 2021, 78, 143–171.
- Cajal, S.R.y. Contribución Al Conocimiento de La Neuroglia Del Cerebro Humano. Trab. Lab. Investig. Biol. 1913, 11, 255–315.
- Kettenmann, H.; Ransom, B.R. The Concept of Neuroglia: A Historical Perspective. In Neuroglia, 2nd ed.; Kettenmann, H., Ransom, B.R., Eds.; Oxford University Press: New York, NY, USA, 2005; pp. 1–16.
- del Rio-Hortega, P. Microglia. In Cytology and Cellular Pathology of the Nervous System; Penfield, W., Ed.; PB Hocker: New York, NY, USA, 1932; pp. 489–534.
- Imamoto, K.; Leblond, C.P. Radioautographic Investigation of Gliogenesis in the Corpus Callosum of Young Rats. II. Origin of Microglial Cells. J. Comp. Neurol. 1978, 180, 139–163.
- Ling, E.A.; Wong, W.C. The Origin and Nature of Ramified and Amoeboid Microglia: A Historical Review and Current Concepts. Glia 1993, 7, 9–18.
- Hickey, W.F.; Kimura, H. Graft-vs.-Host Disease Elicits Expression of Class I and Class II Histocompatibility Antigens and the Presence of Scattered T Lymphocytes in Rat Central Nervous System. Proc. Natl. Acad. Sci. USA 1987, 84, 2082–2086.
- Lassmann, H.; Schmied, M.; Vass, K.; Hickey, W.F. Bone Marrow Derived Elements and Resident Microglia in Brain Inflammation. Glia 1993, 7, 19–24.
- Fujita, S.; Tsuchihashi, Y.; Kitamura, T. Origin, Morphology and Function of the Microglia. In Glial and Neuronal Cell Biology; Vidrio, E.A., Fedroff, S., Eds.; Alan R. Liss: New York, NY, USA, 1981; pp. 141–169.
- Fedoroff, S. Development of Microglia. In Neuroglia; Kettenmann, H., Ransom, B.R., Eds.; Oxford Univ Press: New York, NY, USA, 1995; pp. 162–181.
- Prinz, M.; Mildner, A. Microglia in the CNS: Immigrants from Another World. Glia 2011, 59, 177–187.
- Takahashi, K.; Yamamura, F.; Naito, M. Differentiation, Maturation, and Proliferation of Macrophages in the Mouse Yolk Sac: A Light-Microscopic, Enzyme-Cytochemical, Immunohistochemical, and Ultrastructural Study. J. Leukoc. Biol. 1989, 45, 87–96.
- Monier, A.; Adle-Biassette, H.; Delezoide, A.-L.; Evrard, P.; Gressens, P.; Verney, C. Entry and Distribution of Microglial Cells in Human Embryonic and Fetal Cerebral Cortex. J. Neuropathol. Exp. Neurol. 2007, 66, 372–382.
- Cuadros, M.A.; Navascués, J. The Origin and Differentiation of Microglial Cells during Development. Prog. Neurobiol. 1998, 56, 173–189.
- Marín-Teva, J.L.; Cuadros, M.A.; Martín-Oliva, D.; Navascués, J. Microglia and Neuronal Cell Death. Neuron Glia Biol. 2011, 7, 25–40.
- Chugani, D.C.; Kedersha, N.L.; Rome, L.H. Vault Immunofluorescence in the Brain: New Insights regarding the Origin of Microglia. J. Neurosci. 1991, 11, 256–268.
- Milligan, C.E.; Cunningham, T.J.; Levitt, P. Differential Immunochemical Markers reveal The Normal Distribution of Brain Macrophages and Microglia in The Developing Rat Brain. J. Comp. Neurol. 1991, 314, 125–135.
- Ling, E.A. The Origin and Nature of Microglia. In Advances in Cellular Neurobiology; Fedoroff, S., Hertz, L., Eds.; Elsevier: New York, NY, USA, 1981; Volume 2, pp. 33–82.
- Perry, V.H.; Gordon, S. Macrophages and Microglia in the Nervous System. Trends Neurosci. 1988, 11, 273–277.
- Graeber, M.B.; Streit, W.J. Microglia: Immune Network in the CNS. Brain Pathol. 1990, 1, 2–5.
- Lawson, L.J.; Perry, V.H.; Dri, P.; Gordon, S. Heterogeneity in the Distribution and Morphology of Microglia in the Normal Adult Mouse Brain. Neuroscience 1990, 39, 151–170.
- Dos Santos, S.E.; Medeiros, M.; Porfirio, J.; Tavares, W.; Pessôa, L.; Grinberg, L.; Leite, R.E.P.; Ferretti-Rebustini, R.E.L.; Suemoto, C.K.; Filho, W.J.; et al. Similar Microglial Cell Densities across Brain Structures and Mammalian Species: Implications for Brain Tissue Function. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 4622–4643.
- Courtney, J.-M.; Morris, G.P.; Cleary, E.M.; Howells, D.W.; Sutherland, B.A. An Automated Approach to Improve the Quantification of Pericytes and Microglia in Whole Mouse Brain Sections. eNeuro 2021, 8, ENEURO.0177-21.2021.
- von Bartheld, C.S.; Bahney, J.; Herculano-Houzel, S. The Search for True Numbers of Neurons and Glial Cells in the Human Brain: A Review of 150 Years of Cell Counting. J. Comp. Neurol. 2016, 524, 3865–3895.
- Streit, W.J.; Graeber, M.B.; Kreutzberg, G.W. Functional Plasticity of Microglia: A Review. Glia 1988, 1, 301–307.
- Raivich, G.; Gehrmann, J.; Kreutzberg, G.W. Increase in Macrophage Colony-Stimulating Factor and Granulocyte-Macrophage Colony-Stimulating Factor Receptors in the Regenerating Rat Facial Nucleus. J. Neurosci. Res. 1991, 30, 682–686.
- Perry, V.H.; Holmes, C. Microglial Priming in Neurodegenerative Disease. Nat. Rev. Neurol. 2014, 10, 217–224.
- Xu, Y.; Jin, M.-Z.; Yang, Z.-Y.; Jin, W.-L. Microglia in Neurodegenerative Diseases. Neural Regen. Res. 2021, 16, 270–280.
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s Disease. J. Clin. Investig. 2017, 127, 3240–3249.
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s Disease. J. Cell Biol. 2018, 217, 459–472.
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; López-Cuenca, I.; Rojas, P.; Triviño, A.; Ramírez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214.
- Ho, M.S. Microglia in Parkinson’s Disease. Adv. Exp. Med. Biol. 2019, 1175, 335–353.
- Smajić, S.; Prada-Medina, C.A.; Landoulsi, Z.; Ghelfi, J.; Delcambre, S.; Dietrich, C.; Jarazo, J.; Henck, J.; Balachandran, S.; Pachchek, S.; et al. Single-Cell Sequencing of Human Midbrain Reveals Glial Activation and a Parkinson-Specific Neuronal State. Brain 2022, 145, 964–978.
- Monif, M.; Burnstock, G.; Williams, D.A. Microglia: Proliferation and Activation Driven by the P2X7 Receptor. Int. J. Biochem. Cell Biol. 2010, 42, 1753–1756.
- Nowacki, P.; Koziarska, D.; Masztalewicz, M. Microglia and Astroglia Proliferation within the Normal Appearing White Matter in Histologically Active and Inactive Multiple Sclerosis. Folia Neuropathol. 2019, 57, 249–257.
- Kraft, A.D.; Kaltenbach, L.S.; Lo, D.C.; Harry, G.J. Activated Microglia Proliferate at Neurites of Mutant Huntingtin-Expressing Neurons. Neurobiol. Aging 2012, 33, 621.e17–621.e33.
- Li, T.; Pang, S.; Yu, Y.; Wu, X.; Guo, J.; Zhang, S. Proliferation of Parenchymal Microglia Is the Main Source of Microgliosis after Ischaemic Stroke. Brain 2013, 136, 3578–3588.
- Rawlinson, C.; Jenkins, S.; Thei, L.; Dallas, M.L.; Chen, R. Post-Ischaemic Immunological Response in the Brain: Targeting Microglia in Ischaemic Stroke Therapy. Brain Sci. 2020, 10, 159.
- Zhang, S. Microglial Activation after Ischaemic Stroke. Stroke Vasc. Neurol. 2019, 4, 71–74.
- Dewil, M.; Van Den Bosch, L.; Robberecht, W. Microglia in Amyotrophic Lateral Sclerosis. Acta Neurol. Belg. 2007, 107, 63–70.
- Hovden, H.; Frederiksen, J.L.; Pedersen, S.W. Immune System Alterations in Amyotrophic Lateral Sclerosis. Acta Neurol. Scand. 2013, 128, 287–296.
- Obst, J.; Simon, E.; Mancuso, R.; Gomez-Nicola, D. The Role of Microglia in Prion Diseases: A Paradigm of Functional Diversity. Front. Aging Neurosci. 2017, 9, 207.
- Peggion, C.; Stella, R.; Lorenzon, P.; Spisni, E.; Bertoli, A.; Massimino, M.L. Microglia in Prion Diseases: Angels or Demons? Int. J. Mol. Sci. 2020, 21, 7765.
- Poulen, G.; Aloy, E.; Bringuier, C.M.; Mestre-Francés, N.; Artus, E.V.F.; Cardoso, M.; Perez, J.-C.; Goze-Bac, C.; Boukhaddaoui, H.; Lonjon, N.; et al. Inhibiting Microglia Proliferation after Spinal Cord Injury Improves Recovery in Mice and Nonhuman Primates. Theranostics 2021, 11, 8640–8659.
- Xu, L.; Wang, J.; Ding, Y.; Wang, L.; Zhu, Y.-J. Current Knowledge of Microglia in Traumatic Spinal Cord Injury. Front. Neurol. 2021, 12, 796704.
- Svensson, M.; Mattsson, P.; Aldskogius, H. A Bromodeoxyuridine Labelling Study of Proliferating Cells in the Brainstem Following Hypoglossal Nerve Transection. J. Anat. 1994, 185 Pt 3, 537–542.
- Gehrmann, J.; Banati, R.B. Microglial Turnover in the Injured CNS: Activated Microglia Undergo Delayed DNA Fragmentation Following Peripheral Nerve Injury. J. Neuropathol. Exp. Neurol. 1995, 54, 680–688.
- Liu, L.; Rudin, M.; Kozlova, E.N. Glial Cell Proliferation in the Spinal Cord after Dorsal Rhizotomy or Sciatic Nerve Transection in the Adult Rat. Exp. Brain Res. 2000, 131, 64–73.
- Kreutzberg, G.W. Microglia: A Sensor for Pathological Events in the CNS. Trends Neurosci. 1996, 19, 312–318.
- Moran, L.B.; Graeber, M.B. The Facial Nerve Axotomy Model. Brain Res. Brain Res. Rev. 2004, 44, 154–178.
- Nakajima, K.; Ishijima, T. Events Occurring in the Axotomized Facial Nucleus. Cells 2022, 11, 2068.
- Raivich, G.; Jones, L.L.; Kloss, C.U.; Werner, A.; Neumann, H.; Kreutzberg, G.W. Immune Surveillance in the Injured Nervous System: T-Lymphocytes Invade the Axotomized Mouse Facial Motor Nucleus and Aggregate around Sites of Neuronal Degeneration. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 5804–5816.
- Jones, K.J.; Lovett-Racke, A.E.; Walker, C.L.; Sanders, V.M. CD4 + T Cells and Neuroprotection: Relevance to Motoneuron Injury and Disease. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2015, 10, 587–594.
- Yamamoto, S.; Nakajima, K.; Kohsaka, S. Macrophage colony-stimulating Factor as an Inducer of Microglial Proliferation in Axotomized Rat Facial Nucleus. J. Neurochem. 2010, 115, 1057–1067.
- Nakajima, K. Characterization of Microglia Isolated from a Primary Culture of Embryonic Rat Brain by a Simplified Method. Biomed. Res. 1989, 10, 411–423.
- Ganter, S.; Northoff, H.; Männel, D.; Gebicke-Härter, P.J. Growth Control of Cultured Microglia. J. Neurosci. Res. 1992, 33, 218–230.
- Rieske, E.; Graeber, M.B.; Tetzlaff, W.; Czlonkowska, A.; Streit, W.J.; Kreutzberg, G.W. Microglia and Microglia-Derived Brain Macrophages in Culture: Generation from Axotomized Rat Facial Nuclei, Identification and Characterization in Vitro. Brain Res. 1989, 492, 1–14.
- Nakajima, K.; Graeber, M.B.; Sonoda, M.; Tohyama, Y.; Kohsaka, S.; Kurihara, T. In Vitro Proliferation of Axotomized Rat Facial Nucleus-Derived Activated Microglia in an Autocrine Fashion. J. Neurosci. Res. 2006, 84, 348–359.
- Yamamoto, S.; Kohsaka, S.; Nakajima, K. Role of Cell Cycle-Associated Proteins in Microglial Proliferation in the Axotomized Rat Facial Nucleus. Glia 2012, 60, 570–581.
More
