Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Suzana Maria Loures de Oliveira Marcionilio and Version 2 by Dean Liu.
The use of new technologies for the removal of pollutants from wastewater has become globally necessary due to the complexity and facilities defined by conventional treatments. Advanced oxidative processes, specifically the Fenton process, have become widely applied given their low cost and ease of use.
- scientometrics
- wastewater
- advanced oxidative processes
- pollution
1. Introduction
According to the 2017 UNESCO report on water-resource development [1] contemporary production and lifestyles are closely associated with strict demands for high drinking water quality, but production and lifestyle have led to serious water pollution. Therefore, the same report states that about 80% of the wastewater is still globally returned to the environment without adequate treatment. Then, to mitigate the resulting pollution, regulatory agencies currently require on-site treatment of generated effluents. Several technologies are used for effluent treatment, including filtration, reverse osmosis, adsorption, and coagulation–flocculation [2][3][4][5][2,3,4,5]. However, new processes are being established to compare the results and increase the efficiency of the removal of persistent, refractory, and nonbiodegradable pollutants, such as drugs, pesticides, dyes, organic solvents, phenols, oily effluents, and agroindustrial wastes, such as stillage [6][7][6,7].
To degrade certain types of pollutants, chemical treatment, which generates oxidizing agents, particularly hydroxyl radicals (•OH), must be used. Currently, the most effective methods for degrading organic pollutants in wastewater and contaminated soil are advanced oxidative processes (AOPs), which include the Fenton approach. The Fenton process produces •OH through a homogeneous reaction (takes place via the reaction from a mixture of hydrogen peroxide and ferrous salts). These oxidants are highly reactive and nonselective, reacting with a wide range of organic compounds and, consequently, degrading them into water, carbon dioxide, and inorganic ions [8]. For the by-products, the type of reaction that occurs between •OH and the intermediate products will depend on the structure of the target compound.
Treatment processes based on the Fenton reaction have been effectively applied to treat various types of industrial effluents and municipal sewage treatment plants, as a single-, pre- and/or post-treatment methods [9]. For example, it can be used to treat leachate from landfill, from vinasse, as post-treatment of biodigested vinasse, in the treatment of effluents from the wood industry, in the removal of endocrine disruptors as well as to remove other environmental micro pollutants from the pharmaceutical industry, from hospital wastes, from university laboratories, from pesticides residues, and others [9][10][9,10].
2. Time Trends in Studies Using Fenton
Considering WoS and Scopus databases, the search identified a total of 1073 and 1653 articles in indexed journals, respectively, until 2022, with the earliest indexed publication on the theme in Scopus dating back to 1992. Among these articles, 141 and 390 contained the term “Fenton” in their title but these did not employ the technique in their study. These papers were excluded from further analysis as they were not aligned with the objectives of this systematic review. In total, 932 and 1263 articles (WoS and Scopus databases, respectively) on the implementation of the Fenton technique to remove any form of pollutant were considered. In regard to the use of the WoS database, it is important to indicate that WoS is mainly designed for researchers to find published literature, and it could be restricted for bibliometric studies. Then, it is not suitable to directly use all different types and levels of the WoS database for bibliometric study. For example, Data Citation Index, Derwent Innovations Index, Zoological Record, Social Sciences Citation Index, Arts & Humanities Citation Index, Conference Proceedings Citation Index—Social Sciences & Humanities, Book Citation Index—Social Sciences & Humanities, Current Chemical Reactions, and Index Chemicus. In fact, a minor number of articles was identified by the WoS search. Then, the information was collected once again with the same methodology previously used but the Scopus database was utilized. Comparing the results obtained in the two databases (Figure 1), it is observed that WoS is less comprehensive than Scopus. This may be due to the Scopus database having a larger, internationally diverse database as well as a range of intelligent tools that can be used to aid the research process, such as journal rankings, author profiles, the number of articles published by a journal in a given year, and the frequency of use of scientific terms. Therefore, Scopus was used to elaborate the systematic review, examining the results obtained. Additionally, WoS results were reported as Supplementary Materials (SM) in order to illustrate the similarities.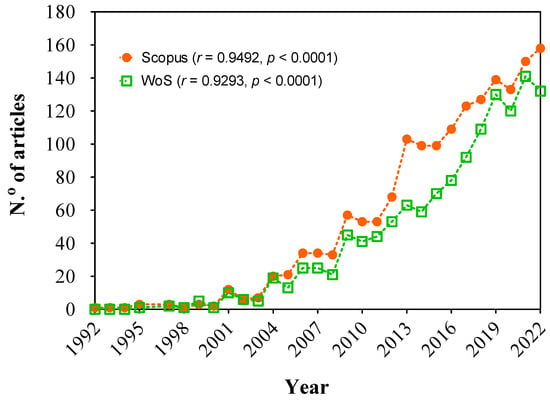
Figure 1. Comparison of the number of articles, between WoS and Scopus databases, using the same search methodology. Temporal trend in number of articles published in WoS and Scopus database.
Table 1. Temporal trend in number of articles published between 1992 and 2022, and Pearson correlation (r) in relation to number of articles published per year × type of compounds degraded by Fenton.
| Variable | No. of Articles | r | p |
|---|---|---|---|
| No. of articles | 1263 | 0.93 | <0.001 |
| Dye | 227 | 0.85 | <0.001 |
| Drugs | 114 | 0.81 | <0.001 |
| Phenol | 62 | 0.81 | <0.001 |
| Organic compounds | 216 | 0.87 | <0.001 |
| Hygiene and cleaning | 11 | 0.42 | 0.02 |
| Pesticide | 22 | 0.59 | <0.001 |
| Inorganic compounds/metals | 40 | 0.82 | <0.001 |
| Vinasse | 5 | 0.32 | 0.09 |
| Other compounds | 418 | 0.69 | <0.001 |
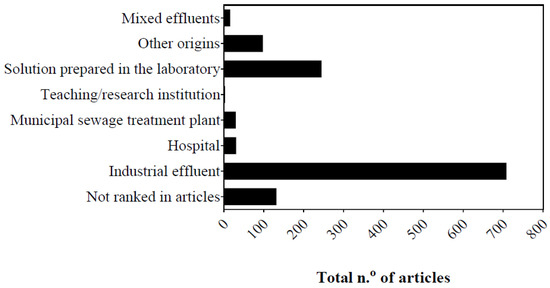
Figure 2. Temporal trend in number of articles published between 1992 and 2022 in Scopus database, considering origin of effluents degraded by Fenton.
3. Size and Reaction Conditions of Fenton Reaction Studies
Most studies on Fenton reactions have been conducted on the laboratory-bench scale, representing 98.3% of the studies analyzed, followed by those on the pilot and industrial scales. On the laboratory-bench scale, a smaller volume of effluent is used under controlled laboratory conditions, even though these effluents are representative of industrial effluents, as shown in Figure 2. Only 1.3% of the analyzed publications were conducted on the pilot scale, which is a method used for limiting the problems experienced with laboratory-bench-scale studies. However, pilot-scale studies are hindered by the operational costs associated with their implementation and execution. For example, Ferreira et al., (2020) [41][48] discussed the effect in a pilot plant process combining Fenton reaction with other AOPs and, then, the results showed that these kind of combinations are promising options for reducing the water footprint of biorecalcitrant industrial wastewaters. Moreover, 0.07% of direct studies have been conducted on the industrial scale, 0.07% of studies have used a combination of scales, and 0.31% of the studies did not specify the scale used. Most studies (80.9%) used traditional reaction conditions (1022), followed by Fenton-like reactions (140) (Figure 3).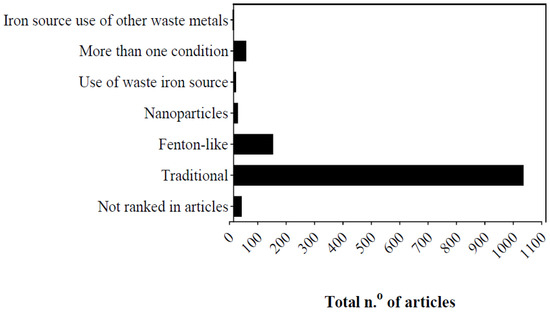
Figure 3. Fenton reaction arrangements on 30-year time scale.
