Gastroesophageal reflux disease (GERD) has the highest prevalence among diseases of the digestive system and is characterized by a significant decrease in patients’ quality of life, comparable to arterial hypertension and coronary heart disease. One in every ten cases of reflux esophagitis leads to the formation of Barrett’s esophagus, which is associated with a high risk of esophagus adenocarcinoma. The key factors determining the progression of the disease are the frequency and duration of the reflux of the stomach’s contents. As a result, refluxate, which includes hydrochloric acid, pepsin, and, in the case of concomitant duodeno-gastric reflux, bile acids and lysolecithin, is thrown into the overlying sections of the digestive tract.
- gastroesophageal reflux disease
- mucosal resistance
- reflux esophagitis
1. Introduction
2. Components of the Mucosal Barrier That Provide Resistance to the Esophageal Mucosa in Conditions of GERD
The most superficial, pre-epithelial level of protection is the mucus layer, which neutralizes the incoming acid and protects the squamous epithelium of the esophagus from contact with the reflux content [15]. Its key components are mucins, bicarbonates and non-bicarbonate buffers, prostaglandin E2, epidermal growth factor, and transforming growth factor alpha [16]. The main protective glycoproteins, mucins, enter the esophagus with saliva and are secreted by the esophagus’s glands. Mucins are present in a secreted form, which forms a protective layer over the epithelium, and in a form associated with the cell membrane, which is part of the glycocalyx and localized on the surface of epithelial cells [15]. It has been established that aggressive molecules in the composition of the refluxate, primarily hydrochloric acid, stimulate the secretion of mucins MUC3 and MUC5AC, while an increase in the secretion of mucins is associated with the restoration of the protective properties of the mucous membrane and vice versa; this is significantly reduced under conditions of the progression of esophagitis [17]. The resident microbiota are also commonly referred to as pre-epithelial protection factors, although their population is significantly smaller in comparison with other parts of the digestive tract [18]. The presence of data on the dominance of streptococci and the frequent presence of other taxa typical of the microbiota of the oropharynx are associated with the composition of the microflora of the oral cavity and pharynx, where a high prevalence of streptococci is found, along with taxonomic units, such as Veillonella, Fusobacterium, Gemella, Granulicatella, and Rothia, indicating that the microbiota of the esophagus are mainly of oral origin [19,20][19][20]. However, not all bacteria associated with the oral mucosa can colonize the esophageal mucosa, while some members of the esophageal microbiota are absent or present in small numbers in typical oral microflora, indicating the existence of microbiota. The esophagus is a separate microbiological ecosystem [21]. The next stage of protection is the mucosa of the esophagus proper (epithelial level), represented by the stratified squamous non-keratinized epithelium, which consists of three different layers: the surface layer of squamous epithelium cells, cells of the spiny layer, and the layer of basal cells [22]. The basal layer is usually represented by 1–3 layers of cells and comprises immature cells with relatively large nuclei and a relatively small amount of cytoplasm. These cells are the source of renewal for the epithelial layer, and the only cells in the esophageal epithelium that are capable of dividing with the subsequent migration of daughter cells towards the upper layers of the epithelium. During the process of such migration, the nuclei decrease in size, and the cell eventually enters the layer of superficially located mature cells [23]. In addition to the compact arrangement of cells, the presence of a layer of intercellular glycocalyx also provides additional protection against the penetration of aggressive refluxate components [24]. However, the most important mechanism for the formation of the integrity of the epithelial layer involves a special molecular complex that ensures the formation of cell contacts in the superficial and spinous layer. This structure, which forms intercellular contacts and regulates the diameter of the intercellular space, is known as the apical junction complex and includes three main components: tight-junction proteins, intercellular adhesion proteins, and desmosomes [25]. Tight-junction proteins play an important role in the formation of intercellular tight-junction complexes and are represented by occludins (OCLN), zonulins, adhesion junction molecules (JAM-A, JAM-B, JAM-C), and claudins, mainly claudin-nom-1 (CLDN1), claudin-2 (CLDN2), and claudin-4 (CLDN4). Claudins and occludins are transmembrane proteins and bind to cytoskeletal proteins (actin) through intracellular proteins, including zonulins ZO-1, ZO-2, and cingulin. Tight junctions make it possible to form a physical barrier that regulates the penetration of electrolytes and water between cells and prevents the penetration of bacteria and toxins through the epithelium [26,27,28][26][27][28]. The most significant tight-junction proteins in the mucosa of the esophagus are claudin-1 and claudin-4 [29]. Intercellular adhesion proteins provide the structural integrity of the epithelial lining by binding epithelial cells to each other. Desmosomes in the stratified squamous epithelium not only isolate cells, but also carry out protein and ion transport through intercellular spaces. Desmosomes are represented by desmosomal cadherins with intercellular and extracellular domains that regulate the rate of ion exchange, proliferation, and polarization of epithelial cells [26,27,30][26][27][30]. The post-epithelial level of protection is provided by the blood supply to the mucosa and the mechanisms for maintaining the acid–base state of the tissue [16]. Thus, ionic H+-transporters are basolaterally located in the cell membranes of the esophageal epithelium and are able to remove excess H+ ions, increasing the cellular pH to normal values [15,31][15][31]. The mucosal bloodstream, in addition to providing nutrients and oxygen, delivers bicarbonates to the tissue and removes metabolic by-products, including hydrogen ions, lactic acid, and CO2. A number of studies have shown a compensatory increase in the blood supply to the esophageal mucosa when it is exposed to hydrochloric acid [22].3. Methods for Diagnosing Reflux Esophagitis and Assessing the Resistance of the Esophageal Mucosa
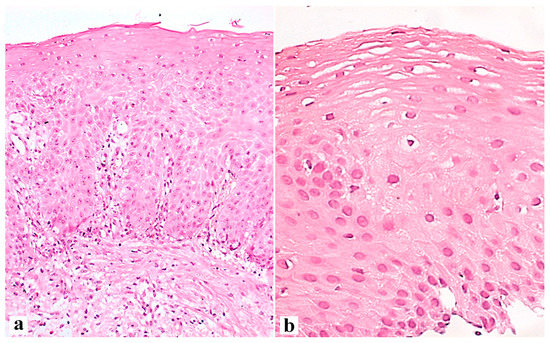
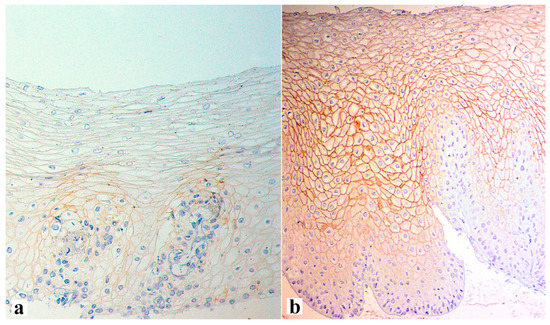
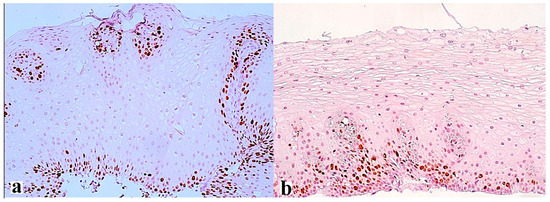
The interaction between the aggressive properties of refluxate and the protective properties of the mucosa in the esophagus under conditions of pathological gastroesophageal reflux causes the formation of reflux esophagitis, which can be assessed both at the macroscopic level via endoscopy and at the microscopic level by examining biopsies of the esophageal mucosa. During esophagogastroscopy, the severity of reflux esophagitis is assessed in accordance with the Los Angeles classification [3]. Identification of reflux esophagitis grade S-D is one of the most convincing diagnostic signs of GERD [32]. In addition to routine esophagogastroscopy, from the standpoint of assessing the stability of the esophageal mucosa under conditions of pathological gastroesophageal reflux, the novel method of endoscopic basic impedancemetry (BI) can be used. This method involves determining the basic impedance of the mucosa of the esophagus at rest in the absence of swallowing or reflux phenomena. Farré et al., based on in vivo and in vitro studies of acid perfusion in animals and humans, suggested that BI correlates with transepithelial resistance, a known marker of esophageal mucosal integrity [33]. It was found that patients with GERD (both erosive and non-erosive) had lower BI values compared with functional heartburn, which can serve as a reliable biomarker for GERD. The relationship between BI and common markers of GERD was further studied, and an association between reflux with long-duration acid exposure and DIS with low BI was found [34]. Subsequently, Savarino E. et al. confirmed the correlation between BI and histopathological markers of GERD, noting a lower BI in patients with erosive and non-erosive GERD compared with patients with functional heartburn [35]. Taking a biopsy and histological examination of biopsies of the mucous membrane of the esophagus is currently not a mandatory method for examining a patient suffering from GERD. Nevertheless, when performing an endoscopy, a biopsy should be taken in patients with a discrepancy between clinical and endoscopic data, with refractory GERD (lack of clinical and endoscopic remission within 1–2 months of treatment with a standard dose of PPI), an atypical course of erosive esophagitis, suspicion of the formation of Barrett’s esophagus, or the presence of neoplasms. High-resolution endoscopy (HD), narrow band endoscopy (NBI), and magnifying endoscopy can detect metaplastic and dysplastic areas of the esophageal epithelium and be used for targeted biopsy [36,37,38][36][37][38]. On the one hand, damage resulting from the contact of the mucous membrane with acid stimulates an increase in the proliferative potential and a significant thickening of the cell layer of the basal layer of the epithelium, i.e., basal cell hyperplasia. On the other hand, exposure to acid and bile salts stimulates the secretion of pro-inflammatory cytokines via epithelial cells, in particular interleukins-1, 6, 8, and 10, and tumor necrosis factor-alpha, increasing the presence of T-cells and neutrophils in the tissue [39]. Pro-inflammatory cytokines released by epithelial cells not only increase damage to the latter, but also activate mesenchymal and endothelial cells, stimulating the production of even more inflammatory mediators, with the involvement of immune cells and the formation of a vicious circle [10,22,40][10][22][40]. Reactive oxygen species released by immune cells react with the surrounding proteins and fatty acids of cell membranes, causing lipid peroxidation with the development of oxidative stress. Damage to the apical junction complexes and a decrease in the expression of tight-junction proteins, specifically claudin-1, claudin-2, claudin-4, zonulin, occludin, and cell adhesion proteins, together with the expansion of intercellular spaces in patients with GERD, along with additional factors that reduce epithelial protection, allow aggressive refluxate molecules to penetrate into the deeper layers of the mucosa [22,41][22][41]. A combination of basal cell hyperplasia, increased length of the papillae, intraepithelial inflammation, intercellular oedema with dilated intercellular spaces (spongiosis), balloon cells, and vascular changes in the squamous mucosa comprises the classical set of signs for a reflux pattern of injury. Because the histologic features are not specific, a number of additional features must be assessed before a definitive diagnosis of reflux esophagitis can be made. The three major features of reflux esophagitis are basal cell hyperplasia, inflammatory cells in the squamous epithelium, and elongation of the lamina propria papillae. Inflammatory infiltrate in lamina propria may comprise neutrophils, eosinophils, and lymphocytes. Together with dilated intercellular spaces and erosion, these signs can now be used as semi-quantitative diagnostic criteria for reflux esophagitis. Many individuals without reflux may show mild epithelial hyperplasia, elongation of the papillae, and occasional eosinophils 2–3 cm proximal to the lower esophageal sphincter as a result of physiologic reflux [42]. An illustration of histological signs of reflux esophagitis and a decrease in the expression of the claudin-1 protein in a patient with GERD is shown in Figure 1, Figure 2 and Figure 3.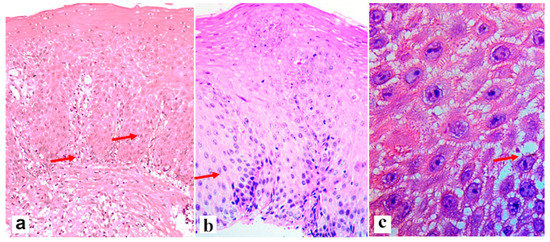
References
- Yadlapati, R.; Gyawali, C.P.; Pandolfino, J.E.; CGIT GERD Consensus Conference Participants. AGA Clinical Practice Update on the Personalized Approach to the Evaluation and Management of GERD: Expert Review. Clin. Gastroenterol. Hepatol. 2022, 20, 984–994.e1.
- Delshad, S.D.; Almario, C.V.; Chey, W.D.; Spiegel, B.M.R. Prevalence of Gastroesophageal Reflux Disease and Proton Pump Inhibitor-Refractory Symptoms. Gastroenterology 2020, 158, 1250–1261.e2.
- Vakil, N.; van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R.; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am. J. Gastroenterol. 2006, 101, 1900–1943.
- Fuchs, K.H.; DeMeester, T.R.; Otte, F.; Broderick, R.C.; Breithaupt, W.; Varga, G.; Musial, F. Severity of GERD and disease progression. Dis. Esophagus 2021, 34, doab006.
- Prichard, D.O.; Byrne, A.M.; Murphy, J.O.; Reynolds, J.V.; O’Sullivan, J.; Feighery, R.; Doyle, B.; Eldin, O.S.; Finn, S.P.; Maguire, A.; et al. Deoxycholic acid promotes development of gastroesophageal reflux disease and Barrett’s oesophagus by modulating integrin-αv trafficking. J. Cell. Mol. Med. 2017, 21, 3612–3625.
- Bilski, J.; Pinkas, M.; Wojcik-Grzybek, D.; Magierowski, M.; Korbut, E.; Mazur-Bialy, A.; Krzysiek-Maczka, G.; Kwiecien, S.; Magierowska, K.; Brzozowski, T. Role of Obesity, Physical Exercise, Adipose Tissue-Skeletal Muscle Crosstalk and Molecular Advances in Barrett’s Esophagus and Esophageal Adenocarcinoma. Int. J. Mol. Sci. 2022, 23, 3942.
- Sun, D.; Wang, X.; Gai, Z.; Song, X.; Jia, X.; Tian, H. Bile acids but not acidic acids induce Barrett’s esophagus. Int. J. Clin. Exp. Pathol. 2015, 8, 1384–1392.
- Zheng, Z.; Shang, Y.; Wang, N.; Liu, X.; Xin, C.; Yan, X.; Zhai, Y.; Yin, J.; Zhang, J.; Zhang, Z. Current Advancement on the Dynamic Mechanism of Gastroesophageal Reflux Disease. Int. J. Biol. Sci. 2021, 17, 4154–4164.
- Zhou, J.; Shrestha, P.; Qiu, Z.; Harman, D.G.; Teoh, W.-C.; Al-Sohaily, S.; Liem, H.; Turner, I.; Ho, V. Distinct Microbiota Dysbiosis in Patients with Non-Erosive Reflux Disease and Esophageal Adenocarcinoma. J. Clin. Med. 2020, 9, 2162.
- Rieder, F.; Biancani, P.; Harnett, K.; Yerian, L.; Falk, G.W. Inflammatory mediators in gastroesophageal reflux disease: Impact on esophageal motility, fibrosis, and carcinogenesis. Am. J. Physiol. Gastrointest Liver Physiol. 2010, 298, G571–G581.
- Nejat Pish-Kenari, F.; Qujeq, D.; Maghsoudi, H. Some of the effective factors in the pathogenesis of gastro-oesophageal reflux disease. J. Cell. Mol. Med. 2018, 22, 6401–6404.
- Blevins, C.H.; Dierkhising, R.A.; Geno, D.M.; Johnson, M.L.; Vela, M.F.; Ravi, K.; Iyer, P.G.; Katzka, D.A. Obesity and GERD impair esophageal epithelial permeability through 2 distinct mechanisms. Neurogastroenterol. Motil. 2018, 30, e13403.
- Badgery, H.; Chong, L.; Iich, E.; Huang, Q.; Georgy, S.R.; Wang, D.H.; Read, M. Recent insights into the biology of Barrett’s esophagus. Ann. N. Y. Acad. Sci. 2020, 1481, 198–209.
- GadEl-Hak, N.A.; El-Hemaly, M.; Hamdy, E.; AbdEl-Raouf, A.; Mostafa, M.; Haleem, M. Bile reflux measurement and its contribution to the severity of reflux esophagitis. Saudi J. Gastroenterol. 2007, 13, 180–186.
- Günther, C.; Neumann, H.; Vieth, M. Esophageal epithelial resistance. Dig. Dis. 2014, 32, 6–10.
- Storonova, O.A.; Trukhmanov, A.S.; Ivashkin, V.T. Esophageal mucosa protective factors at the treatment of gastroesophageal reflux disease. Clin. Persp. Gastroenterol. Hepatol. 2014, 5, 37–42.
- Niv, Y.; Ho, S.B.; Fass, R.; Rokkas, T. Mucin Expression in the Esophageal Malignant and Pre-malignant States: A Systematic Review and Meta-analysis. J. Clin. Gastroenterol. 2018, 52, 91–96.
- Di Pilato, V.; Freschi, G.; Ringressi, M.N.; Pallecchi, L.; Rossolini, G.M.; Bechi, P. The esophageal microbiota in health and disease. Ann. N. Y. Acad. Sci. 2016, 1381, 21–33.
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017.
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143.
- Corning, B.; Copland, A.P.; Frye, J.W. The Esophageal Microbiome in Health and Disease. Curr. Gastroenterol. Rep. 2018, 20, 39.
- Orlando, R.C. Review article: Oesophageal mucosal resistance. Aliment. Pharmacol. Ther. 1998, 12, 191–197.
- Blevins, C.H.; Iyer, P.G.; Vela, M.F.; Katzka, D.A. The Esophageal Epithelial Barrier in Health and Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 608–617.
- Orlando, R.C. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 873–882.
- Rusu, A.D.; Georgiou, M. The multifarious regulation of the apical junctional complex. Open Biol. 2020, 10, 190278.
- Simanenkov, V.I.; Maev, I.V.; Tkacheva, O.N.; Alekseenko, S.A.; Andreev, D.N.; Bordin, D.S.; Vlasov, T.D.; Vorobyeva, N.M.; Grinevich, V.; Gubonina, I.V.; et al. Syndrome of increased epithelial permeability in clinical practice. Multidisciplinary national Consensus. Cardiovasc. Ther. Prev. 2021, 20, 2758.
- Zhao, X.; Zeng, H.; Lei, L.; Tong, X.; Yang, L.; Yang, Y.; Li, S.; Zhou, Y.; Luo, L.; Huang, J.; et al. Tight junctions and their regulation by non-coding RNAs. Int. J. Biol. Sci. 2021, 17, 712–727.
- Krug, S.M.; Fromm, M. Special Issue on “The Tight Junction and Its Proteins: More than Just a Barrier”. Int. J. Mol. Sci. 2020, 21, 4612.
- Oshima, T.; Miwa, H. Gastrointestinal mucosal barrier function and diseases. J. Gastroenterol. 2016, 51, 768–778.
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181.
- Becskeházi, E.; Korsós, M.M.; Erőss, B.; Hegyi, P.; Venglovecz, V. OEsophageal Ion Transport Mechanisms and Significance Under Pathological Conditions. Front. Physiol. 2020, 11, 855.
- Gyawali, C.P.; Kahrilas, P.J.; Savarino, E.; Zerbib, F.; Mion, F.; Smout, A.J.P.M.; Vaezi, M.; Sifrim, D.; Fox, M.R.; Vela, M.F.; et al. Modern diagnosis of GERD: The Lyon Consensus. Gut 2018, 67, 1351–1362.
- Farre, R.; Blondeau, K.; Clement, D.; Vicario, M.; Cardozo, L.; Vieth, M.; Mertens, V.; Pauwels, A.; Silny, J.; Jimenez, M.; et al. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut 2011, 60, 885–892.
- Zhong, C.; Duan, L.; Wang, K.; Xu, Z.; Ge, Y.; Yang, C.; Han, Y. Esophageal intraluminal baseline impedance is associated with severity of acid reflux and epithelial structural abnormalities in patients with gastroesophageal reflux disease. J. Gastroenterol. 2013, 48, 601–610.
- Savarino, E.; de Bortoli, N.; Zentilin, P.; Furnari, M.; Marchi, S.; Mastracci, L.; Fiocca, R.; Savarino, V. Esophageal baseline impedance values correlate with presence and severity of microscopic esophagitis in patients with gastro-esophageal reflux disease. Gastroenterology 2014, 146, S-4.
- Hoffman, A.; Basting, N.; Goetz, M.; Tresch, A.; Mudter, J.; Biesterfeld, S.; Galle, P.; Neurath, M.; Kiesslich, R. High-definition endoscopy with i-Scan and Lugol’s solution for more precise detection of mucosal breaks in patients with reflux symptoms. Endoscopy 2009, 41, 107–112.
- Sharma, P.; Wani, S.; Bansal, A.; Hall, S.; Puli, S.; Mathur, S.; Rastogi, A. A feasibility trial of narrow band imaging endoscopy in patients with gastroesophageal reflux disease. Gastroenterology 2007, 133, 454–674.
- Swager, A.; Curvers, W.L.; Bergman, J.J. Diagnosis by endoscopy and advanced imaging. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 97–111.
- Kaymak, T.; Hruz, P.; Niess, J.H. Immune system and microbiome in the esophagus: Implications for understanding inflammatory diseases. FEBS J. 2022, 289, 4758–4772.
- Morozov, S.; Sentsova, T. Local inflammatory response to gastroesophageal reflux: Association of gene expression of inflammatory cytokines with esophageal multichannel intraluminal impedance-pH data. World J. Clin. Cases 2022, 10, 9254–9263.
- Tobey, N.A.; Hosseini, S.S.; Argote, C.M.; Dobrucali, A.M.; Awayda, M.S.; Orlando, R.C. Dilated intercellular spaces and shunt permeability in nonerosive acid-damaged esophageal epithelium. Am. J. Gastroenterol. 2004, 99, 13–22.
- Triantos, C.; Koukias, N.; Karamanolis, G.; Thomopoulos, K. Changes in the esophageal mucosa of patients with non erosive reflux disease: How far have we gone? World J. Gastroenterol. 2015, 21, 5762–5767.
- Han, D.; Zhang, C. The Oxidative Damage and Inflammation Mechanisms in GERD-Induced Barrett’s Esophagus. Front. Cell Dev. Biol. 2022, 10, 885537.
- Chan, M.W.; Pouw, R.E. Risk-stratification models for Barrett’s esophagus: Will we get to the perfect classifier? Gastrointest. Endosc. 2022, 95, 1123–1125.
- Reid, B.J.; Li, X.; Galipeau, P.C.; Vaughan, T.L. Barrett’s oesophagus and oesophageal adenocarcinoma: Time for a new synthesis. Nat. Rev. Cancer 2010, 10, 87–101.
- Binato, M.; Fagundes, R.; Gurski, R.; Meurer, L.; Edelweiss, M.I. Immunohistochemical overexpression of the p53 protein and Ki-67 (MIB-1) antigen in patients with GERD and chronic esophagitis. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 236–243.
- Binato, M.; Gurski, R.R.; Fagundes, R.B.; Meurer, L.; Edelweiss, M.I. P53 and Ki-67 overexpression in gastroesophageal reflux disease—Barrett’s esophagus and adenocarcinoma sequence. Dis. Esophagus. 2009, 22, 588–595.
- Fujii, T.; Nakagawa, S.; Hanzawa, M.; Sueyoshi, S.; Fujita, H.; Shirouzu, K.; Yamana, H. Immunohistological study of cell cycle-related factors, oncogene expression, and cell proliferation in adenocarcinoma developed in Barrett’s esophagus. Oncol. Rep. 2003, 10, 427–431.
- Polkowski, W.; van Lanschot, J.J.; Ten Kate, F.J.; Baak, J.P.; Tytgat, G.N.; Obertop, H.; Voorn, W.; Offerhaus, G. The value of p53 and Ki67 as markers for tumour progression in the Barrett’s dysplasia-carcinoma sequence. Surg. Oncol. 1995, 4, 163–171.
- Clemons, N.J.; Phillips, W.A.; Lord, R.V. Signaling pathways in the molecular pathogenesis of adenocarcinomas of the esophagus and gastroesophageal junction. Cancer Biol. Ther. 2013, 14, 782–795.
- Kaz, A.M.; Grady, W.M.; Stachler, M.D.; Bass, A.J. Genetic and Epigenetic Alterations in Barrett’s Esophagus and Esophageal Adenocarcinoma. Gastroenterol. Clin. N. Am. 2015, 44, 473–489.
- Feber, A.; Xi, L.; Luketich, J.D.; Pennathur, A.; Landreneau, R.J.; Wu, M.; Swanson, S.J.; Godfrey, T.E.; Litle, V.R. MicroRNA expression profiles of esophageal cancer. J. Thorac. Cardiovasc. Surg. 2008, 2, 255–260.
- Grady, W.M.; Yu, M. Molecular Evolution of Metaplasia to Adenocarcinoma in the Esophagus. Dig. Dis. Sci. 2018, 63, 2059–2069.
- Duits, L.C.; Lao-Sirieix, P.; Wolf, W.A.; O’Donovan, M.; Galeano-Dalmau, N.; Meijer, S.L.; Offerhaus, G.J.A.; Redman, J.; Crawte, J.; Zeki, S.; et al. A biomarker panel predicts progression of Barrett’s esophagus to esophageal adenocarcinoma. Dis. Esophagus 2019, 32, doy102.
- Murata, A.; Baba, Y.; Watanabe, M.; Shigaki, H.; Miyake, K.; Karashima, R.; Imamura, Y.; Ida, S.; Ishimoto, T.; Iwagami, S.; et al. p53 immunohistochemical expression and patient prognosis in esophageal squamous cell carcinoma. Med. Oncol. 2013, 30, 728.
- Madani, K.; Zhao, R.; Lim, H.J.; Casson, A.G. Prognostic value of p53 mutations in oesophageal adenocarcinoma: Final results of a 15-year prospective study. Eur. J. Cardiothorac. Surg. 2010, 37, 1427–1432.
- Wang, L.; Yu, X.; Li, J.; Zhang, Z.; Hou, J.; Li, F. Prognostic significance of p53 expression in patients with esophageal cancer: A meta-analysis. BMC Cancer 2016, 16, 373.
