Activated partial thromboplastin time (aPTT) is a fundamental screening test for coagulation disturbances. An increased aPTT ratio is quite common in clinical practice. How the detection of prolonged activated aPTT with a normal prothrombin time is interpreted is therefore very important. In daily practice, the detection of this abnormality often leads to delayed surgery and emotional stress for patients and their families and may be associated with increased costs due to re-testing and coagulation factor assessment. An isolated, prolonged aPTT is seen in (a) patients with congenital or acquired deficiencies of specific coagulation factors, (b) patients receiving treatment with anticoagulants, mainly heparin, and (c) individuals/patients with circulating anticoagulants.
- activated partial thromboplastin time (aPTT)
- coagulation factor defect
- mixing test
1. Introduction
2. Activated Partial Thromboplastin Time (aPTT): Rationale, Procedure, and Aims
2.1. What Is the Rationale of aPTT?
The activated partial thromboplastin time is sensitive to deficiencies in the activities of factors of the so called “intrinsic and common pathways”: factors II, V, VIII, IX, X, XI, XII, fibrinogen, high-molecular-weight kininogen (HMWK), and prekallikrein (PK) (Figure 1, [1][6]).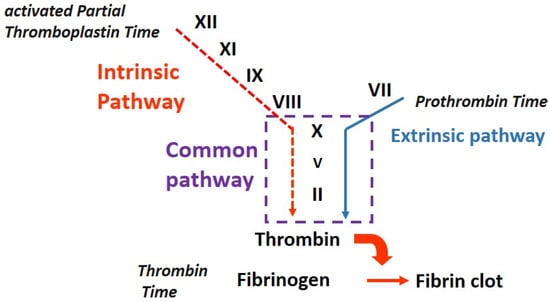
-
Congenital, for example, Factor VIII (hemophilia A) or Factor IX (hemophilia B);
-
Acquired due to a neutralizing antibody (acquired hemophilia) or the effect of an anticoagulant therapy (unfractionated or low-molecular-weight heparin, LMWH, or direct oral anticoagulants, DOACs).
2.2. How Is aPTT Performed?
-
An activator, a substance able to sustain an activation reaction activation of the zymogens belonging to the so called “contact pathway”; these are mostly factor XII but may also be HMWK and PK.
-
Phospholipids, including phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), and sphyngomyelin (SM), are incorporated into the reagent used for testing to reproduce in vitro the role of platelet in vivo.
- o
-
PS serves as a surface for the assembly and activation of coagulation factors, specifically those involved in the intrinsic pathway. It provides a platform for the formation of the intrinsic tenase complex and supports the activation of Factor X, which is crucial for clot formation.
- o
-
PC contributes to the overall stability and structure of the lipid vesicles used in the aPTT reagent. It helps maintain the integrity of the phospholipid membrane and aids in the presentation of other coagulation factors during the assay.
- o
-
PE is involved in the formation of the phospholipid membrane used in the aPTT assay. It contributes to the overall structure and fluidity of the membrane, which are important for the proper assembly and activation of coagulation factors.
- o
-
SM, like PC, is a key component of the lipid vesicles used in the aPTT reagent. It contributes to the structure and stability of the phospholipid membrane, facilitating the presentation of other necessary coagulation factors during the assay.
-
Calcium chloride is used to reintroduce in the reaction calcium ions previously depleted by the anticoagulant (3.2% trisodium citrate) present in the blood.
-
Citrated plasma is also used. The recommended anticoagulant for blood collection for coagulation analyses is trisodium citrate in 1 + 9 ratio with blood.
-
The addition of an activator and phospholipids to citrated plasma, determining the generation of Factors XIIa and XIa.
-
After incubation at 37 °C, the plasma is recalcified by adding calcium chloride; beginning from this moment, the activated partial thromboplastin time is recorded as the time in seconds needed to generate the fibrin clot.
2.3. Why Is aPTT Performed?
3. Preanalytical Cause of Prolongation of aPTT and Other Coagulation Tests
3.1. Hemolysis, Hyperbilirubinemia, and Hypertriglyceridemia
Hemolysis interferes with both optical and mechanical measurement methods via optical interference due to the presence of cell-free hemoglobin absorbance and via biologic interference due to the release of molecules able to activate platelet and coagulation factors [5][9]. Hyperbilirubinemia and hypertriglyceridemia both interfere with optical methods. To overcome this interference, modern optical analyzers have the capability to increase the reading wavelength over 650 nm. Regardless, extreme hyperbilirubinemia and hypertriglyceridemia may make performing the coagulation test with optical analyzers unfeasible [5][9].3.2. Blood/Anticoagulant Ratio and Hemoconcentration
As in the performance of other laboratory coagulation tests, for aPTT, an adequate amount of blood should be placed in the test tube; the optimal ratio of blood to anticoagulant should be 9:1. The importance of this ratio is confirmed by the fact that hemoconcentration (a hematocrit higher than 55%, which could be present in patients with heart disease and in newborns, for example) prolongs aPTT as the plasma volume per blood volume is decreased, leading to an excess of anticoagulant in the sample if no correction is applied (the necessary volume of the anticoagulant is consequently decreased) [6][8].4. Isolated, Prolonged aPTT: Prevalence and Causes
4.1. Isolated, Prolonged aPTT: A Truly Unexpected Finding?
In an Italian retrospective study [7][26], 5.8% of 8.069 patients undergoing elective surgery had an abnormal aPTT; 2.9% (240 patients) had an aPTT ratio higher than 1.3 and for this reason, they were worthy of further investigation. An old review by Munro et al. [8][27] which considered 29 papers regarding the value of routine pre-operative coagulation testing showed an incidence of aPTT abnormalities of 15.6%. A recent large Danish study [9][24] showed a prolonged aPTT in 12% of 18.642 aPTT measurements performed on 10.697 patients (excluding those affected by known coagulation disorders); 79% of these abnormal aPTTs were reported to be moderately or severely prolonged (40–45 or >45 s, respectively).4.2. Isolated, Prolonged aPTT: What Are the Causes?
4.2.1. Heparin Contamination
Heparin contamination can be a source of error and is often observed when blood is collected from a central venous line. The suspicion of this preanalytical problem should be an indication to repeat sampling from a peripheral vein [9][24]. However, it should be taken into account that the heparin contamination of samples destinated for use in coagulation tests could also happen if the blood is collected from a direct venipuncture that is performed after the venous lines are flushed with an amount of heparin solution containing enough heparin enough to induce an anticoagulant effect in the patient.4.2.2. C-reactive Protein
C-reactive protein (CRP) interference with the measurement of aPTT is most likely phospholipid-dependent and depends on both the CRP concentration and aPTT assay type [10][11][12,13].4.2.3. Lupus Anticoagulants
Lupus anticoagulants, a heterogeneous group of immunoglobulins that can bind β2-glycoprotein I, prothrombin, or other proteins in a complex with negatively charged phospholipids, are able to prolong phospholipid-dependent coagulation tests, including aPTT tests [12][18]. The presence of lupus anticoagulants can increase the risk of (venous or arterial) thrombosis.4.2.4. Drug Interferences
As mentioned previously, aPTT is used to monitor therapies using unfractionated heparin (UFH) and argatroban [13][14][28,29]: these drugs prolong aPTT. Low-molecular-weight heparins (LMWHs) are often reported not to affect aPTT, but some commercial brands can have this effect [15][11]. aPTT is also sensitive to direct oral anticoagulants (DOAC), which can cause an isolated prolongation of this clotting time, even if they often also prolong PT/INR, and aPTT is not recommended for monitoring DOAC therapy [16][25]. Anticoagulant therapy with vitamin K antagonists (VKAs) can also affect aPTT, but this type of therapy mainly prolongs PT, limiting the activation of factor VII.4.2.5. Acquired Hemophilia A (AHA) and Acquired Von Willebrand Disease (AVWD)
These acquired deficiencies of clotting factors due to the presence of autoantibodies are very rare diseases (incidences of ~1–2 people per million per year) and are often associated with several pathological conditions, such as autoimmune disorders, malignancies, cardiovascular diseases, pregnancy, and drug administration. AHA and AVWD can lead to severe bleeding events which are sometimes life-threatening and can require immediate intervention with anti-hemorrhagic therapy (a factor concentrate or a bypassing agent) and underlying disorder treatment (e.g., immunosuppression) [17].4.2.6. Hemophilia A and B (HA, HB)
Hemophilia A and B are rare (incidences of ~1 in 5.000 and 1 in 30.000 male births, respectively) X-linked inherited clotting diseases caused by the deficiency of factors VIII and IX; affected patients present a prolonged aPTT and a clinical phenotype with several bleeding symptoms, whether spontaneous (in mild and moderate forms) or provoked by traumas or surgery [18][14].4.2.7. Von Willebrand Disease (VWD)
Von Willebrand Disease is the most common inherited bleeding disorder (affecting up to 1% of the general population) and is characterized by a reduced or abolished synthesis or an altered function of Von Willebrand factor, which plays a role in both primary and secondary hemostasis.4.2.8. Factor XI Deficiency
Factor XI deficiency has a prevalence of 1 in 1 million persons in most populations and is more prevalent in Ashkenazi Jews and French Basques. Its clinical picture is very heterogeneous, without a correlation between factor level and bleeding symptoms. Patients with a severe disorder are at a higher risk of bleeding, but some of them may remain asymptomatic, and patients with a partial deficiency may bleed after trauma or surgery.4.2.9. Contact Pathway Factor Deficiency
Deficiencies in high-molecular-weight kininogen, prekallikrein, or factor XII cause a (sometimes remarkable) prolongation of aPTT but cannot provoke bleedings; these disorders are frequently incidentally diagnosed from screening before surgery and are important for differential diagnosis with the other causes of isolated prolongation of aPTT [19][15].5. Isolated, Prolonged aPTT: Differential Diagnosis
How can We Identify the Cause of Isolated, Prolonged aPTT?
First step—possible pre-analytical interferences? First, evaluate the appropriateness of the sample; exclude that the sample is jaundiced, hemolysis, or hyperlipemic. Verify the correct blood/anticoagulant ratio, namely, the adequate sample filling and hematocrit [5][6][20][8,9,10]. Second step—presence of anticoagulants? Then, the presence of interfering drugs (heparin and DOAC) should be excluded; performing thrombin time and anti-Xa assays will provide information about the type of drug:-
Heparin will be detected by both tests;
-
Direct anti-IIa inhibitor will be detected only via thrombin time;
-
Direct anti-Xa inhibitors will be detected only by an anti-Xa assay.
-
For an immediate mixing test, the plasma is prepared using equal volumes (1:1) of NPP and patient plasma, and aPTT is performed on it at room temperature [4][7]. In parallel, as a control, aPTT is performed on the NPP. Chang et al. [21][34] suggested that the aPTT correction in a mixing test with a 4:1 ratio of NPP and patient plasma can achieve better sensitivity and specificity, mostly in the situations in which the antibody power is relatively weak.
-
The normality range method, in which the aPTT value measured from the mixture must fall within the normal range (a range determined by the laboratory that is effective for the specific combination of the reagent and coagulation analyzer used for the measurement). The advantages of this method are its easiness and immediacy; it has an important diagnostic power in the situations in which the patient plasma aPTT is markedly prolonged.
-
The index of circulating anticoagulant (ICA) method, ICA (or Rosner index), is defined by the following formula:
