Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Kyra Kaiser and Version 2 by Camila Xu.
Silver has an extensive history because it has been used for multiple millennia spanning from the Before Common Era (B.C.E) to the present day. This long-term use of silver stemmed from its anti-deteriorative activity and led to its recognition as the most important antimicrobial agent (i.e., antibacterial, antiviral, antiparasitic, and antifungal) that predated antibiotics.
- nanosilver
- antimicrobial applications
- physicochemical properties
1. Brief History of Silver (Ag) and Its Old Antimicrobial Applications
Silver has an extensive history because it has been used for multiple millennia spanning from the Before Common Era (B.C.E) to the present day (Table 1) [1][2][3][1,2,3]. This long-term use of silver stemmed from its anti-deteriorative activity and led to its recognition as the most important antimicrobial agent (i.e., antibacterial, antiviral, antiparasitic, and antifungal) that predated antibiotics [4][5][6][7][8][9][4,5,6,7,8,9].
Table 1. Overview of the knowledge and applications of silver (Ag) throughout major historical periods. Both household and medical applications are listed, as household use of silver contributed to the foundation of using silver as a therapeutic agent.
| Silver B.C.E. [1][4][7][1,4,7] |
Silver Pre-Industrialization [1][7][1,7] |
Silver during and Post Industrialization [8] |
|
|---|---|---|---|
| Knowledge |
|
|
|
| Applications |
|
|
|
Before Common Era (B.C.E.): The usage of silver for antibacterial purposes in B.C.E. civilizations was primarily through the preservation of food items in silver containers or the addition of a silver coin to beverages for long-term storage [1][9][1,9]. A fundamental discovery was the correlation between containers made of silver and food items remaining safe for consumption. Rulers of various nations (Alexander the Great and Cyrus the Great) only consumed water that was kept in silver vessels [1][6][10][11][1,6,10,11]. Even though bacteria were not known at that time, this connection between the slower decomposition of food with silver containers and cutlery contributed to the medical advancements seen today [8]. Due to the difficulty of interpretation of ancient texts, there are varying claims of the first recorded attempt of using silver as a therapeutic remedy. One of the oldest examples is a reference to silver as a therapeutic agent in 1500 B.C.E, during the Han dynasty in China [12]. Other recorded instances of medical procedures using silver include the 69 B.C.E. Roman Pharmacopeia describing a silver nitrate (AgNO3)-based medicine, the practice of Hippocrates using silver leaf for wound care, and an ancient medical system (Ayurveda) from India listing silver as a therapy component for multiple diseases [1][4][8][13][1,4,8,13].
Pre-industrialization: From B.C.E until the first Industrial Revolution in 1760, silver was used as a novel medical therapy for a broad spectrum of ailments (e.g., ulcers, wound infections, impure blood, heart palpitations, poor breath, epilepsy, and irritation) [1][14][1,14]. For example, Pliny the Elder, a Roman physician, described silver within his 79 C.E. (Common Era) book, Natural History (Book XXXIII), as an effective healing agent within plasters and for wound closing [11][15][11,15]. Ambroise Paré, a French surgeon considered among the fathers of surgery, who served for multiple kings (Henry II, Francis II, Charles IX, and Henry III), used silver and other materials to construct ocular prosthetics [4][16][4,16]. Wealthier individuals in the Middle Ages, who regularly used silver utensils, overexposed themselves to silver and developed argyria (Figure 1), a rare skin condition that changes the color of skin, eyes, nails, and internal organs to a permanent blue-grey [1][17][18][1,17,18].
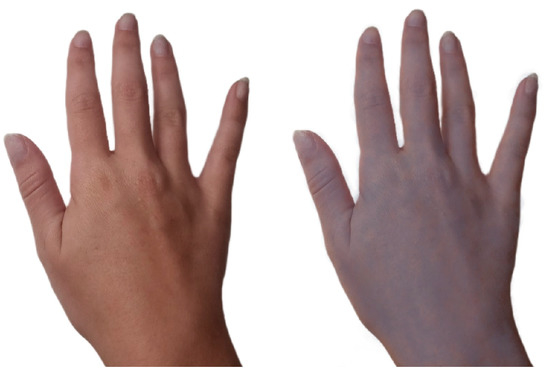
Figure 1. Comparison of an argyria-like skin color characteristic to topical cyanosis (right), versus a simulated standard hand coloring in a healthy patient (left).
During industrialization: Key events such as the discovery of bacteria by Anton Leeuwenhoek in 1676 and the technological advancements associated with the Industrial Revolution in 1760 led to a transformation of medicine [2][19][2,19]. As antibiotics did not yet exist in the medical field, physicians used other agents (e.g., silver, mercury, copper, arsenic, and sulfur compounds) that were later deemed as beneficial, harmful, or entirely ineffective as therapeutic remedies [20]. Public attitudes toward health care were also drastically changed with the first public hospital, Bellevue Hospital, being officially established in New York City, in 1736 [21]. The concept of vaccination had its roots in 1796 through the work of physician Edward Jenner, who made the connection between patients who previously contracted cowpox and their immunity to smallpox [22]. He inoculated an 8-year-old boy with material from the cowpox lesions and concluded that the boy was protected from the illness [22]. This was the origin of transmittable protection, as in vaccination [23]. Vaccines were the most advanced medical agent, up until the 19th century, when the first antibiotic was discovered [24].
Post industrialization: In the 19th century, the physician Robert Koch made the claim that a certain bacterium can cause a specific disease. This led to Koch’s four postulates and the Germ Theory as it is seen today [25][26][25,26]. Following this, the physician Paul Ehrlich synthesized the first antimicrobial compound, salvarsan, in 1910 [24][27][28][24,27,28]. The physician scientist Alexander Fleming discovered the first true antibiotic to treat bacterial infections, penicillin, in 1928 [24][27][28][24,27,28]. Penicillin became available to the public later, in 1945 [29]. In this time, colloidal silver was being employed in hospital settings as an antibacterial agent, and silver salts were being administered to treat various infections and ailments (e.g., conjunctivitis, gonorrhea, gastroenteritis, syphilis, nicotine dependence, and mental illness) [8]. The German physician, Carl Siegmund Franz Credé, formulated in 1881 a 2% AgNO3 solution for neonatal conjunctivitis, which was so effective that it almost ended visual loss from the disease [8][30][8,30]. Other AgNO3 applications in the 1800s included therapies for burns, ulcers, compound fractures, and infections [1][31][1,31]. Physician Marion Sims employed to resolve the dilemma of post-delivery vesico-vaginal fistulas (when silk sutures failed) and administered silver-coated catheters during the healing period [1][31][1,31]. Colloidal silver (i.e., Ag particles suspended within a liquid) was first employed in 1891, by the surgeon B.C. Crede, as an antiseptic measure on wounds [1][12][32][1,12,32].
Silver (Ag) forms: As the scientific understanding of silver expanded over the course of history, the forms of Ag utilized also shifted (Figure 2). Initially, Ag was utilized in macro form (bulk Ag metal), when casting and forging household items (e.g., vessels, jewelry, and coins), or in atomic form (salt solutions of Ag+ ions), when treating wounds and other ailments. This was followed by the development and administration of micro- or nano-forms of Ag in water (colloidal Ag), as antibiotics were not yet available [8][28][8,28]. The first colloid of Ag was synthesized in the laboratory in 1889, by the chemist M. C. Lea [33][34][33,34]. In this redox reaction, citrate-capped AgNPs were intentionally created with dimensions of about 1–100 nanometers (nm) that changed their properties when compared to the Ag+ or bulk Ag forms [35]. However, the term nanotechnology was coined much later in 1974 by the Japanese professor Norio Taniguchi [36]. The first micro- and nanoparticles were visualized and characterized in 1981, after the invention of the first scanning tunneling microscope (STM). Nowadays, ionic silver (Ag+) and nanosilver (e.g., colloidal AgNPs) are the most emphasized forms of antimicrobial silver, which kill or inhibit the growth of microorganisms including pathogenic bacteria, viruses, and fungi, but cause little to no damage to the host.
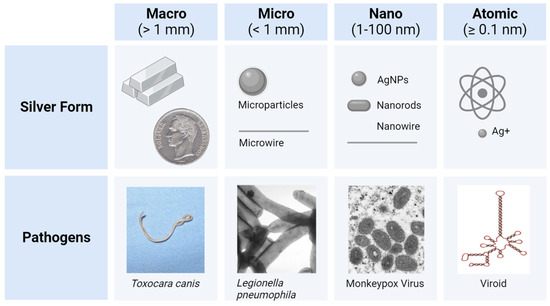
Figure 2. The forms of silver (Ag) utilized from B.C.E until present day, having sizes ranging from visible to the naked eye (1 mm and above) to approximately 0.1 nm (atomic radius and above) [35][37][38][39][40][35,37,38,39,40]. Various pathogens within each category (e.g., the Toxocara canis [usually 2–10 cm] and the pinworm Enterobius vermicularis [usually 1–3 mm] for macro pathogens >> 1 mm) depict a size comparison for the Ag forms [41][42][43][44][41,42,43,44]. Comparative scales are approximated.
2. Modern Antimicrobial Applications of Nanosilver
The antimicrobial activity of nanosilver such as colloidal silver nanoparticles (AgNPs) is linked to its unique, size-related physicochemical properties such as the very large surface-to-volume ratios and the potential release of Ag+ ions from the nanosurface under favorable redox conditions. These properties are currently exploited in the manufacturing of everyday consumer products and other antimicrobial applications (Figure 3) [4][5][4,5].
Antimicrobial consumer products: In 2023, 5367 consumer products have been identified worldwide as containing nanomaterials by the manufacturer, and over 1000 of these products exploit the unique properties of nanosilver (e.g., antimicrobial, optical, and catalytical) [45][46][45,46]. Antimicrobial consumer products containing silver (Figure 3) can be found in the health (24.08%), textile (17.53%), cosmetic (13.38%), appliance (9.31%), environmental (8.30%), and construction (7.93%) sectors [46]. In the last few decades, the U.S. Food and Drug Administration (FDA) has approved many of these products containing antimicrobial Ag+ and nanosilver such as AgNPs. Examples include wound dressings, facial masks, textile fibers, sanitizers, coatings of surgical tools, dental implants, and urinary catheters (Table 2) [45][47][45,47].
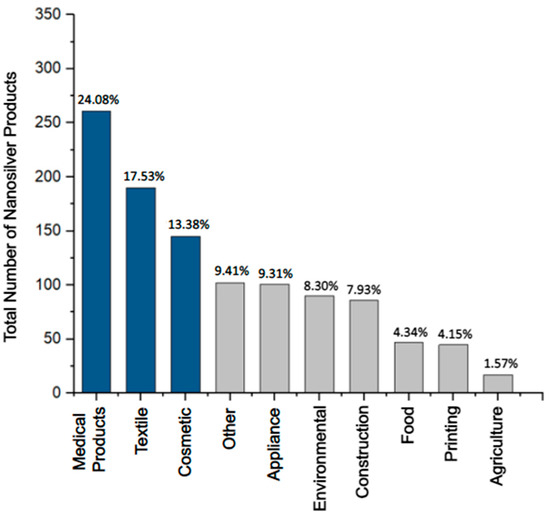
Figure 3. The most prevalent categories of consumer products containing nanosilver (U.S. FDA-approved and non-approved) make up 1084 registered products [46]. The top three sectors, the medical-, textile-, and cosmetics-related products (dark blue), are the most prominent categories, making up ~55% of the total number of consumer products containing nanosilver. The lesser seven categories (grey) make up ~45% of products.
“Silver wound dressings” represent the most web-searched (n = 2214—Table 2) and one of the most heavily used consumer products containing Ag in the medical sector. A large variety of U.S. FDA-approved (e.g., Silverlon, Aquacel Ag Advantage, and Acticoat) and non-approved wound dressings are offered through prescriptions as well as over the counter [48][49][50][48,49,50]. Silver-based wound dressings are used as both preventative and curative measures against bacterial infection of acute and chronic wounds. Textiles, the second most widespread application of nanosilver, have been used in many types of clothing (e.g., facemasks, socks, shirts, athletic wear, and towels) [45]. An illustrative example associated with nanosilver use is disinfectants in facemasks to prevent the spread of pathogens and the formation of malodor caused by bacterial colonies that inhabit the surface of the skin [51]. Manufacturers of cosmetics, the third largest sector, have employed nanosilver for the same antimicrobial benefits [52]. Nanosilver can be found in lotions, face masks, soaps, sunscreens, etc. [45].
Because the adverse effects of Ag on human health are not yet fully understood, concerns have been raised about the growing exposure to nanosilver during the manufacture or prolonged utilization of nanosilver-based consumer products [53]. Furthermore, the environmental health impacts of nanosilver remain under debate as nanosilver properties can change in the environment, leading to altered toxicity and stability [54][55][56][54,55,56]. The regulation of nanosilver-based consumer products has been compounded by the challenging task of tracking products that do not specify the nanomaterial as an ingredient, especially when in minute quantities, and by the product distribution under different brand names [57]. Nevertheless, the integration of nanosilver into consumer products continues to experience a vertiginous increase. An estimated 1000 tons of nanosilver is produced worldwide [58].
Table 2. The top applications of antimicrobial silver (Ag) together with illustrative products for each of the three major categories: health, textiles, and cosmetics. The vendor, the number of PubMed search results and selected key words, Ag form, [Ag quantities], product purpose, and U.S. FDA approval status are reported [49][59][60][49,59,60].
| Product Type | Search Result | Vendor | [Ag] and Ag Form | Purpose | U.S. FDA Approval |
|---|---|---|---|---|---|
| Silver-based wound dressings | “silver wound dressing” n = 2214 |
|
|
|
YES |
| Ankle socks with silver | “silver textile” n = 1155 |
|
|
|
NO |
| Platinum silver nanocolloid cream | “silver cosmetic” n = 2292 |
|
|
|
NO |
Other antimicrobial applications: Lately, AgNPs and Ag+ have received increased attention due to their potential use in the fight against two major global health threats, namely antibiotic resistance and viral infections, where treatments are either limited or not available [61]. For instance, non-cytotoxic concentrations of AgNPs were reported to act against a broad spectrum of viruses of different families regardless of their tropism, clade, and resistance to antiretrovirals [61][62][63][61,62,63]. Relevant examples include HIV-1, hepatitis B (HBV), Tacaribe virus, herpes simplex virus, mpox, smallpox, H1N1 influenza A, respiratory syncytial viruses, vaccinia virus, and dengue virus (DENV). In these studies, AgNPs were found to bind specifically or nonspecifically to proteins in the envelope of virions and thereby deactivate them (virucidal activity). These target proteins are mainly responsible for the viral interaction with host cells [13][61][62][63][13,61,62,63]. During the pre-viral entry into host cells, AgNPs competitively attach to the cells and lyse the membrane of the virions (antiviral activity). In the case of the post-viral entry, AgNPs mainly inhibit the viral fusion with the cell membrane, and in several cases interfered with the stages of the viral replication cycle such as the synthesis of viral RNA (antiviral activity). At the molecular level, these mechanisms relied on the chemical interaction of AgNPs or Ag+ ions released by AgNPs with sulfur, nitrogen, or phosphorus-containing biomolecules including proteins and genetic material. Hence, AgNPs have multiple mechanisms of action, which suggests that resistance to AgNPs will be less likely to arise when compared to specific antiviral or antibiotic therapies [64][65][66][64,65,66].
The World Health Organization (WHO) has published a list of high-priority (first tier), antibiotic-resistant pathogens that present the greatest threat to human health. These include strains in the Acinetobacter, Pseudomonas, and various Enterobacteriaceae genera (Klebsiella, Escherichia coli (E. coli), Serratia, and Proteus) [67]. Most of these pathogens are Gram-negative strains that exhibit increased resistance when compared to the Gram-positive strains. Gram-negative bacteria have an outer membrane that contains lipopolysaccharide (LPS), which creates a permeability barrier against external, harmful factors [68]. For example, Pseudomonas aeruginosa (P. aeruginosa), a Gram-negative species that nanosilver-based products are commonly tested against, is listed as Priority 1 because the organism is CRITICAL due to its resistance to carbapenem antibiotics that are used as “last line” or “last resort” antibiotics [67][69][67,69]. Four of the six multi-drug-resistant (MDR) pathogens that are primarily responsible for infections originating from hospitalization are also Gram-negative bacteria, labeled as ESKAPE pathogens (Enterococcus faecium (E. faecium), Staphylococcus aureus (S. aureus), Klebsiella pneumoniae (K. pneumoniae), Acinetobacter baumannii (A. baumannii), P. aeruginosa, and Enterobacter species) [70].
