Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Sabrin R. M. Ibrahim.
Marine-derived fungi are renowned as a source of astonishingly significant and synthetically appealing metabolites that are proven as new lead chemicals for chemical, pharmaceutical, and agricultural fields. Aspergillus sydowii is a saprotrophic, ubiquitous, and halophilic fungus that is commonly found in different marine ecosystems. This fungus can cause aspergillosis in sea fan corals leading to sea fan mortality with subsequent changes in coral community structure.
- fungi
- Aspergillus sydowii
- metabolites
1. Sesquiterpenes
Phenolic bisabolane sesquiterpenoids are among the main constituents reported from this fungus. They are a rare class of terpenes that have a p-alkylated benzene connected with 1C and 8C side chains at C-5 and C-2, respectively. Their structural variability is due to cyclization, reduction, or oxidation at various alkyl chain carbons to yield carboxylic acid, alcohol, lactone, double bond, pyran, and furan functionalities. Besides their fascinating skeletons, they show various bioactivities. It is noteworthy that most of the reported bisabolanes were separated from marine-derived A. sydowii as discussed below.
In 1978, Hamasaki and his group separated and characterized compounds 1 and 2 as optically inactive metabolites from A. sydowii acetone extract by spectral and chemical means. These compounds were soluble in saturated NaHCO3 and positively reacted with bromophenol blue [11][1].
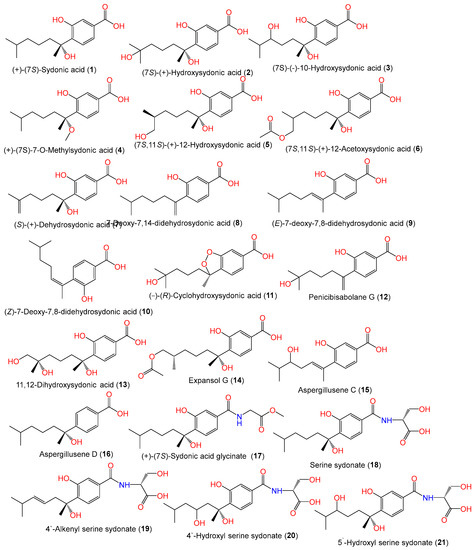
Figure 1.
Structures of sesquiterpenoids (
1
–
21
) reported from
A. sydowii.
Aspergillusene D (16) with a 7S-configuration was reported as a new sesquiterpenoid from Phakellia fusca-associated A. sydowii SCSIO-41301 by Liu et al., along with compounds 1, 5 8, 9, 10, and 22 that were characterized based on spectral and ECD (electronic circular dichroism) analyses [35][2]. Xu et al. separated compound 17, along with compounds 1, 8, and 23, from A. sydowii CUGB-F126 isolated from the Bohai Sea, Tianjin, using SiO2 (silica gel)/Sephadex LH-20/HPLC (high-performance liquid chromatography). Compound 17 is a new sydonic acid analog with a glycinate moiety [15][3].
Sun et al. developed a new approach that integrated computational programs (MS (mass spectrometry)-DIAL and MS-FINDER) and web-based tools (MetaboAnalyst and GNPS) for the identification of A. sydowii–Bacillus subtilis coculture metabolites, wherein 25 biosynthesized metabolites were detected and purified by SiO2/ODS CC/HPLC. Among them, compounds 1, 2, 3, and 18–21 were characterized by spectral and CD (circular dichroism) analyses [58][4]. Further, Hu et al. separated and characterized new bisabolene-type sesquiterpenoids 24 and 25 as well as the known analogs 2 and 23 from A. sydowii EN-434 obtained from Symphyocladia latiuscula marine red alga using RP-18 (reversed phase-18)/SiO2 CC (silica gel column chromatography) and spectral and ECD data. Compounds 24 and 25 have 7S/8S and 7R*/10R* configurations, respectively [32][5]. Fourteen new phenolic bisabolanes with varied structures, labeled 28–41, were separated and characterized by Niu et al. from the deep-sea sediment-derived A. sydowii MCCC-3A00324 (Figure 2).
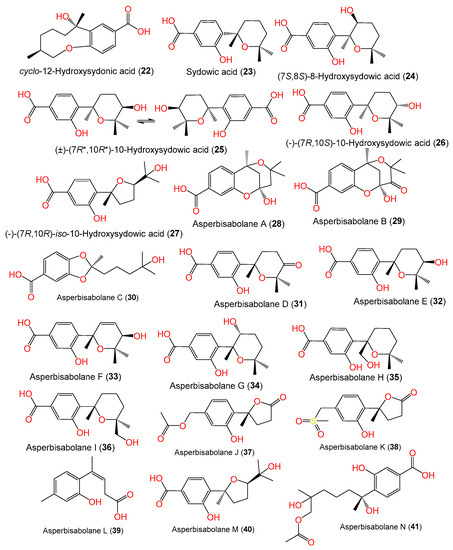
Figure 2.
Structures of sesquiterpenoids (
22
–
41
) reported from
A. sydowii
.
Compounds 28 and 29 are the first bisabolanes with a 6/6/6 tricyclic skeleton, whereas compound 30 features a novel seco-bisabolane with a rare dioxolane moiety, and compound 38 has an unusual methylsulfonyl moiety [57][6]. Trisuwan et al. purified—from A. sydowii PSUF154 isolated from gorgonian sea fan of genus Annella—new bisabolane-type sesquiterpenes 4, 42, and 43, along with 1. Compound 42 has 2-substituted 6-methyl-2-heptenyl and 1,2,4-trisubstituted benzene. Compound 43‘s benzofuran moiety results from the ether linkage of C-1 OH of the tri-substituted phenyl and 2-substituted 6-methyl-2-heptenyl moieties. Compound 4 is a methyl ether of compound 1 with a 7S configuration [56][7]. The first phenolic bisabolane sesquiterpene glycoside, β-D-glucopyranosyl aspergillusene A (44), was purified from sponge-derived A. sydowii [36][8] and assigned using spectral and chemical methods [36][8].
Chung et al. stated that the addition of 5-azacytidine (a DNA methyltransferase inhibitor) to the culture of marine sediment-derived A. sydowii obtained from Hsinchu, Taiwan, significantly promoted the production of various metabolites [54][9]. Investigation of the EtOAc (ethyl acetate) extract of 5-azacytidine-treated culture broth by SiO2 CC and HPLC yielded new bisabolane sesquiterpenoids 5, 46, and 47, along with 1, 42, 45, and 49, that were assigned based on spectral analyses. The S-configuration of compounds 5 and 46 was assigned using optical rotation comparison, whereas compound 46 ([α]D +1.87) is a methyl derivative of compound 45 ([α]D +7.2) and compound 5 ([α]D +3.9) is C-12 hydroxy analog of compound 1 ([α]D +23) (Figure 3). On the other hand, compound 47 is closely similar to the previously reported compound 8 except for the absence of the C-3 carboxylic group in compound 47 [54][9]. Compounds 5, 46, and 47 were proposed to be biosynthesized from farnesyl diphosphate (FPP) created from the addition of an IPP (isopentenyl diphosphate) unit to a GPP (geranyl diphosphate) (Scheme 1). Then, cyclization and folding of the carbon chain through an electrophilic attack on double bonds produced the bisabolane nucleus that then underwent a series of carboxylation, hydration, oxidation, and reduction to give compounds 5, 46, and 47 [54][9].
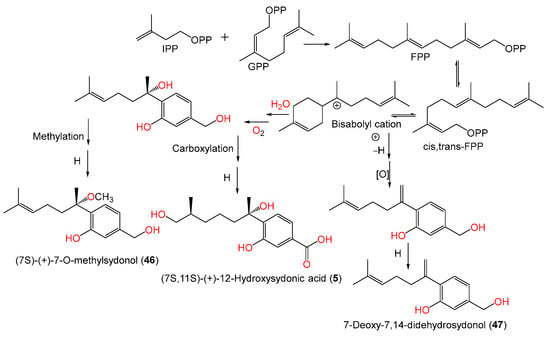
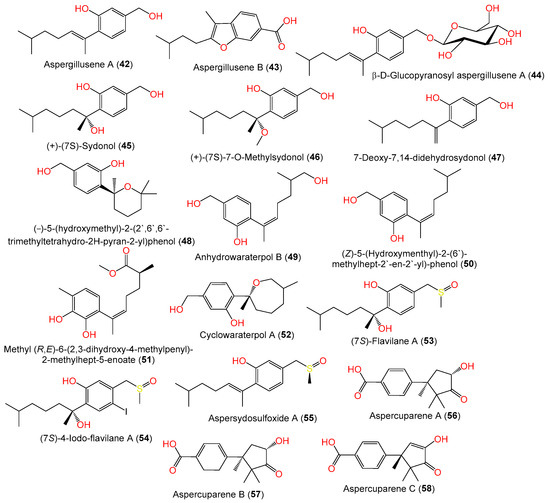
Figure 3.
Structures of sesquiterpenoids (
42
–
58
) reported from
A. sydowii
.
A new bisabolane sesquiterpenoid, compound 15, in addition to compounds 1, 7, 6, 42, 47, 49, 50, and 52, were purified from A. sydowii ZSDS1-F6 EtOAc extract using SiO2/Sephadex LH-20/RP-HPLC by Wang et al. [45][10]. Compound 51 is a new aromatic bisabolene-type sesquiterpenoid with 11S-configuration purified and characterized from the sea-derived A. sydowii SW9 [41][11]. In 2022, Liu et al. purified a rare iodine- and sulfur-containing derivative (7S)- 4-iodo-flavilane A (54) along with compound 53. Compound 54 is 4-iodinated analog of compound 53 and its absolute S-configuration was proven by ECD analysis [38][12]. Furthermore, three undescribed cuparene-type sesquiterpenes, labeled 56–58, were isolated from fermented cultured EtOAc extract of the sea sediment-derived A. sydowii MCCC-3A00324 using SiO2/RP-18/Sephadex LH-20 CC/HPLC and assigned using spectral and ECD analyses. They represent rare cuparene-type sesquiterpenoids having a C-10 keto group and were discovered for the first time from filamentous fungi [57][6].
2. Mono- and Triterpenoids and Sterols
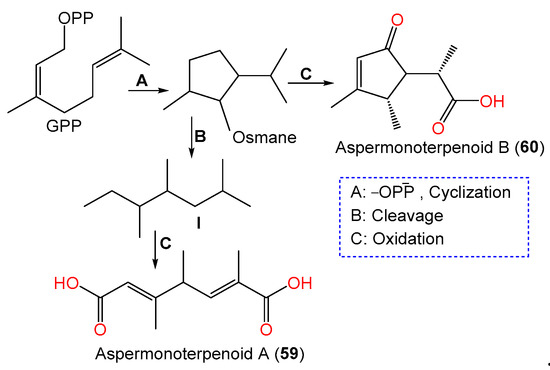
In 2020, the chemical investigation of deep-sea sediment-isolated A. sydowii MCCC-3A00324 by Niu et al. led to the separation of new osmane-type monoterpenoids aspermonoterpenoids A (59) and B (60) by SiO2 CC/HPLC and their structures were determined by spectral, ECD, and specific rotation analyses (Table 2, Figure 4). Compounds 59 and 60 are the first osmane monoterpenes reported from fungi, whereas compound 59 features a novel skeleton, which is possibly derived after the cleavage of the cyclopentane ring and oxidation reaction of the osmane monoterpenoid. They have 4S and 4S/5R/6S configurations, respectively [60][13].
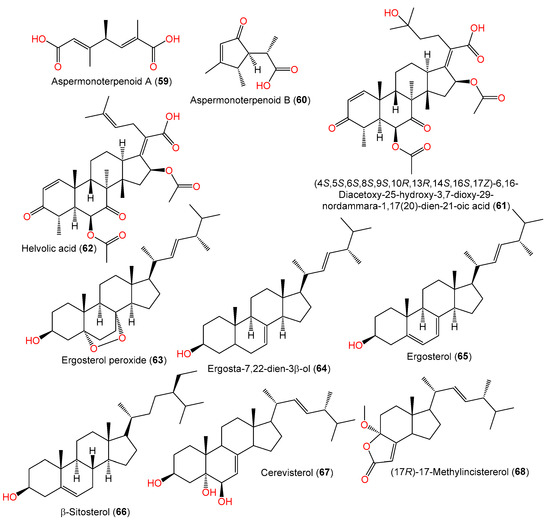
Figure 4.
Structures of mono- (
59
and
60
) and triterpenoids (
61
and
62
) and sterols (
63
–
68
) reported from
A. sydowii.
Table 2.
Mono- and triterpenoids and sterols reported from
A. sydowii
(molecular weight and formulae, strain, host, and location).
| Compound Name | Mol. Wt. | Mol. Formula | Strain, Host, Location | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Monoterpenoids | |||||||||
| Verrucella umbraculum | (gorgonian), Sanya, Hainan, China | [ | 31 | ][24] | Aspermonoterpenoid A (59) | 198 | C10H14O4 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | |
| Cyclotryprostatin E (101) | 443 | C23H29N3O6 | [ | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China60][13] | |||||
| [ | 31 | ] | [ | 24] | Aspermonoterpenoid B (60) | 182 | |||
| Fumitremorgin B (102) | C | 47910H14O3 | C27HMCCC 3A00324, deep-sea sediment, South Atlantic Ocean | 33N3O5 | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China[60][13] | ||||
| [ | 31 | ] | [ | 24] | Triterpenoids | ||||
| (4S,5S |
126
) reported from
A. sydowii.
Acremolins are rare alkaloids with a 5/6/5 tricyclic core, possessing an imidazole moiety fused with a methyl guanine moiety. Interestingly, acremolins were reported from Aspergillus species Aspergillus sp. S-3-75 and SCSIO-Ind09F01 and A. sydowii SP-1 [40,67][26][31]. From the Antarctic A. sydowii SP-1, a new alkaloid acremolin C (128) along with compound 110 were separated using SiO2 CC/ODS/HPLC and characterized by spectral methods. Compound 128 is a regio-isomer of acremolin B previously reported by Tian et al. from the deep-sea-derived fungus Aspergillus sp. SCSIO and has a isopropyl group at C-2′ instead of C-1′ (Figure 10) [40,67][26][31]. In 2022, Niu et al. purified and characterized, from the deep-sea-derived A. sydowii MCCC-3A00324, a new acremolin alkaloid acremolin D (129) along with compounds 110, 126, 127, 135, and 136 using SiO2 CC/HPLC and spectral and ECD data. Compound 129 is closely related to compound 127 in that one CH3 group in 127 has been replaced by an acetoxy methylene group [65][27].
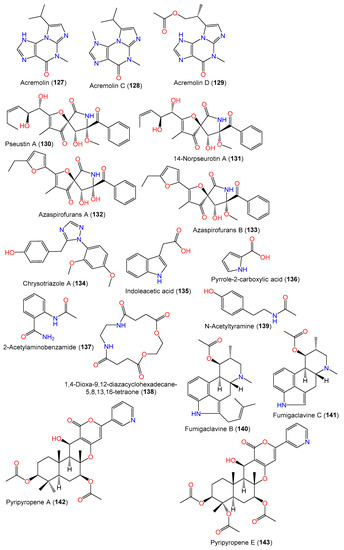
Figure 10.
Structures of alkaloids (
127
–
143
) reported from
A. sydowii.
New hetero-spirocyclic γ-lactam analogs azaspirofurans A (132) and B (133) were separated from the marine sediment-derived A. sydowii D2-6 using SiO2/Sephadex LH-20 CC and were characterized based on spectral and chemical evidence (Figure 10). These compounds featured an ethyl furan ring linked to 1-oxa-7-azaspiro[4,4][32][32]non2-ene-4,6-dione core [43][29].
5. Phenyl Ether Derivatives
Phenyl ethers are a group of simple polyketides that are widely reported in various Aspergillus species and have shown significant bioactivities (Table 5).
Table 5.
Phenyl ether derivatives reported from
Aspergillus sydowii
(molecular weight and formulae, strain, host, and location).
| Compound Name | Mol. Wt. | Mol. Formula | Strain, Host, Location | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Violaceol I (144) | 262 | C14H14O5 | MF357, sea sediment, East China Sea, China | [37][33] | ||||||
| - | - | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [36][8] | |||||||
| Violaceol II (145) | 248 | C13H12O5 | MF357, sea sediment, East China Sea, China | [37][33] | ||||||
| 6-Methoxyspirotryprostatin B (103) | 393 | C22H23N3 | ||||||||
| O | 4 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48][14] | |||||||
| - | - | J05B-7F-4, | Stelletta sp. (marine sponge), South Korea | [36][8] | ,6S,8S,9S,10R,13R,14S,16S,17Z)-6,16-Diacetoxy-25-hydroxy-3,7-dioxy-29-nordammara-1,17(20)-dien-21-oic acid (61) | 572 | C | 18-Oxotryprostatin A (104)32H44O9 | PFW1-13, driftwood, beach of Baishamen, Hainan, China | 395[48] |
| Diorcinol (146) | C | 23022H25N3O4 | [ | 14] | ||||||
| C | PFW1-13, driftwood, Baishamen beach, Hainan, China | 14H14O3 | [ | 48][ | Marine sediment, Hsinchu, Taiwan14] | |||||
| [ | 54 | ] | [ | 9] | Helvolic acid (62) | 554 | C32H42O8 | PFW1-13, driftwood, beach of Baishamen, Hainan, China | [48][14] | |
| 14-Hydroxyterezine D (105) | 341 | C19H23N3O3 | PFW1-13, driftwood, Baishamen beach, Hainan, China | Sterols | ||||||
| - | - | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [48][14] | |||||||
| [ | 36 | ] | [ | 8] | Spirotryprostatin A (106 | ) | 365 | C21 | ||
| H | 23N3O2 | -PFW1-13, driftwood, Baishamen beach, Hainan, China | - | |||||||
| [ | 48 | FNA026, seawater, Xiamen, China | ] | [14] | ||||||
| [ | 9 | ] | [ | 34] | Ergosterol peroxide (63) | 430 | Terezine D (107)C | 32528H46O3 | C19HC1-S01-A7, seawater, West Pacific Ocean | 23 |
| N | 3O2 | [ | 55][15] | |||||||
| PFW1-13, driftwood, Baishamen beach, Hainan, China | [ | 48 | ] | [ | - | -14 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean] | |||
| [ | 60 | ] | [ | 13] | Ergosta-7,22-dien-3β-ol (64) | 398 | C28H46O | C1-S01-A7, seawater, West Pacific Ocean | [55][15] | |
| Fumitremorgin C ( | Ergosterol (65) | 396 | C28H44O | C1-S01-A7, seawater, West Pacific Ocean | [55][15] | |||||
| β-Sitosterol (66) | 414 | C29H50O | C1-S01-A7, seawater, West Pacific Ocean | [55][15] | ||||||
| Cerevisterol (67) | 430 | C28H46O3 | YH11-2, deep-sea fungus, Guam, South Japan | [44][16] | ||||||
| (17R)-17-Methylincistererol (68) | 346 | C22H34O3 | YH11-2, deep-sea fungus, Guam, South Japan | [44][16] |
These metabolites were proposed to be biosynthesized from a GPP that underwent subsequent hydrolysis/oxygenation/cyclization to yield the monocyclic osmane monoterpenoid ring. Then, carbon–carbon bond cleavage of osmane gives intermediate I and its further oxygenation yields compound 59, whilst the osmane oxygenation forms compound 60 [60][13] (Scheme 2).
Zhang et al. purified and characterized compound 61, a new 29-nordammarane-type triterpenoid, in addition to its known analog, compound 62, from the marine-derived A. sydowii PFW1-13 [48][14]. Compound 61 is structurally similar to compound 62 with a 1,1,2-trisubstituted ethanol unit instead of a trisubstituted ethenyl unit, suggesting that compound 61 is a C24–C25 hydrated derivative of compound 62 [48][14]. Its configuration was assigned as 4S/5S/6S/8S/9S/10R/13R/14S/16S/17Z based on comparing its optical rotation (−118.9) with that of compound 62 (optical rotation −105.1) [48][14].
Wang et al., in 2019, reported the separation of ergosterol derivatives 63–66 from deep-sea water-isolated A. sydowii [55][15], while compounds 68 and 69 were separated by Li et al.; compound 69 was assumed to be a sterol degradation product [44][16].
3. Xanthone and Anthraquinone Derivatives
Xanthones are commonly found in lichen, fungi, plants, and bacteria [61][17]. In fungi, xanthones are mostly derived from acetyl-CoA through a series of polyketide synthase-catalyzed chemical transformations [62][18]. These metabolites were found to demonstrate diverse bioactivities.
Compounds 69 and 71 were reported from the EtOAc extract of 5-azacytidine-treated A. sydowii culture broth [54][9]. Additionally, from liverwort Scapania ciliate-accompanied A. sydowii, new xanthone derivatives, labeled 72, 76, and 77, and known compounds 74 and 78 were isolated by SiO2/Sephadex LH-20 CC/HPLC and assigned by spectral data. Compounds 76 and 77 are examples of sulfur-containing xanthones; compound 77 has an additional acetyl group at C-13 and compound 72 features C-2-OH instead of the methylthio moiety as in compound 76 [63][19]. New hydrogenated xanthones, aspergillusones A (86) and B (87), along with compounds 69, 71, 73, 88, and 90, were purified from a strain associated with the gorgonian sea fan of the genus Annella by Trisuwan et al. Compound 86 is a 11-deoxy derivative of compound 88 with an optical rotation of −1.6 and the same C-7 and C-8 absolute configuration, whereas compound 87 is a 1-hydroxy analog of compound 90 with 7R/8R and −46.3 optical rotation (Figure 5) [56][7].
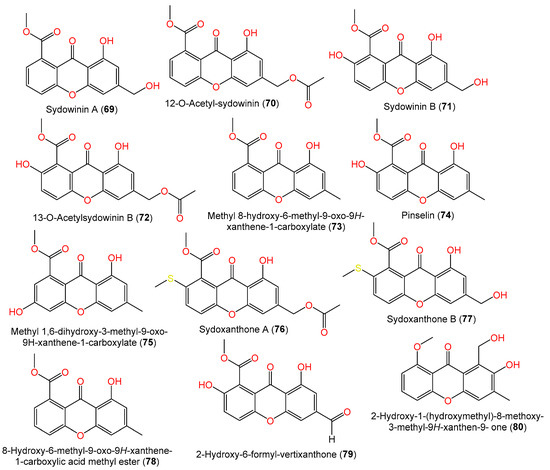
Figure 5.
Structures of xanthones (
69
–
80
) reported from
A. sydowii.
In 2019, Wang et al. purified two novel xanthones, labeled 70 and 79, along with the known xanthones 71, 72, 74, 86, 88, and 89 and quinones 91, 94, and 96, from seawater-derived A. sydowii C1-S01-A7 using SiO2/Sephadex LH-20/RP-18/HPLC; the compounds were elucidated by spectral analyses (Table 3). Compound 79 is similar to previously reported 2-hydroxyvertixanthone with an additional formyl moiety at C-6, whereas compound 70 is similar to compound 69 with one more acetyl group at C-12 [55][15].
Table 3.
Xanthones and quinones reported from
Aspergillus sydowii
(molecular weight and formulae, strain, host, and location).
| Compound Name/Chemical Class | Mol. Wt. | Mol. Formula | Strain, Host, and Location | Ref. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xanthones | ||||||||||||||
| Sydowinin A (69) | 300 | C16H12O6 | Cultured, IFO 4284, Japan | [29][20] | ||||||||||
| - | - | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56][7] | |||||||||||
| 12-O-Acetyl-sydowinin A (70) | 342 | C18H14O7 | C1-S01-A7, seawater, West Pacific Ocean | [55][15] | ||||||||||
| Sydowinin B (71) | 316 | C16H12O7 | Cultured, IFO 4284, Japan | [29][20] | ||||||||||
| - | - | Marine sediment, Hsinchu, Taiwan | [54][9] | |||||||||||
| - | - | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56][7] | |||||||||||
| - | - | Marine sediment, Hsinchu, Taiwan | [54][9] | |||||||||||
| 108- | - | ) | 379 | |||||||||||
| 4-Carboxydiorcinal ( | C1-S01-A7, seawater, West Pacific Ocean | 147)C | 27422H25N3 | [55][15] | ||||||||||
| O | 3 | C | PFW1-13, driftwood, Baishamen beach, Hainan, China | 15H14O5[48 | J05B-7F-4, Stelletta][14] | 13-O-Acetylsydowinin B (72) | 358 | |||||||
| sp. (marine sponge), South Korea | [ | 36 | ] | [8] | 12,13-Dihydroxyfumitremorgin C (109)C18H14O8 | 411 | C22H25N3OScapania ciliata | |||||||
| 5 | (Chinese liverwort), Maoer Mountain, Guangxi, China | [63][19] | ||||||||||||
| PFW1-13, driftwood, Baishamen beach, Hainan, China | FNA026, seawater, Xiamen, China | [ | 48][14] | |||||||||||
| [ | 9 | ] | [ | 34] | (11- | S,14- | S)-Cyclo-(L-Trp-L-Phe) (110) | 333 | C | |||||
| Diorcinolic acid (148) | 20 | 318H19N3O2 | C16H14O7J05B-7F-4, Stelletta sp. (marine sponge), South Korea | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand[ | [56][7] | |||||||||
| J05B-7F-4, | Stelletta | sp. (marine sponge), South Korea | 36 | ][8] | ||||||||||
| [ | 36 | ] | [ | 8] | - | - | C1-S01-A7, seawater, West Pacific Ocean | [55][15] | ||||||
| - | - | MSX19583, spruce litter, Colorado, USA | [33][25] | Methyl 8-hydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate (73) | 284 | C16H12O5 | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56][7 | ||||||
| - | - | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [36][8] | |||||||||||
| - | - | ZSDS1-F6, unidentified marine sponge, Xisha Islands, China | [45][10] | |||||||||||
| Glyceryl diorcinolic acid (149) | 392 | C19H20O9 | FNA026, seawater, Xiamen, China | [9][34] | ] | |||||||||
| 4-Methoxycarbonyl diorcinol (150) | 288 | C16H16O5 | FNA026, seawater, Xiamen, China | [9][34] | Pinselin (74) | |||||||||
| 10-Deoxygerfelin (151) | 300 | C16H12 | 274O6 | C15H14O5PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] | CPCC 401353, cultured, China[7] | ||||||||
| [ | 59 | ] | [ | 35] | - | - | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63][19] | ||||||
| - | - | |||||||||||||
| Cordyol C (152) | 246 | C14H14 | SP-1, marine sediment, Antarctic Great Wall Station | O4 | J05B-7F-4, Stelletta sp. (marine sponge), South Korea[40][26] | - | ||||||||
| [ | 36 | ] | [ | 8] | - | C1-S01-A7, seawater, West Pacific Ocean | [55 | ] | -[ | - | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [65][ | ||
| - | 27 | - | 15] | |||||||||||
| ] | ||||||||||||||
| FNA026, seawater, Xiamen, China | [ | 9 | ] | [34] | Methyl 1,6-dihydroxy-3-methyl-9-oxo-9H-xanthene-1-carboxylate (75) | 300 | Didehydrobisdethiobis(methylthio)gliotoxin (111)C16H12O6 | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | 356 | C15H20N | ||||
| 2 | O4S2 | [ | 56][7] | |||||||||||
| PFW1-13, driftwood, Baishamen beach, Hainan, China | [ | 48 | ] | - | -[ | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean14] | ||||||||
| [ | 60 | ] | [ | 13] | Sydoxanthone A (76) | 388 | Verruculogen (112)C19H16O7S | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63] | 354 | C15H18N2O4S2[19] | |||
| PFW1-13, driftwood, Baishamen beach, Hainan, China | ||||||||||||||
| Cordyol E (153) | 244 | C15H16O3 | [ | 48][14] | ||||||||||
| J05B-7F-4, | Stelletta | sp. (marine sponge), South Korea | [ | 36][8] | Sydoxanthone B (77) | Cyclo-(S-Pro-S-Ile) (346 | 114)C17H14O6S | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | 210 | C11H | ||||
| Cordyol F (154) | 18 | N | 276 | C15H16[63][19] | ||||||||||
| 2 | O | 2 | Cultured, China | O5[28] | ||||||||||
| FNA026, seawater, Xiamen, China | [ | 9 | ] | [34] | 8-Hydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylic acid methyl ester (78) | 284 | C | Cyclo-(S-Pro-R-Leu) (113)16H12O5 | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63][ | 21019] | |||
| C | 11 | 2-Hydroxy-6-formyl-vertixanthone (79) | 314 | C16H10O7 | C1-S01-A7, seawater, West Pacific Ocean | [55][15] | ||||||||
| 2-Hydroxy-1-(hydroxymethyl)-8-methoxy-3-methyl-9H | ||||||||||||||
| Cordyol C-3-O-α-D-ribofuranoside (155) | H | 18N2O2 | 378Cultured, China | [28] | ||||||||||
| C | 19 | H22O8 | FNA026, seawater, Xiamen, China | [9][34] | WIN 64821 (115) | 664 | C40H36N6O4 | MSX19583, spruce litter, Colorado, USA | [33][25] | -xanthen-9-one (80) | 286 | C16H14O5 | ||
| Diorcinol-3-O-α-D-ribofuranoside (156) | 362 | C19H22O7 | FNA026, seawater, Xiamen, China | [9][34] | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35][ | 2] | |||||||
| - | - | C1-S01-A7, seawater, West Pacific Ocean | [ | |||||||||||
| 4-Methoxycarbonyl diorcinol-3-O-α-D-glucoside (157) | 450 | C22H26O10 | 55 | ][15] | ||||||||||
| FNA026, seawater, Xiamen, China | [ | 9 | ] | [34] | 2-Hydroxy-1-(hydroxymethyl)-7,8-dimethoxy-3-methyl-9H-xanthen-9-one (81) | 316 | Bisdethiobis(methylthio)-acetylaranotin (116)C17H | 53416 | ||||||
| Disydonol B (158) | C | 486O24H | C30266 | N2O8S2SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | HCultured, China | 46O[35[28] | ||||||||
| 5 | FNA026, seawater, Xiamen, China | ] | [ | 2] | ||||||||||
| [ | 55 | ] | [ | 15] | Austocystin A (82) | 372 | [4-(2-Methoxyphenyl)-1-piperazinyl][(1-methyl-1H-indol-3-yl)]-methanone (117) | 349C19H13ClO6 | C21H23N3OSCSIO 00305, Verrucella unbracculum (gorgonian), South China Sea, Sanya, Hainan, China | 2[24] | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China[21] | |||
| [ | 31 | ] | ||||||||||||
| 2-(Ethoxycarbonyl)-4′-carboxydiorcinal (159) | [ | 348 | 24 | ] | ||||||||||
| C | 17 | H16O8 | FNA026, seawater, Xiamen, China | [9][34] | 6-Methoxyl austocystin A (83) | 402 | Fumiquinazoline A (118)C20H15ClO7 | 445 | C24H23SCSIO 00305, Verrucella unbracculum (gorgonian), South China Sea, Sanya, Hainan, China | [ | ||||
| 7-Ethyldiorcinol (160) | N | 5O4 | 24 | ] | SCSIO 00305, Verrucella umbraculum[21] | |||||||||
| (gorgonian), Sanya, Hainan, China | 244 | C15H16O3 | [ | 31][24] | ||||||||||
| FNA026, seawater, Xiamen, China | [ | 9 | ] | [34] | Sterigmatocystin (84) | Fumiquinazoline B (119)324 | C18H12O6 | DC08, Dachtylospongia sp. (marine sponge), South Coast, West Sumatra, Indonesia | 445 | C24H23N5O4 | ||||
| 3-Hydroxydiorcinol (161) | 246 | C14H14 | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China | [39][22] | ||||||||||
| O | 4 | [ | 31 | ][24 | FNA026, seawater, Xiamen, China] | |||||||||
| [ | 9 | ] | [ | 34] | Sydowinol (85) | 318 | C | Fumiquinazoline C (12016H14O7 | IFO 4284, Cultured, Japan | )[29][ | 44320] | |||
| Aspergilol E (162) | C | 24H21N5 | 304O4 | C16H16O6SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China | [31][24] | |||||||||
| FNA026, seawater, Xiamen, China | [ | 9 | ] | [34] | Aspergillusone A (86) | 304 | C | Fumiquinazoline D (12116H16O6 | )PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56][7] | ||||
| 443 | ||||||||||||||
| 4-Hydroxy-2-(3′-hydroxy-4-methoxycarbonyl-5′-methylphenoxy)-6-methylbenzoic acid (163) | 332 | C24H21N5O4 | C17H16OSCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China | 7[31][24] | ||||||||||
| FNA026, seawater, Xiamen, China | [ | 9 | ] | [34] | - | - | C1-S01-A7, seawater, West Pacific Ocean | [ | Fumiquinazoline F (122)55 | |||||
| Aspermutarubrol ( | 358 | 164)C21 | 262H18N4O2 | C14H14O5SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China][15] | ||||||||||
| [ | 31 | ] | [ | 24] | ||||||||||
| FNA026, seawater, Xiamen, China | [ | 9 | ] | [34] | Aspergillusone B (87) | Fumiquinazoline G (123338 | ) | 358C16H18O8 | C21H | |||||
| Bisviolaceol II (165) | 18 | 506N4O2 | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [ | C28H26SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China56][ | O97] | ||||||||
| [ | 31 | ] | [ | 24 | 10–31, sediments, deep-sea, cold seep off southwestern Taiwan] | (7 | ||||||||
| [ | 38 | ] | [ | 12] | R,8R)-AGI-B4 (88) | 320 | C | 2-(4-Hydroxybenzyl)-4-(3-acetyl)quinazolin-one (12416H16O7 | )PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56][7 | 294 | C17H14N2O3 | SW9, seawater, Yangma Island, Yantai, China | [41][11] |
| Sydowiol A (166) | 370 | C20H18O7 | MF357, sea sediment, East China Sea, China | ] | ||||||||||
| [ | 37 | ] | [ | 33] | - | 2-(4-Hydroxybenzoyl)-4(3H- | )-quinazolinone ( | |||||||
| Sydowiol B (167125) | 252 | )C15H12N2 | Marine sediment, Hsinchu, Taiwan | O2[54][9] | ||||||||||
| SW9, seawater, Yangma Island, Yantai, China | [ | 384 | C21H20O7 | 41] | MF357, sea sediment, East China Sea, China[11] | - | - | |||||||
| [ | 37 | ] | [ | 33] | 2-(4-Oxo-3,4-dihydroquinazolin-2-yl)benzoic acid (126)C1-S01-A7, seawater, West Pacific Ocean | 266 | ||||||||
| Sydowiol C (168 | C | 15H10N2O3 | ) | 384 | C21H[55][15] | |||||||||
| 20 | O | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [ | 65][27] | ||||||||||
| 7 | MF357, sea sediment, East China Sea, China | [ | 37 | ][33] | 12-O-Acetyl (7R,8R)-AGI-B4 (89) | 362 | Acremolin (127)C18H18O8 | C1-S01-A7, seawater, West Pacific Ocean | [ | 231 | C11H13N555][15] | |||
| (7R,8R)-α-Diversonolic ester (90) | 322 | C16H18O7 | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56][7] | ||||||||||
| Quinones | ||||||||||||||
| Emodin (91) | 270 | C15H10O5 | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63][19] | ||||||||||
| - | - | C1-S01-A7, seawater, West Pacific Ocean | [55][15] | |||||||||||
| Emodic acid (92) | 300 | C15H8O7 | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35][2] | ||||||||||
| Parietinic acid (93) | 314 | C16H10O7 | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35][2] | ||||||||||
| Questin (94) | 284 | C16H12O5 | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63][19] | ||||||||||
| - | - | C1-S01-A7, seawater, West Pacific Ocean | [55][15] | |||||||||||
| - | - | SCSIO 41301, marine sponge Phakellia fusca, Xisha Islands, China | [35][2] | |||||||||||
| 1,6,8-Trihydroxy-3-methylanthraquinone (95) | 270 | C15H10O5 | SCSIO 41301, marine sponge Phakellia fusca, Xisha Islands, China | [35][2] | ||||||||||
| Yicathin C (96) | 312 | C17H12O6 | C1-S01-A7, seawater sample, West Pacific Ocean | [55][15] | ||||||||||
| 1-Hydroxy-6,8-dimethoxy-3-methylanthraquinone (97) | 298 | C17H14O5 | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63][19] | ||||||||||
| (+)-3,3′,7,7′,8,8′-hexahydroxy-5,5′-dimethyl-bianthra-quinone (98) | 538 | C30H18O10 | #2B, leaves, Aricennia marina, Yangjiang, Guangdong, China | [64][23] | ||||||||||
| Xanthoradone A (99) | 490 | C27H22O9 | #2B, leaves, Aricennia marina, Yangjiang, Guangdong, China | [64][23] |
The cultured EtOAc extract of A. sydowii SCSIO-41301 associated with Phakellia fusca provided new xanthones 80 and 81. Compound 80 is related to versicone A with 3-OH instead of the isopentene group in versicone A, while compound 81 has an additional 6-OCH3 group compared to compound 80 [35][2]. The new mycotoxin 6-methoxyl austocystin A (83) and the related known compound 82 were isolated from Verrucella umbraculum-associated A. sydowii SCSIO-00305 (Figure 6). Compound 83 is similar to compound 82 except for the presence of an additional C6-OCH3. Their 1′R/2S configuration was assigned based on X-ray analysis [24][21].
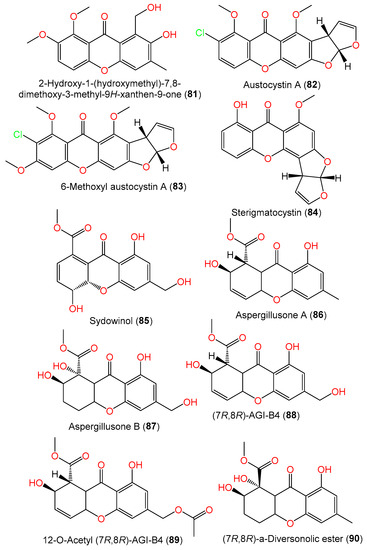
Figure 6.
Structures of xanthones (
81
–
90
) reported from
A. sydowii.
Additionally, compounds 92–95 are anthraquinones reported by Liu et al. from a Phakellia fusca-associated fungal strain [35][2] (Figure 7). Compounds 98 and 99 are quinone derivatives separated from A. sydowii #2B associated with Aricennia marina by Wang et al. [64][23].
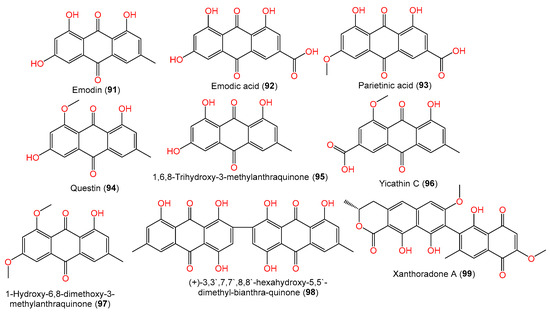
Figure 7.
Structures of quinones (
91
–
99
) reported from
A. sydowii.
4. Alkaloids
Alkaloids have drawn considerable attention because of their unique structural features and varied bioactivities. Interestingly, alkaloids belonging to various classes were reported from A. sydowii.
From the culture broth of coral Verrucella umbraculum-accompanied A. sydowii SCSIO-00305, using bio-guided fractionation, a new indole diketopiperazine member, cyclotryprostatin E (101), and compounds 100, 102, and 117–123 were purified using RP-18 CC/HPLC and characterized by spectral data interpretation [31][24] (Figure 8). Compound 101 is similar to compound 100 bar the replacement of the tri-substituted double bond in compound 100 with an oxygen-bonded quaternary carbon; compound 117 possesses indolyl, piperazinyl, and 1,2-disubstituted phenyl groups [31][24].
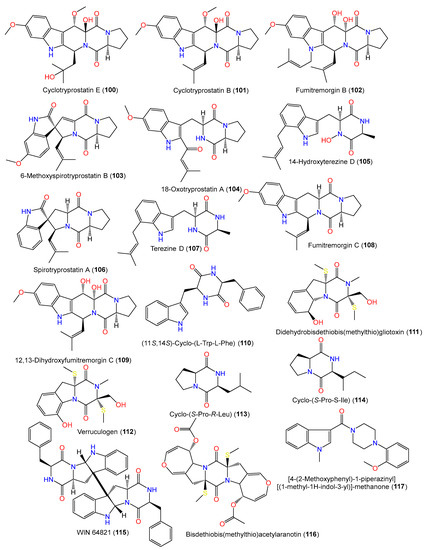
Figure 8.
Structures of alkaloids (
100
–
117
) reported from
A. sydowii.
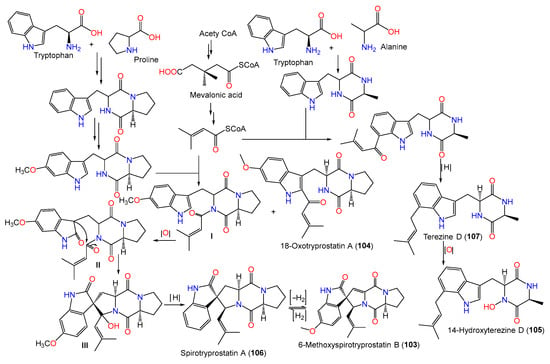
In 2008, Zheng et al. purified new diketopiperazines 103–105 and a new oxaspiro [4.4]lactam-containing alkaloid, labeled 131, along with compounds 106–109, 111, 112, 130, and 140–143 from the EtOAc extract of A. sydowii PFW1-13 isolated from driftwood sourced from Baishamen beach, Hainan, China, using SiO2/Sephadex LH-20 CC/HPLC [48][14]. The configurations of compounds 103–105 and 131 were assigned based on NMR (nuclear magnetic resonance) and CD spectral analyses, and the specific rotation was 3S/12S/18S for compound 103 and 9S/12S for compounds 104 and 105, while compound 131 was identified as a 14-nor-derivative of compound 130 with 5S/8S/9R/10S/11S/12Z configuration [48][14].
Biosynthetically, compounds 103–105 were postulated to be generated through a mixed mevalonic acid/amino acid pathway. Compound 105 is generated from the oxidation of compound 107, which results from mevalonic acid, tryptophan, and alanine. A cyclo(Trp-Pro) is formed from proline and tryptophan and is further oxidized and methylated to produce ethoxylated cyclo(Trp-Pro). Then, the latter reacts with mevalonic acid to yield compound 104 and intermediate I. An intramolecular aldol reaction of intermediate I yields intermediate III, which is deoxygenated to produce compound 106. Additionally, the dehydrogenation of compound 106 gives compound 103 (Scheme 3).
Kaur et al. separated a new diketopiperazine dimer WIN 64821 (115) and the known compound 110 using SiO2 CC and RP-HPLC from the CH3OH/CH3CN extract of A. sydowii MSX-19583 obtained from spruce litter; the compounds were assigned by spectral and ECD analyses and Marfey’s Method (Table 4). Compound 115 is structurally similar to the ditryptophenaline reported in various Aspergillus species and derived from tryptophan and phenylalanine subunits [33][25].
Table 4. Alkaloids reported from Aspergillus sydowii (molecular weight and formulae, strain, host, and location).
| Compound Name | Mol. Wt. | Mol. Formula | Strain, Host, and Location | Ref. |
|---|---|---|---|---|
| Cyclotryprostatin B (100) | 425 | C23H27N3O5 | SCSIO 00305, | |
| O | ||||
| MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | ||||
| [ | ||||
| 65 | ||||
| ] | ||||
| [ | ||||
| 27 | ||||
| ] | ||||
| Acremolin C ( | ||||
| 128 | ||||
| ) | ||||
| 245 | ||||
| C | ||||
| 12 | ||||
| H | ||||
| 15 | ||||
| N | ||||
| 5 | ||||
| O | ||||
| SP-1, marine sediment, Antarctic Great Wall Station | ||||
| [ | ||||
| 40 | ||||
| ] | ||||
| [ | ||||
| 26 | ||||
| ] | ||||
| Acremolin D (129) | 289 | C13H15N5O3 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [65][27] |
| Pseustin A (130) | 431 | C22H25NO8 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48][14] |
| 14-Norpseurotin A (131) | 417 | C21H23NO8 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48][14] |
| Azaspirofurans A (132) | 411 | C21H19NO7 | D2-6, Marine sediment, Jiaozhou Bay, China | [43][29] |
| Azaspirofurans B (133) | C22H21NO7 | D2-6, Marine sediment, Jiaozhou Bay, China | [43][29] | |
| Chrysotriazole A (134) | 311 | C17H17N3O3 | SW9, seawater, Yangma Island, Yantai, China | [41][11] |
| Indoleacetic acid (135) | 175 | C10H9NO2 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [65][27] |
| Pyrrole-2-carboxylic acid (136) | 111 | C5H5NO2 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [65][27] |
| 2-Acetylaminobenzamide (137) | 178 | C9H10N2O2 | C1-S01-A7, seawater, West Pacific Ocean | [55][15] |
| 1,4-Dioxa-9,12-diazacyclohexadecane-5,8,13,16-tetraone (138) | 286 | C12H18N2O6 | Cultured, China | [28] |
| N-Acetyltyramine (139) | 179 | C10H13NO2 | Cultured, China | [28] |
| Fumigaclavine B (140) | 366 | C23H30N2O2 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48][14] |
| Fumigaclavine C (141) | 298 | C18H22N2O2 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48][14] |
| Pyripyropene A (142) | 525 | C29H35NO8 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48][14] |
| Pyripyropene E (143) | 569 | C30H35NO10 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48][14] |
A new quinazolinone alkaloid, labeled 124, as well as related alkaloid 125 and triazole analog 134 were separated and characterized from the mycelia EtOAc extract of seawater-derived A. sydowii SW9 using SiO2/Rp-18/Sephadex LH-20 CC and spectral analyses (Figure 9). Compound 124 is an acetyl derivative of 2-(4-hydroxybenzyl)quinazolin-4(3H)-one, previously reported from Cordyceps-associated Isaria farinose [41,66][11][30].
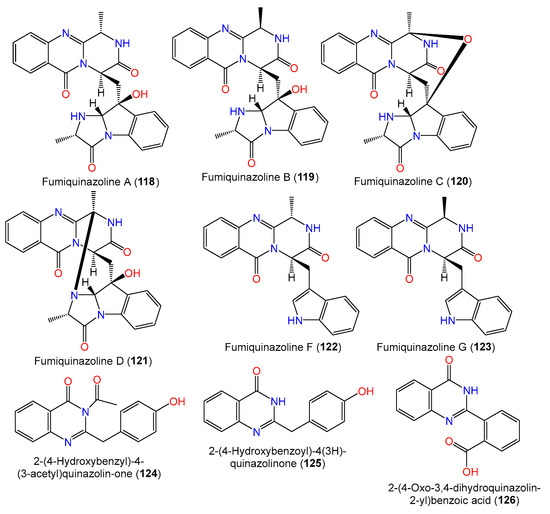
Figure 9.
Structures of quinazoline alkaloids (
118
–
A new biphenyl ether derivative diorcinolic acid (148) together with compounds 144–147, 152, and 153 were separated from marine sponge Stelletta sp.-associated A. sydowii (Figure 11). Compound 149 featured two ether-linked 1,3-dioxy-6-carboxy-5-methylphenyl units. It was assigned as dicarboxylated diorcinol (carboxylated orcinol‘s ether-linked dimer) [36][8]. Bioassay-guided separation of the East China Sea sediment-derived A. sydowii MF357 yielded new tris-pyrogallol ethers sydowiols A–C (166–168) and related bis-pyrogallol ethers 144 and 145 that were characterized based on detailed spectral analysis and symmetry considerations [37][33]. On the other hand, the LC–UV–MS-guided separation of EtOAc extract of China Sea-derived A. sydowii resulted in new diphenyl ethers 155–157 and 159–161 along with compounds 146, 147, 149, 150, 152, and 162–164 using SiO2/Sephadex LH-20 CC/HPLC; the compounds were assigned using spectral and chemical methods. Compounds 155 and 156 are rare glycosides, possessing a D-ribose moiety, whereas compound 157 has a D-glucose moiety [9][34].
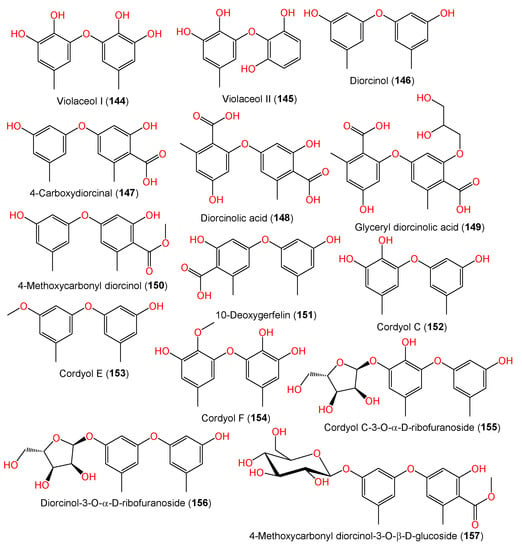
Figure 11.
Structures of phenyl ether derivatives (
144
–
157
) reported from
A. sydowii.
From cold seep-derived A. sydowii 10–31, bisviolaceol II (165), a new tetraphenyl ether derivative, was isolated and characterized by Liu et al. using SiO2/Sephadex LH-20 CC/HPLC and spectral tools, respectively [38][12] (Figure 12).
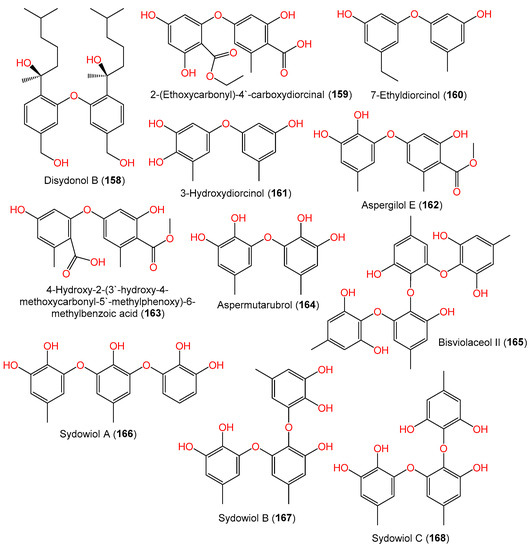
Figure 12.
Structures of phenyl ether derivatives (
158
–
168
) reported from
A. sydowii.
References
- Hamasaki, T.; Nagayama, K.; Hatsuda, Y. Two New Metabolites, Sydonic Acid and Hydroxysydonic Acid, from Aspergillus Sydowi. Agric. Biol. Chem. 1978, 42, 37–40.
- Liu, N.; Peng, S.; Yang, J.; Cong, Z.; Lin, X.; Liao, S.; Yang, B.; Zhou, X.; Zhou, X.; Liu, Y. Structurally Diverse Sesquiterpenoids and Polyketides from a Sponge-Associated Fungus Aspergillus sydowii SCSIO41301. Fitoterapia 2019, 135, 27–32.
- Xu, X.; Zhao, S.; Yin, L.; Yu, Y.; Chen, Z.; Shen, H.; Zhou, L. A New Sydonic Acid Derivative from a Marine Derived-Fungus Aspergillus sydowii. Chem. Nat. Compd. 2017, 53, 1056–1058.
- Sun, Y.; Liu, W.; Shi, X.; Zheng, H.; Zheng, Z.; Lu, X.; Xing, Y.; Ji, K.; Liu, M.; Dong, Y. Inducing Secondary Metabolite Production of Aspergillus sydowii through Microbial Co-Culture with Bacillus Subtilis. Microb. Cell Factories 2021, 20, 42.
- Hu, X.; Li, X.; Meng, L.; Wang, B. Antioxidant Bisabolane-Type Sesquiterpenoids from Algal-Derived Fungus Aspergillus sydowii EN-434. J. Oceanol. Limnol. 2020, 38, 1532–1536.
- Niu, S.; Yang, L.; Zhang, G.; Chen, T.; Hong, B.; Pei, S.; Shao, Z. Phenolic Bisabolane and Cuparene Sesquiterpenoids with Anti-Inflammatory Activities from the Deep-Sea-Derived Aspergillus sydowii MCCC 3A00324 Fungus. Bioorg. Chem. 2020, 105, 104420.
- Trisuwan, K.; Rukachaisirikul, V.; Kaewpet, M.; Phongpaichit, S.; Hutadilok-Towatana, N.; Preedanon, S.; Sakayaroj, J. Sesquiterpene and Xanthone Derivatives from the Sea Fan-Derived Fungus Aspergillus sydowii PSU-F154. J. Nat. Prod. 2011, 74, 1663–1667.
- Liu, S.; Wang, H.; Su, M.; Hwang, G.J.; Hong, J.; Jung, J.H. New Metabolites from the Sponge-Derived Fungus Aspergillus sydowii J05B-7F-4. Nat. Prod. Res. 2017, 31, 1682–1686.
- Chung, Y.; Wei, C.; Chuang, D.; El-Shazly, M.; Hsieh, C.; Asai, T.; Oshima, Y.; Hsieh, T.; Hwang, T.; Wu, Y. An Epigenetic Modifier Enhances the Production of Anti-Diabetic and Anti-Inflammatory Sesquiterpenoids from Aspergillus sydowii. Bioorg. Med. Chem. 2013, 21, 3866–3872.
- Wang, J.; Lin, X.; Qin, C.; Liao, S.; Wan, J.; Zhang, T.; Liu, J.; Fredimoses, M.; Chen, H.; Yang, B. Antimicrobial and Antiviral Sesquiterpenoids from Sponge-Associated Fungus, Aspergillus sydowii ZSDS1-F6. J. Antibiot. 2014, 67, 581–583.
- Liu, Y.; Zhang, J.; Li, C.; Mu, X.; Liu, X.; Wang, L.; Zhao, Y.; Zhang, P.; Li, X.; Zhang, X. Antimicrobial Secondary Metabolites from the Seawater-Derived Fungus Aspergillus sydowii SW9. Molecules 2019, 24, 4596.
- Liu, Y.; Fang, S.; Wang, B.; Ji, N. Phenol Derivatives from the Cold-Seep Fungus Aspergillus sydowii 10–31. Phytochem. Lett. 2022, 52, 63–66.
- Niu, S.; Yang, L.; Chen, T.; Hong, B.; Pei, S.; Shao, Z.; Zhang, G. New Monoterpenoids and Polyketides from the Deep-Sea Sediment-Derived Fungus Aspergillus sydowii MCCC 3A00324. Mar. Drugs 2020, 18, 561.
- Zhang, M.; Wang, W.; Fang, Y.; Zhu, T.; Gu, Q.; Zhu, W. Cytotoxic Alkaloids and Antibiotic Nordammarane Triterpenoids from the Marine-Derived Fungus Aspergillus Sydowi. J. Nat. Prod. 2008, 71, 985–989.
- Wang, W.; Gao, M.; Luo, Z.; Liao, Y.; Zhang, B.; Ke, W.; Shao, Z.; Li, F.; Chen, J. Secondary Metabolites Isolated from the Deep Sea-Derived Fungus Aspergillus sydowii C1-S01-A7. Nat. Prod. Res. 2019, 33, 3077–3082.
- Tian, L.; Cai, S.; Li, D.; Lin, Z.; Zhu, T.; Fang, Y.; Liu, P.; Gu, Q.; Zhu, W. Two New Metabolites with Cytotoxicities from Deep-Sea Fungus, Aspergillus Sydowi YH11-2. Arch. Pharm. Res. 2007, 30, 1051–1054.
- Huang, Q.; Wang, Y.; Wu, H.; Yuan, M.; Zheng, C.; Xu, H. Xanthone Glucosides: Isolation, Bioactivity and Synthesis. Molecules 2021, 26, 5575.
- Badiali, C.; Petruccelli, V.; Brasili, E.; Pasqua, G. Xanthones: Biosynthesis and Trafficking in Plants, Fungi and Lichens. Plants 2023, 12, 694.
- Song, X.; Zhang, X.; Han, Q.; Li, X.; Li, G.; Li, R.; Jiao, Y.; Zhou, J.; Lou, H. Xanthone Derivatives from Aspergillus sydowii, an Endophytic Fungus from the Liverwort Scapania Ciliata S. Lac and their Immunosuppressive Activities. Phytochem. Lett. 2013, 6, 318–321.
- Hamasaki, T.; Sato, Y.; Hatsuda, Y. Isolation of New Metabolites from Aspergillus Sydowi and Structure of Sydowic Acid. Agric. Biol. Chem. 1975, 39, 2337–2340.
- Amin, M.; Liang, X.; Ma, X.; Dong, J.; Qi, S. New Pyrone and Cyclopentenone Derivatives from Marine-Derived Fungus Aspergillus sydowii SCSIO 00305. Nat. Prod. Res. 2021, 35, 318–326.
- Handayani, D.; Dwinatrana, K.; Rustini, R. Antibacterial Compound from Marine Sponge Derived Fungus Aspergillus sydowii DC08. Rasayan J. Chem. 2022, 15, 2485–2492.
- Wang, Y.; Zhong, Z.; Zhao, F.; Zheng, J.; Zheng, X.; Zhang, K.; Huang, H. Two New Pyrone Derivatives from the Mangrove-Derived Endophytic Fungus Aspergillus sydowii# 2B. Nat. Prod. Res. 2022, 36, 3872–3878.
- He, F.; Sun, Y.; Liu, K.; Zhang, X.; Qian, P.; Wang, Y.; Qi, S. Indole Alkaloids from Marine-Derived Fungus Aspergillus sydowii SCSIO 00305. J. Antibiot. 2012, 65, 109–111.
- Kaur, A.; Raja, H.A.; Darveaux, B.A.; Chen, W.; Swanson, S.M.; Pearce, C.J.; Oberlies, N.H. New Diketopiperazine Dimer from a Filamentous Fungal Isolate of Aspergillus sydowii. Magn. Reson. Chem. MRC 2015, 53, 616.
- Li, W.; Luo, D.; Huang, J.; Wang, L.; Zhang, F.; Xi, T.; Liao, J.; Lu, Y. Antibacterial Constituents from Antarctic Fungus, Aspergillus sydowii SP-1. Nat. Prod. Res. 2018, 32, 662–667.
- Niu, S.; Chen, Z.; Pei, S.; Shao, Z.; Zhang, G.; Hong, B. Acremolin D, a New Acremolin Alkaloid from the Deep-Sea Sediment Derived Aspergillus sydowii Fungus. Nat. Prod. Res. 2022, 36, 4936–4942.
- Gao, T.; Cao, F.; Yu, H.; Zhu, H. Secondary Metabolites from the Marine Fungus Aspergillus sydowii. Chem. Nat. Compd. 2017, 53, 1204–1207.
- Ren, H.; Liu, R.; Chen, L.; Zhu, T.; Zhu, W.M.; Gu, Q.Q. Two New Hetero-Spirocyclic Γ-Lactam Derivatives from Marine Sediment-Derived Fungus Aspergillus Sydowi D2–6. Arch. Pharm. Res. 2010, 33, 499–502.
- Ma, C.; Li, Y.; Niu, S.; Zhang, H.; Liu, X.; Che, Y. N-Hydroxypyridones, Phenylhydrazones, and a Quinazolinone from Isaria Farinosa. J. Nat. Prod. 2011, 74, 32–37.
- Tian, Y.; Qin, X.; Lin, X.; Kaliyaperumal, K.; Zhou, X.; Liu, J.; Ju, Z.; Tu, Z.; Liu, Y. Sydoxanthone C and Acremolin B Produced by Deep-Sea-Derived Fungus Aspergillus Sp. SCSIO Ind09F01. J. Antibiot. 2015, 68, 703–706.
- Ibrahim, S.R.; Mohamed, G.A.; Khedr, A.I. γ-Butyrolactones from Aspergillus Species: Structures, Biosynthesis, and Biological Activities. Nat. Prod. Commun. 2017, 12, 791–800.
- Liu, X.; Song, F.; Ma, L.; Chen, C.; Xiao, X.; Ren, B.; Liu, X.; Dai, H.; Piggott, A.M.; Av-Gay, Y. Sydowiols A–C: Mycobacterium Tuberculosis Protein Tyrosine Phosphatase Inhibitors from an East China Sea Marine-Derived Fungus, Aspergillus sydowii. Tetrahedron Lett. 2013, 54, 6081–6083.
- Wang, Y.; Mou, Y.; Dong, Y.; Wu, Y.; Liu, B.; Bai, J.; Yan, D.; Zhang, L.; Feng, D.; Pei, Y. Diphenyl Ethers from a Marine-Derived Aspergillus sydowii. Mar. Drugs 2018, 16, 451.
- Sun, Y.; Shi, X.; He, L.; Xing, Y.; Guo, Q.; Xiu, Z.; Dong, Y. Biosynthetic Profile in the Co-Culture of Aspergillus sydowii and Bacillus Subtilis to Produce Novel Benzoic Derivatives. Microb. Ecol. 2022, 85, 1288–1299.
More