Caffeic acid phenethyl ester (CAPE) is a phenylpropanoid naturally found in propolis that shows important biological activities, including neuroprotective activity by modulating the Nrf2 and NF-κB pathways, promoting antioxidant enzyme expression and inhibition of proinflammatory cytokine expression. Its simple chemical structure has inspired the synthesis of many derivatives, with aliphatic and/or aromatic moieties, some of which have improved the biological properties. Moreover, new drug delivery systems increase the bioavailability of these compounds in vivo, allowing its transcytosis through the blood-brain barrier, thus protecting brain cells from the increased inflammatory status associated to neurodegenerative and psychiatric disorders.
1. Introduction
The current demographic shift has led to a surge in the prevalence of diseases affecting older adults, including neurodegenerative disorders. Among them, Alzheimer’s disease (AD) is the first cause of dementia and a significant global public health problem, estimated to affect about 131.5 million people worldwide, with an annual incidence of 4 to 6 million new cases [
1]. Furthermore, the second neurodegenerative disorder is Parkinson’s disease (PD), affecting one in every hundred people over 60 years of age. It is estimated that by 2030 there will be 9 million patients with idiopathic PD worldwide [
2]. Moreover, psychiatric disorders with increasing worldwide prevalence, such as major depressive disorder (MDD), are relevant and potentially modifiable risk factors for dementia. Neurodegenerative disorders are characterized by excessive damage of key brain structures, which is followed by neuronal function loss, structural alterations, and decrease of cellular survival [
3]. However, despite researchers increasing knowledge about their pathogenic mechanisms, successful therapeutic approaches to tackle neurodegeneration have remained highly elusive.
A wealth of evidence has indicated that oxidative stress and neuroinflammation may have a crucial role in the pathogenesis of neurodegenerative disorders, as they are associated with complex systems that activate programmed cell death cascades [
4,
5,
6,
7].
Activation of the Nrf2 and NF-κB pathways is expected to have a prominent role in the control of oxidative stress and inflammation. The nuclear erythroid transcription factor 2 (Nrf2) responds to oxidative stress, mediating cytoprotection in mammalian cells by the production of a set of antioxidant genes called phase II genes, which produce phase II proteins associated with ROS stabilization [
8,
9,
10]. In turn, NF-κB controls pro-inflammatory gene expression. The synthesis of cytokines such as the tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, IL-6, and IL-8 is directly mediated by NF-κB, as well as the expression of cyclooxygenase-2 (COX2) [
11].
To date, conventional single drug-based therapeutic approaches for addressing neurodegenerative disorders have not been entirely satisfactory. For instance, in the pharmacological treatment of AD, since the 1990s, only three small molecules as cholinesterase inhibitors, including galantamine, rivastigmine, and donepezil, together with memantine, a glutamatergic antagonist, are available, alongside two antibodies, aducanumab and lecanemab. Therefore, targeting multiple pharmacological pathways such as inflammation and oxidative stress holds great promise for increasing the likelihood of obtaining potent neuroprotective effects. Among the novel and emerging therapeutic approaches, consuming functional foods enriched with natural bioactive molecules has shown great potential as a palliative or preventative measure for the middle-aged population. For instance, propolis is considered a food supplement with relevant therapeutic properties, including antidiabetic, anticancer or antibacterial activities. Propolis is a large mixture of polyphenols, essential oils, and waxes produced by plants as resinous secretions. This is collected by honeybees, which use it as a waterproof substance to seal hive cracks or holes [
12]. Furthermore, bees use propolis as an antiseptic material to prevent infections due to its powerful antibacterial [
13] and antiparasitic properties [
14]. The large molecular variety of propolis depends on many factors, such as location, the season, and plants distributed around the hive [
15]. Despite this, propolis always shows antioxidant, anti-inflammatory, bactericidal, and antifungal properties that could be explained due to the high content and variety of flavonoids with a particular chemical composition [
16,
17,
18].
2. Natural Sources of Caffeic Acid Phenethyl Ester
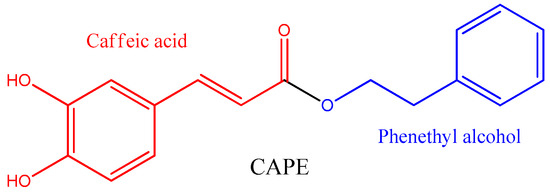
Caffeic acid phenethyl ester (CAPE) is found mainly in propolis. It is a natural polyphenol from the phenylpropanoid family, formed by the esterification of caffeic acid and phenethyl alcohol (
Figure 1) [
19,
20,
21].
Figure 1. Chemical structure of caffeic acid phenethyl ester (CAPE).
CAPE is not the major component of propolis, but it is one of the most potent antioxidants supplied by the caffeic acid moiety, which has a higher antioxidant capacity than related phenylpropanoids such as ferulic acid and p-coumaric acid [
22]. The concentration of CAPE in propolis varies greatly, from 0 to 11 mg/g of propolis collected in different regions of Turkey [
23]. Quantification by high-performance liquid chromatography (HPLC) is a suitable method for its determination [
24].
Table 1 shows the concentration of CAPE in some sources.
The presence of CAPE and caffeic acid was analyzed in 25 species of mushrooms. CAPE was not found in any any of them, unlike caffeic acid, which was quantified in seven mushrooms, including
B. edulis,
S. commune,
F. velutipes,
Agrocybe aegerita,
P. eryngii,
P. cystidiosus and
P. adiposa in concentrations from 0.004 to 0.577 mg g
−1 [
28].
3. Biosynthesis of Caffeic Acid Phenethyl Ester
CAPE displays a broad spectrum of beneficial activities, including antitumor activity by inducing apoptosis in many cancer cell lines [
26,
29,
30,
31]. For example, on human leukemia HL-60 cells, CAPE inhibits DNA, RNA, and protein synthesis with an IC
50 of 1.0 μM, 5.0 μM, and 1.5 μM, respectively [
32]. Moreover, CAPE induces anti-inflammatory and immunomodulatory responses. These activities have been explained by the activation of Nrf2 and the inhibition of NF-κB. Thus, CAPE is commercially available as an NF-κB inhibitor for in vitro and in vivo studies [
33].
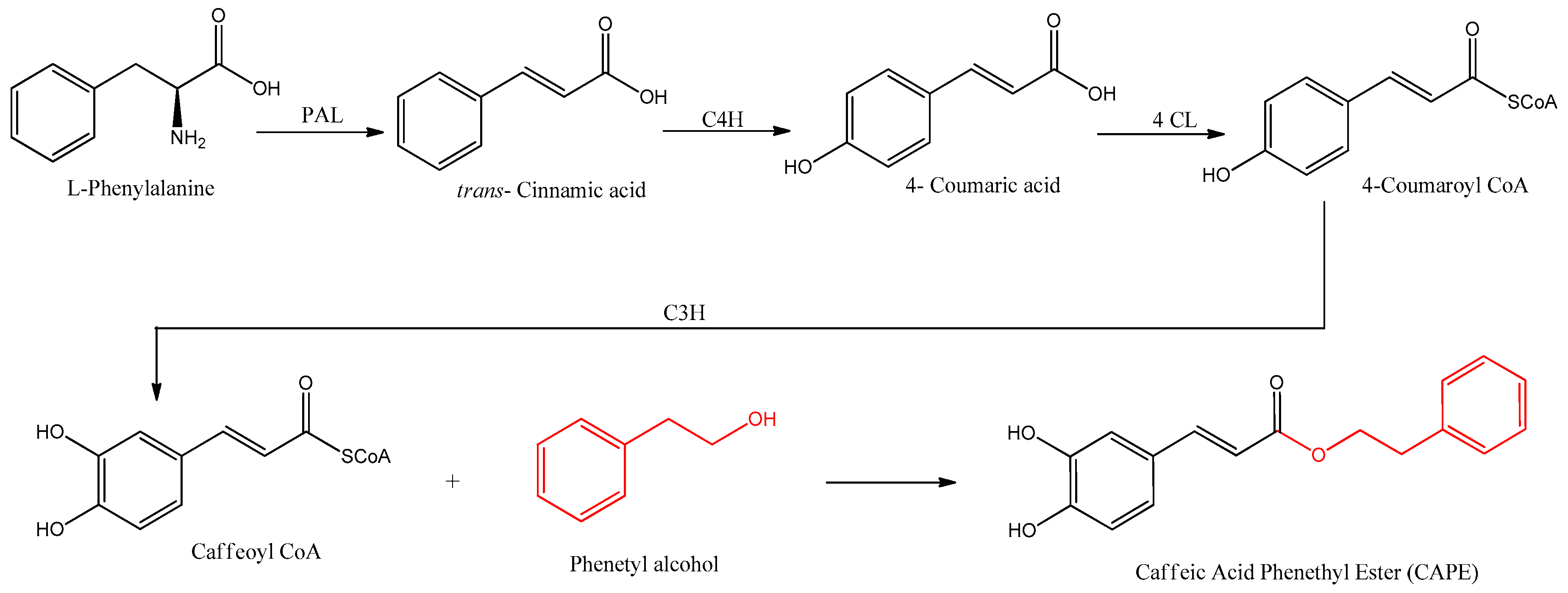
CAPE is naturally synthesized by the phenylpropanoid pathway, beginning with the amino acids phenylalanine or tyrosine, which produce 4-coumaric acid. The biosynthesis of CAPE is summarized in Figure 2.
Figure 2. Biosynthetic pathways for the formation of CAPE. The involved enzymes are abbreviated: PAL = phenylalanine ammonia lyase; C4H = cinnamate 4-hydroxilase; 4CL = 4-coumaric acid CoA-ligase; C3H = p-coumarate 3-hydroxylase.
Coenzyme A and 4-coumaric acid are bound by 4-coumaric acid CoA-ligase (4CL) enzymatic activity, producing 4-coumaroyl CoA with an activated carbonyl. This product is further meta hydroxylated by p-coumarate 3-hydroxylase, producing the intermediate caffeoyl CoA, which is the precursor of caffeic acid by hydrolysis of CoA. In the biosynthesis of CAPE, however, the CoA is substituted in one step with phenethyl alcohol by ester linkage. Phenethyl alcohol is synthesized by two enzymes, an aromatic amino acid decarboxylase such as phenylalanine decarboxylase (PDC), followed by a monoamine oxidase such as phenethylamine oxidase (PEO) [
34].
The absorption and metabolism of CAPE have not yet been well studied, but these processes should be like those of caffeic acid or related caffeic acid esters. The absorption and metabolism of the radiolabel [3-
14C] caffeic acid have been studied in rats, showing that absorption starts in the stomach after 1 h post-ingestion and is quickly absorbed in the first portion of the intestine, obtaining high concentrations in plasma after 2 h, and then is excreted mainly by urine as nine derivatives of type sulfonated and glucuronide, with no accumulation in tissues [
35]. CAPE is hydrolyzed after 6 h in rat plasma, producing caffeic acid by a carboxylesterase [
36].
Caffeic acid esters, such as chlorogenic and rosmarinic acid, have shown a similar pattern, being excreted by urine 4–8 h post-ingestion, with only 3.3 to 4.0% of total polyphenol content remaining [
35,
37]. The pharmacodynamic and pharmacokinetics of murine and human organisms are different, however. For instance, CAPE shows more stability in human than in rat plasma, where its concentration decreases quickly, producing caffeic acid and by-products, suggesting that CAPE is hydrolyzed by enzymes present in rat plasma but not in human [
36].