Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Leontina Elena Filipiuc.
The chemical constituents of the Cannabis plant known as cannabinoids have been extensively researched for their potential therapeutic benefits. The use of cannabinoids applied to the skin as a potential method for both skin-related benefits and systemic administration.
- skin endocannabinoid system
- natural cannabinoids
- phytocannabinoids
- delivery systems
1. Introduction
For over two millennia, the Cannabis plant has been used for both recreational and therapeutic purposes [1[1][2],2], with various archaeological discoveries confirming that it has been cultivated across most of the known world. Ancient Chinese medicine, for example, used the plant for musculoskeletal conditions and to relax and harmonize the mind and body [3], while the Greeks and Romans used it for recreational consumption and medical use [4]. The plant’s psychoactive and analgesic qualities were used by Indians to treat various types of pain [5,6][5][6]. In the first Chinese Pharmacopeia, Cannabis seeds were recommended for the treatment of skin diseases and disorders, such as eczema, psoriasis, and other inflammatory conditions [7]. Nowadays, due to the increasing interest in sustainability and ecological issues, the use of plant-based natural products in dermatology has become a valuable method for improving therapies with respect to acute and chronic skin diseases and disorders, with lower costs for patients and healthcare systems [7]. Cannabis, a representative genus of the Cannabaceae family [8], is an annual herbaceous plant, which includes the Cannabis sativa L. (hemp and marijuana), C. ruderalis Janisch, and C. indica Lam. species [9]. Cannabis is a genus characterized by dioecy [10], producing male and female inflorescences on different individuals that are obligatory out-crossers [11]. The plant is naturally occurring and is part of the local flora in the Indian Peninsula and Central Asia; however, in the rest of the world, it is cultivated for a variety of purposes. Cannabis is used in its unprocessed state as dried flower bulbs, known as marijuana, and as pieces of resin, known as hashish [12].
What makes each variety or strain of the Cannabis plant unique in its own way is the presence of three different classes of chemical compounds that exhibit biological activity: phytocannabinoids (pCBs), flavonoids, and terpenes/terpenoids [13]; they may act either individually and/or synergistically [14]. Depending on the plant content in each of these molecules, various modulatory or potentiation pharmacological effects have been observed [15,16][15][16]. A recent classification of Cannabis plant constituents identified 545 entities divided into chemical classes based on their structural similarities [17]. Thus, more than 100 Cannabis constituents belong to the pCB class, which is mostly extracted from female plants, with the most well-known being tetrahydrocannabinol (THC) with its two derivatives Δ8-tetrahydrocannabinol (Δ8-THC) and Δ9-tetrahydrocannabinol (Δ9-THC); cannabidiol (CBD); and cannabigerol (CBG) [18,19][18][19]. The rest of the natural compounds are classified into seven other main classes: cannabinol (CBN), cannabielsoin (CBE), cannabichromene (CBC), cannabicyclol (CBL), cannabinodiol (CBND), cannabitriol (CBT), and other types of cannabinoids [20].
The pharmacokinetics and metabolism of natural cannabinoids depend on the route of administration [13]. pCBs are known to have high lipophilicity, low aqueous solubility, rapid metabolism, poor bioavailability, and erratic pharmacokinetics [21], being temperature-, light-, and auto-oxidation-sensitive [22]. For example, the oral bioavailability of CBD ranges between 13% and 19% [23] due to its incomplete oral absorption and high hepatic clearance [2], while its bioavailability increases at 34–46% when it is absorbed intranasally [24]. Also, after topical in vivo gel applications, CBD provided significant plasma levels [24]. Consequently, pCB formulations emphasize a low oral bioavailability that led to the necessity of other administration routes to improve pCBs’ bioavailability and efficacy, such as the local (topical), transcutaneous (transdermal), pulmonary, and transmucosal routes [25] that include intranasal and rectal routes, with the last one demonstrating double the rate of bioavailability compared to the oral route [22]. Topical and transdermal administration demonstrated several advantages, such as a higher bioavailability rate, prolonged steady-state plasma concentration, and a reduction in psychoactive impacts of the drug due to its passive diffusion in the main barrier of the skin, stratum corneum [22]. To evaluate the efficacy of pCB topical applications, a plethora of studies emphasizing the mechanisms of action at the molecular level, redox transformation, electronic structure, and stability; and the cytotoxic, phototoxic, and UVA or UVB photoprotective effects of pCBs have been performed [26]. To emphasize the mechanisms of action of pCBs at the molecular level, it has been demonstrated that CBD inhibits nuclear factor kappa B (NF-κB) and the genes involved in the expression of molecules with pro-inflammatory roles, such as cytokines and metalloproteinases [27]. Clinical studies emphasized that topical treatment with CBD-enriched ointment significantly improved skin parameters and disease symptomatology, proving that the topical application is a safe and non-invasive alternative for improving the quality of life in patients with various skin disorders [28].
Cannabinoids work on the endocannabinoid system (ECS) [62][29]. The endocannabinoid receptors and their endogenous ligands were discovered and identified after the first compounds in the class were isolated and characterized. The endogenous endocannabinoid system (ECS) is represented by a molecular signaling network consisting of endocannabinoids, specific G-protein-coupled receptors (GPCRs), cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2), and specific enzymes responsible for the synthesis, transport, and degradation of endocannabinoids [59][30]. Various studies have also identified non-CB1/CB2 receptors modulated by endocannabinoids, such as GPCRs (GPR18, GPR55, and GPR119), transient potential vanilloid receptors (TRPV1 and TRPV3), transient receptor potential ankyrin 1 (TRPA1), nuclear hormone receptors like peroxisome proliferator-activated receptors (PPARs), and others [2,63][2][31]. ECS plays a critical role in a number of physiological processes, including immune modulation, vasomotion and vasodilation, motor control, muscle fiber formation, cognitive memory, learning, anxiety, appetite, gastro-intestinal motility, sleep, fertility, lipogenesis, and the formation of insulin and muscle fibers, as well as intestinal and bronchial motility [15,64,65,66,67,68][15][32][33][34][35][36]. It also plays a key role in pathological processes like pain, inflammation, and cancer [69,70,71][37][38][39]. Minor pCBs, such as CBG, CBC, THCV, and cannabigerolic acid (CBGA), exert their anti-inflammatory effect in human keratinocytes via the molecular deregulation of endocannabinoid (ECB) signaling and the mitogen-activated protein kinase (MAPK) pathway [29][40].
Cannabinoids’ beneficial or harmful effects are determined by how they act on the CB receptors and by their affinity for a particular receptor. The cannabinoids can also interact with a multitude of other receptors, which explains the variety of effects encountered in these compounds [20]. Being fully represented in the skin, the ECS contributes significantly to the body’s homeostasis [59][30]. By controlling, among other things, cell growth, differentiation, and survival; immune and inflammatory responses; and sensory phenomena, the ECS is also involved in the physiology and pathology of skin functions [72][41].
The skin is an accessible organ for the non-invasive or minimally invasive administration of medications [73][42]. However, the variability in skin permeability remains a challenge in optimizing transdermal drug delivery that targets the epidermis, dermis, deeper tissues, and systemic circulation [74][43]. Hence, the skin can be approached from both points of view as a therapeutic target and as a route of systemic administration.
The skin has the advantage of being an easy and convenient route of administration for a wide range of active ingredients, including natural or synthetic cannabinoids. Various techniques for the enhancement of skin permeabilization are available for the improvement of bioavailability and the efficacy of therapeutic cannabinoids, such as the use of organosilane particles as transdermal delivery vehicles [75][44], oleic acid and ethanol as good chemical enhancers, ethosomes and nanocryogels as controlled release strategies, and microneedle arrays for both the topical and transdermal delivery of incorporated cannabinoids [76][45]. Topical applications of cannabinoids provide therapeutic benefits for the patient in multiple pathologies like psoriasis, eczema, and atopic dermatitis, as shown in Figure 1. These advantages are obtained due to the remarkable role of the ECS in improving dermatological conditions symptoms such as itching, inflammation, and pain.
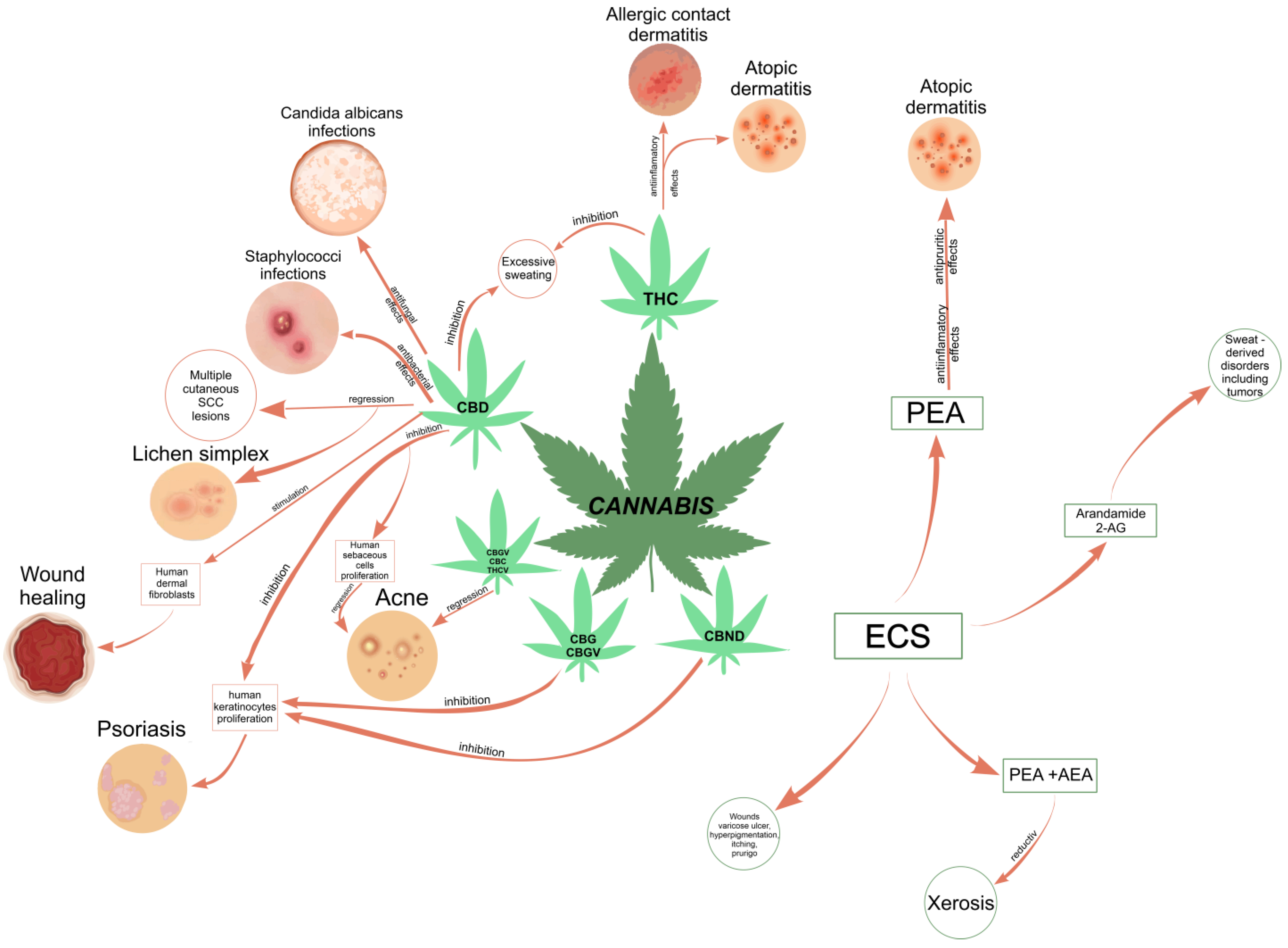
Figure 1.
Correlation diagram between the most common phytocannabinoids and the skin conditions they could treat
[1]
.
Complementary and alternative medicine (CM and CAM) therapies have an increasing presence in dermatology [77][46], gaining popularity among patients in recent years. Natural remedies are seen as safer than conventional medications, with few if any notable adverse effects. However, dermatologists are wary of prescribing cannabinoid-based remedies due to the lack of studies regarding the formulation and standardization of extracts and safety profiles [78][47]. There are a few reported clinical cases of allergies related to topical cannabis contact, such as pruritus, urticaria, or angioedema, which were confirmed via specific allergy tests [79][48]. Similar o many other natural products or extracts, cannabis-based medicines have the potential to cause skin allergies in some individuals. However, thanks to advances in formulation techniques, exposure to some cannabis plant constituents may be significantly reduced.
32. Transdermal Delivery of Cannabinoids
The undeniable development with respect to increasing cannabinoid bioavailability, whether natural or synthetic, has increased their potential of being introduced into therapy, with the ultimate objective of increasing bioavailability and efficacy and lowering toxicity and/or adverse effects. Cannabinoid study has become a mainstream discipline as a result of the growing interest in the use of cannabinoids in pharmaceutical formulations, which show promise in the treatment of a number of diseases. These studies revealed a couple of key concepts that need to be considered when designing or optimizing novel pharmaceutical formulations with the purpose of enhancing cannabinoid transport. These concepts will be further discussed in more detail. Using cannabinoid compounds with no affinity for CB1 receptors from CNS (central nervous system), which drastically limits the selection of effective solutions, or formulating them so that they cannot cross the BBB are two practical ways to avoid the psychotropic action of cannabinoid compounds. Another interesting perspective in order to avoid the psychotropic effects of common cannabinoids is represented by the design and synthesis of dualsteric/bitopic ligands that are able to selectively target CB2 receptors versus CB1, as Gado et al. reported [226][49]. When released into the bloodstream, cannabinoids have the ability to cross the BBB, where they will exert their undesirable effects on the CNS. The topical formulations of such substances may thus offer a unique alternative to avoid their binding to CB1 receptors at the central level by employing the structures of the epidermis as a reservoir for cannabinoids. This paradigm is obviously particularly important, as it prevents the rapid release of a large amount of substance into the bloodstream with the detrimental consequences already mentioned, but it is equally important to develop formulations that enable the controlled release of the active ingredient from these deposits as a way to extend the duration of the expected effect. Pharmaceutical formulations for external application need to ensure effective transdermal delivery of the therapeutically relevant substance. In this regard, the oil–water partition coefficient, logP, which describes the degree of lipophilicity of a molecule or molecular assembly [227[50][51],228], is of particular interest, along with other physicochemical properties, including the melting point, aqueous solubility, pKa, and molecular weight (Mw). In order to be able to penetrate through the skin’s layers, particularly the SC, the optimal log P value of a chemical entity should be within the range of 1–3 [229,230][52][53]. Consequently, transdermal formulations of cannabinoids should reduce their log P from values of 6–7 to those within the optimum range. The ongoing research with respect to obtaining appropriate solutions for the use of cannabinoids for therapeutic purposes has resulted in the development of a wide range of formulations, with each one having its advantages and disadvantages. The most promising outcomes from the perspective of therapeutic efficacy which don’t cause significant discomfort for the patient and at a competitive production price, should serve as a basis for the further development of technologies envisioned for introduction the of new cannabinoid therapeutic agents into the clinic. Nanotechnology allowed the encapsulation of cannabinoids in nanocarriers, improving their physical–chemical stability and bioavailability while also preventing degradation. In this regard, several nanotechnology-based formulation strategies have been developed, of which lipid-based nanoparticles have proven to be the most attractive for transdermal delivery. As shown in Figure 2, such formulations can be tailored in various approaches, and these range from simple micro- or nanoemulsions to complex architectures, such as liposomes, solid lipid nanoparticles (SLNs), lipid nanocapsules (LNCs), or self-emulsifying drug delivery systems (SEDDS) [22,231][22][54]. Despite the fact that they exhibit obvious benefits over conventional topical formulations, typical liposomes are believed to be unsuitable as transdermal delivery systems of drugs. Unmodified liposomes have been retained in the SC because of their rigid structure, which prevents diffusion into the deeper skin layers. In addition, the use of regular liposomes is limited by their poor stability, poor encapsulation efficiency, and decreased loading capacity [232][55]. To develop an innovative class of liposomal formulations with enhanced properties, several approaches have therefore been evolved, with one of them being focused on deformable liposomal formulations including transfersomes, ethosomes, invasomes, mentosomes, and niosomes. Ogunsola and colab. have depicted a method for the preparation and characterization of flexible liposomes, also named transfersomes, and they are able to pass through the small intercellular spaces between SC cells (20–40 nm) [233][56]. This study has established the dependence between the degree of skin permeability and the ratio between phosphatidylcholine (PC) and Tween 80 (a non-ionic surfactant), the main constituents in the composition of the liposomes prepared by the research group. To be included in transdermal formulations, future research should focus on optimizing the preparation method of this type of liposomes to counteract their main weaknesses, such as instability against oxidative degradation [234][57].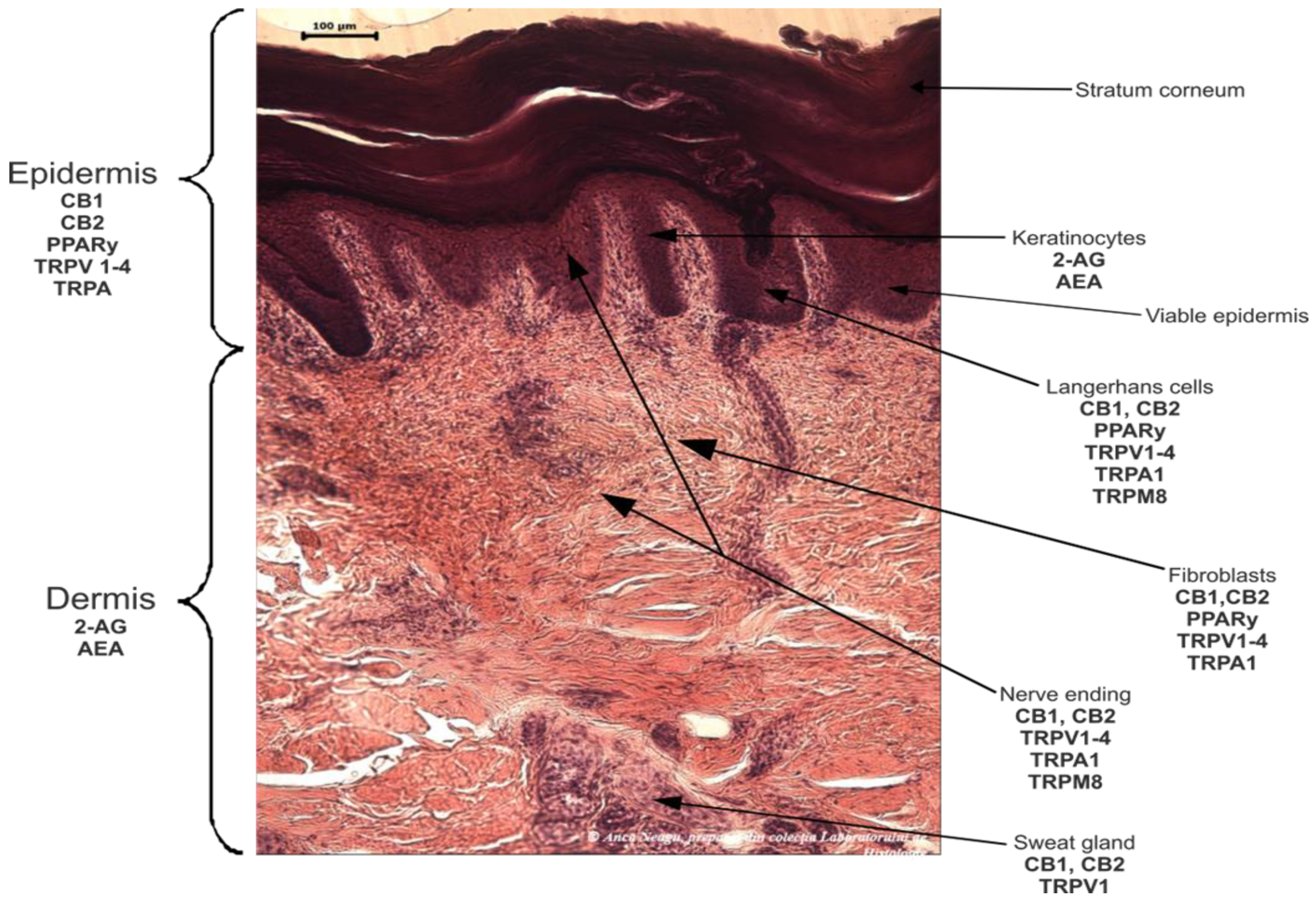
Figure 2. The passage of substances through the layers of the skin depending on the way they are formulated. SLN, solid lipid nanoparticles; NLC, nanostructured lipid carriers.
Sermsaksasithorn and colab. have approached the transdermal administration of CBD from Cannabis sativa with the help of a transdermal patch, aiming to alleviate the effect on psoriasis, within a randomized double-blind controlled trial [235][58]. The authors thought out the benefits of employing transdermal patches, which are similar to those of any other transdermal delivery system; however, the patches also provide better dose control. The most suitable substances for such delivery systems are lipophilic molecules with low molecular weight, and they also have lower melting points and higher volatility, characteristics associated with excellent skin penetration and relatively simple formulation [236][59]. Isaac and Holvey emphasized the impact of using transde rmal patches in psychiatric medication, through which the legal concerns of the use of drugs with psychotropic action are overcome, preventing the possibility of drug abuse and facilitating the least restrictive affordable administration of prescribed medication as well. From an economic point of view, the cost of a transdermal formulation, particularly in the form of patches, has always been a subject of debate as the costs are much higher compared to the same treatment administered orally.
Nevertheless, the formulation of cannabinoids with the help of nanotechnology, along with many other drugs, is in continuous progress, allowing unique solutions to numerous challenges that would otherwise have remained unsolved. Future strategies in the field should provide at the same time accessible, reproducible, and cost-reduced solutions.
References
- Silviu-Iulian Filipiuc; Anca-Narcisa Neagu; Cristina Mariana Uritu; Bogdan-Ionel Tamba; Leontina-Elena Filipiuc; Ivona Maria Tudorancea; Andreea Nicoleta Boca; Mădălina Florina Hâncu; Vlad Porumb; Walther Bild; et al. The Skin and Natural Cannabinoids–Topical and Transdermal Applications. Pharm. 2023, 16, 1049.Abel, E.L.; Emboden, W. Marihuana. The First Twelve Thousand Years. Ernest L. Abel. Q. Rev. Biol. 1981, 56, 514.
- Filipiuc, L.E.; Ababei, D.C.; Alexa-Stratulat, T.; Pricope, C.V.; Bild, V.; Stefanescu, R.; Stanciu, G.D.; Tamba, B.-I. Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics 2021, 13, 1823.
- Johal, H.; Vannabouathong, C.; Chang, Y.; Zhu, M.; Bhandari, M. Medical cannabis for orthopaedic patients with chronic musculoskeletal pain: Does evidence support its use? Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X2093796.
- Butrica, J.L. The Medical Use of Cannabis Among the Greeks and Romans. J. Cannabis Ther. 2002, 2, 51–70.
- Turgeman, I.; Bar-Sela, G. Cannabis for cancer–illusion or the tip of an iceberg: A review of the evidence for the use of Cannabis and synthetic cannabinoids in oncology. Expert Opin. Investig. Drugs 2019, 28, 285–296.
- Zuardi, A.W. History of cannabis as a medicine: A review. Rev. Bras. Psiquiatr. 2006, 28, 153–157.
- Martins, A.; Gomes, A.; Boas, I.; Marto, J.; Ribeiro, H. Cannabis-Based Products for the Treatment of Skin Inflammatory Diseases: A Timely Review. Pharmaceuticals 2022, 15, 210.
- McPartland, J.M. Cannabis Systematics at the Levels of Family, Genus, and Species. Cannabis Cannabinoid Res. 2018, 3, 203–212.
- Gloss, D. An Overview of Products and Bias in Research. Neurotherapeutics 2015, 12, 731–734.
- Pollio, A. The Name of Cannabis: A Short Guide for Nonbotanists. Cannabis Cannabinoid Res. 2016, 1, 234–238.
- Punja, Z.K.; Holmes, J.E. Hermaphroditism in Marijuana (Cannabis sativa L.) Inflorescences—Impact on Floral Morphology, Seed Formation, Progeny Sex Ratios, and Genetic Variation. Front. Plant Sci. 2020, 11, 718.
- Farag, S.; Kayser, O. The Cannabis Plant: Botanical Aspects. In Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 3–12. ISBN 9780128008270.
- Chayasirisobhon, S. Mechanisms of Action and Pharmacokinetics of Cannabis. Perm. J. 2021, 25, 1–3.
- Milay, L.; Berman, P.; Shapira, A.; Guberman, O.; Meiri, D. Metabolic Profiling of Cannabis Secondary Metabolites for Evaluation of Optimal Postharvest Storage Conditions. Front. Plant Sci. 2020, 11, 583605.
- Tamba, B.I.; Stanciu, G.D.; Urîtu, C.M.; Rezus, E.; Stefanescu, R.; Mihai, C.T.; Luca, A.; Rusu-zota, G.; Leon-constantin, M.M.; Cojocaru, E.; et al. Challenges and opportunities in preclinical research of synthetic cannabinoids for pain therapy. Medicina 2020, 56, 24.
- Russo, E.B. History of Cannabis and Its Preparations in Saga, Science, and Sobriquet. Chem. Biodivers. 2007, 4, 1614–1648.
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31.
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2006, 147, 163–171.
- Curran, H.V.; Freeman, T.P.; Mokrysz, C.; Lewis, D.A.; Morgan, C.J.A.; Parsons, L.H. Keep off the grass? Cannabis, cognition and addiction. Nat. Rev. Neurosci. 2016, 17, 293–306.
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Switzerland, 2017; Volume 103, pp. 1–36.
- Palrasu, M.; Wright, L.; Patel, M.; Leech, L.; Branch, S.; Harrelson, S.; Khan, S. Perspectives on Challenges in Cannabis Drug Delivery Systems: Where Are We? Med. Cannabis Cannabinoids 2022, 5, 102–119.
- Mahmoudinoodezh, H.; Telukutla, S.R.; Bhangu, S.K.; Bachari, A.; Cavalieri, F.; Mantri, N. The Transdermal Delivery of Therapeutic Cannabinoids. Pharmaceutics 2022, 14, 438.
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An Overview of Some Pharmacological Aspects. J. Clin. Pharmacol. 2002, 42, 11S–19S.
- Paudel, K.S.; Hammell, D.C.; Agu, R.U.; Valiveti, S.; Stinchcomb, A.L. Cannabidiol bioavailability after nasal and transdermal application: Effect of permeation enhancers. Drug Dev. Ind. Pharm. 2010, 36, 1088–1097.
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 2018, 23, 2478.
- Vacek, J.; Vostalova, J.; Papouskova, B.; Skarupova, D.; Kos, M.; Kabelac, M.; Storch, J. Antioxidant function of phytocannabinoids: Molecular basis of their stability and cytoprotective properties under UV-irradiation. Free Radic. Biol. Med. 2021, 164, 258–270.
- Jîtcă, G.; Ősz, B.E.; Vari, C.E.; Rusz, C.-M.; Tero-Vescan, A.; Pușcaș, A. Cannabidiol: Bridge between Antioxidant Effect, Cellular Protection, and Cognitive and Physical Performance. Antioxidants 2023, 12, 485.
- Palmieri, B.; Laurino, C.; Vadalà, M. A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars. Clin. Ther. 2019, 170, e93–e99.
- Makhakhe, L. Topical cannabidiol (CBD) in skin pathology—A comprehensive review and prospects for new therapeutic opportunities. S. Afr. Fam. Pract. 2022, 64, 4.
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942.
- Irving, A.; Abdulrazzaq, G.; Chan, S.L.F.; Penman, J.; Harvey, J.; Alexander, S.P.H. Cannabinoid Receptor-Related Orphan G Protein-Coupled Receptors. Adv. Pharmacol. 2017, 80, 223–247.
- Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Della Pina, S.; Tambaro, S.; Memo, M.; Mastinu, A. Cannabidiol: Recent advances and new insights for neuropsychiatric disorders treatment. Life Sci. 2019, 224, 120–127.
- Taylor, A.H.; Ang, C.; Bell, S.C.; Konje, J.C. The role of the endocannabinoid system in gametogenesis, implantation and early pregnancy. Hum. Reprod. Update 2007, 13, 501–513.
- Gillies, R.; Lee, K.; Vanin, S.; Laviolette, S.R.; Holloway, A.C.; Arany, E.; Hardy, D.B. Maternal exposure to Δ9-tetrahydrocannabinol impairs female offspring glucose homeostasis and endocrine pancreatic development in the rat. Reprod. Toxicol. 2020, 94, 84–91.
- González-Mariscal, I.; Krzysik-Walker, S.M.; Doyle, M.E.; Liu, Q.R.; Cimbro, R.; Santa-Cruz Calvo, S.; Ghosh, S.; Cieala, A.; Moaddel, R.; Carlson, O.D.; et al. Human CB1 Receptor Isoforms, present in Hepatocytes and β-cells, are Involved in Regulating Metabolism. Sci. Rep. 2016, 6, 33302.
- Joshi, N.; Onaivi, E.S. Endocannabinoid System Components: Overview and Tissue Distribution. Adv. Exp. Med. Biol. 2019, 1162, 1–12.
- Deiana, S. Potential Medical Uses of Cannabigerol: A Brief Overview. In Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 958–967. ISBN 9780128008270.
- Bodine, M.; Kemp, A.K. Medical Cannabis Use in Oncology; StatPearls: Treasure Island, FL, USA, 2022.
- Donvito, G.; Nass, S.R.; Wilkerson, J.L.; Curry, Z.A.; Schurman, L.D.; Kinsey, S.G.; Lichtman, A.H. The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain. Neuropsychopharmacology 2018, 43, 52–79.
- Tortolani, D.; Di Meo, C.; Standoli, S.; Ciaramellano, F.; Kadhim, S.; Hsu, E.; Rapino, C.; Maccarrone, M. Rare Phytocannabinoids Exert Anti-Inflammatory Effects on Human Keratinocytes via the Endocannabinoid System and MAPK Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 2721.
- del Río, C.; Millán, E.; García, V.; Appendino, G.; DeMesa, J.; Muñoz, E. The endocannabinoid system of the skin. A potential approach for the treatment of skin disorders. Biochem. Pharmacol. 2018, 157, 122–133.
- Alkilani, A.; McCrudden, M.T.; Donnelly, R. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470.
- Roberts, M.S. Targeted drug delivery to the skin and deeper tissues: Role of physiology, solute structure and disease. Clin. Exp. Pharmacol. Physiol. 1997, 24, 874–879.
- Khabir, Z.; Partalis, C.; Panchal, J.V.; Deva, A.; Khatri, A.; Garcia-Bennett, A. Enhanced Skin Penetration of Cannabidiol Using Organosilane Particles as Transdermal Delivery Vehicles. Pharmaceutics 2023, 15, 798.
- Tijani, A.O.; Thakur, D.; Mishra, D.; Frempong, D.; Chukwunyere, U.I.; Puri, A. Delivering therapeutic cannabinoids via skin: Current state and future perspectives. J. Control. Release 2021, 334, 427–451.
- Landis, E.; Davis, S.; Feldman, S.; Taylor, S. Complementary and Alternative Medicine Use in Dermatology in the United States. J. Altern. Complement. Med. 2014, 20, 392–398.
- Nickles, M.; Lio, P. Cannabinoids in Dermatology: Hope or Hype? Cannabis Cannabinoid Res. 2020, 5, 279–282.
- Shao, K.; Stewart, C.; Grant-Kels, J.M. Cannabis and the skin. Clin. Dermatol. 2021, 39, 784–795.
- Gado, F.; Ferrisi, R.; Polini, B.; Mohamed, K.A.; Ricardi, C.; Lucarini, E.; Carpi, S.; Domenichini, F.; Stevenson, L.A.; Rapposelli, S.; et al. Design, Synthesis, and Biological Activity of New CB2 Receptor Ligands: From Orthosteric and Allosteric Modulators to Dualsteric/Bitopic Ligands. J. Med. Chem. 2022, 65, 9918–9938.
- Chen, X.; Zhu, L.; Li, R.; Pang, L.; Zhu, S.; Ma, J.; Du, L.; Jin, Y. Electroporation-enhanced transdermal drug delivery: Effects of logP, pKa, solubility and penetration time. Eur. J. Pharm. Sci. 2020, 151, 105410.
- Uritu, C.M.; Calin, M.; Maier, S.S.; Cojocaru, C.; Nicolescu, A.; Peptanariu, D.; Constantinescu, C.A.; Stan, D.; Barboiu, M.; Pinteala, M. Flexible cyclic siloxane core enhances the transfection efficiency of polyethylenimine-based non-viral gene vectors. J. Mater. Chem. B 2015, 3, 8250–8267.
- N’Da, D. Prodrug Strategies for Enhancing the Percutaneous Absorption of Drugs. Molecules 2014, 19, 20780–20807.
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and Transdermal Drug Delivery Systems: Current and Future Prospects. Drug Deliv. 2006, 13, 175–187.
- Reddy, T.S.; Zomer, R.; Mantri, N. Nanoformulations as a strategy to overcome the delivery limitations of cannabinoids. Phyther. Res. 2023, 37, 1526–1538.
- Kuznetsova, D.A.; Vasilieva, E.A.; Kuznetsov, D.M.; Lenina, O.A.; Filippov, S.K.; Petrov, K.A.; Zakharova, L.Y.; Sinyashin, O.G. Enhancement of the Transdermal Delivery of Nonsteroidal Anti-inflammatory Drugs Using Liposomes Containing Cationic Surfactants. ACS Omega 2022, 7, 25741–25750.
- Ogunsola, O.A.; Kraeling, M.E.; Zhong, S.; Pochan, D.J.; Bronaugh, R.L.; Raghavan, S.R. Structural analysis of “flexible” liposome formulations: New insights into the skin-penetrating ability of soft nanostructures. Soft Matter 2012, 8, 10226.
- Kumar, A. Transferosome: A Recent Approach for Transdermal Drug Delivery. J. Drug Deliv. Ther. 2018, 8, 100–104.
- Sermsaksasithorn, P.; Asawanonda, P.; Phutrakool, P.; Ondee, T.; Payungporn, S.; Pongpirul, K.; Hirankarn, N.; Medicine, S.; Management, C.D.; Botany, P.; et al. Efficacy and Safety of Cannabis Transdermal Patch for Alleviating Psoriasis Symptoms: Protocol for a Randomized Controlled Trial (CanPatch). medRxiv 2023, 1–15.
- Bunge, A.; Vecchia, B. Evaluating the Transdermal Permeability of Chemicals. In Transdermal Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 2002.
More
