Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Ana Novo Barros.
Chlorophylls play a crucial role in photosynthesis and are abundantly found in green fruits and vegetables that form an integral part of our diet. Although limited, existing studies suggest that these photosynthetic pigments and their derivatives possess therapeutic properties. These bioactive molecules exhibit a wide range of beneficial effects, including antioxidant, antimutagenic, antigenotoxic, anti-cancer, and anti-obesogenic activities.
- chlorophylls
- chlorophyllin
- health
- biological activity
1. Introduction
Chlorophyll, Figure 1, a complex green pigment found in plants, algae, and certain bacteria, plays a crucial role in the process of photosynthesis by absorbing light energy and converting it into chemical energy [1]. While early beliefs about the bioavailability and stability of chlorophyll under differaent conditions limited research on its health effects, recent studies have shed light on the potential benefits of chlorophyllin as a chemopreventive agent [2]. Nagini et al. have provided insights into its molecular mechanisms [2]. Although in vitro and in vivo studies suggest its anticancer effects, evidence of its efficacy in humans remains scarce [2]. Dietary supplements containing chlorophyll and chlorophyllin are available and generally considered safe, with no reported adverse side effects over several decades of human use [3]. However, skepticism about their effectiveness persists due to the lack of robust scientific evidence supporting their claimed health benefits [3].
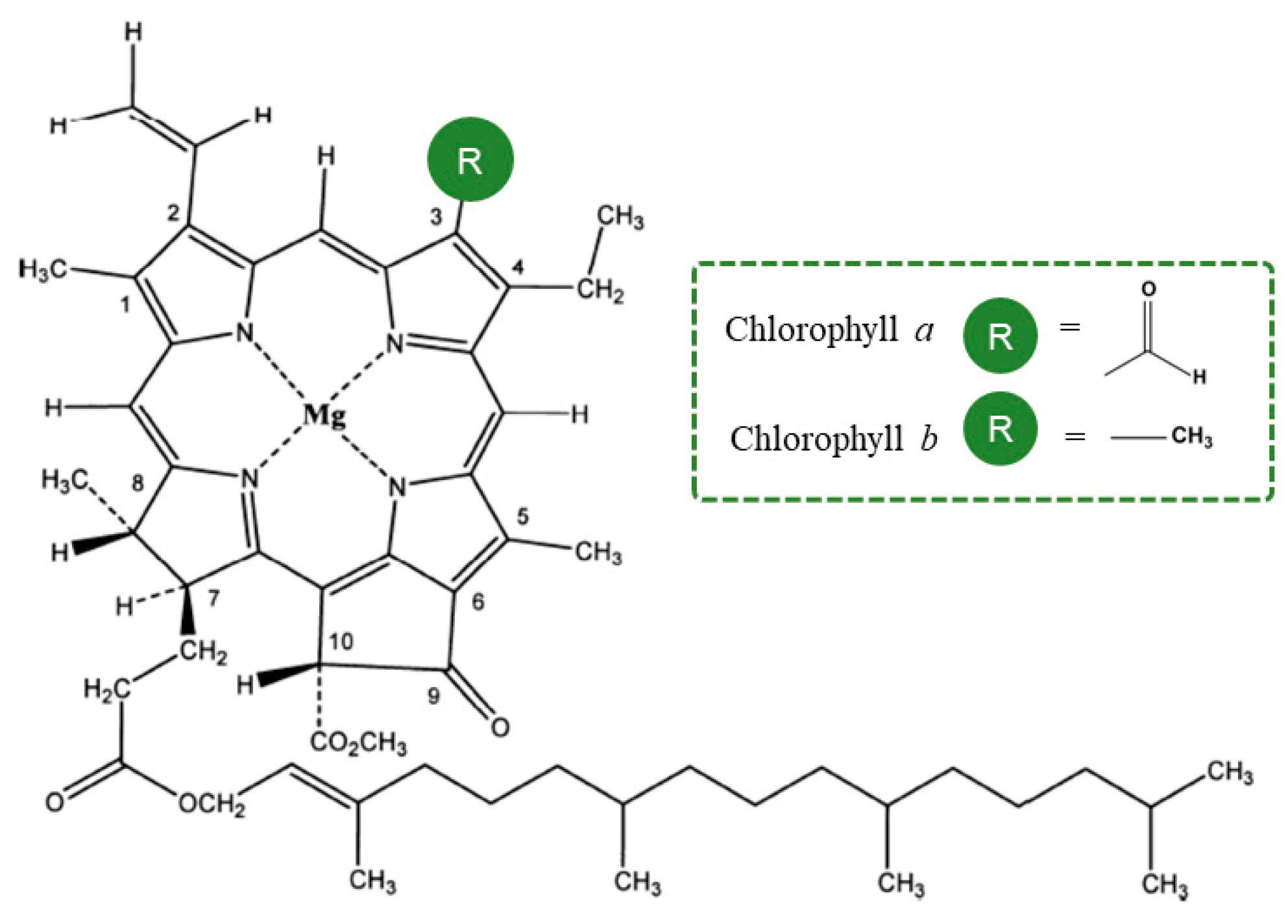
Figure 1.
Structure from Chlorophyll
a
and Chlorophyll
b
.
Despite the potential health benefits associated with chlorophyll, a significant number of chlorophyll-rich vegetables, leafy materials, and fruits are lost throughout the food supply chain [4]. This loss occurs despite the underutilized potential of these agro-food residues [5,6][5][6]. Harnessing and utilizing this discarded material could contribute to the transition towards a more sustainable circular economy.
2. Chlorophyll: Chemical Properties and Metabolism
Chlorophyll is a complex molecule made up of a porphyrin ring, a magnesium ion, and an attached hydrocarbon tail. The porphyrin ring is responsible for absorbing light energy and the magnesium ion acts as an electron acceptor. Chlorophyll has many forms such as chlorophyll a, chlorophyll b, chlorophyll c, chlorophyll d and chlorophyll e [1]. The most common form of chlorophyll found in plants is chlorophyll a. Its chemical structure includes a porphyrin ring with a central magnesium ion, and an attached hydrocarbon tail known as a phytol. The porphyrin ring is made up of four nitrogen-containing groups called pyrrole, and the phytol tail is composed of isoprenoid units [7]. Chlorophyll a absorbs light most efficiently in the red and blue regions of the spectrum, with peak absorption at around 430 and 662 nanometers, respectively. Chlorophyll b is another form of chlorophyll found in plants, algae, and some bacteria. Its chemical structure is similar to that of chlorophyll a, but it has a slightly different porphyrin ring. This difference results in chlorophyll b absorbing light in the blue-green region of the spectrum, with peak absorption at around 453 nanometers. Chlorophyll b also has a role in photosynthesis, but its main function is to protect chlorophyll a from excess light. In addition to chlorophyll a and b, there are other forms of chlorophyll such as chlorophyll c, chlorophyll d, and chlorophyll e. These are found in a variety of organisms such as algae, and they have different absorption spectra and different functions. Chlorophyll c absorbs light in the blue-green region of the spectrum, chlorophyll d absorbs light in the red region of the spectrum, and chlorophyll e absorbs light in the far-red region of the spectrum [7]. Photosynthesis is the process by which plants, algae, and some bacteria convert light energy into chemical energy. The light energy is absorbed by chlorophyll and other pigments, which excite electrons in the pigment molecules. After absorbing light energy, the excited electrons in chlorophyll are utilized to facilitate the synthesis of ATP (adenosine triphosphate) and NADPH (nicotinamide adenine dinucleotide phosphate), which are essential components for the subsequent phases of photosynthesis. These energy-rich molecules play a crucial role in the production of glucose and the release of oxygen as byproducts. The first stage of photosynthesis is known as the light-dependent reactions which take place in the thylakoid membrane of chloroplasts. The light energy absorbed by chlorophyll and other pigments is used to drive the transfer of electrons, which results in the production of ATP and NADPH. The second stage of photosynthesis is known as the light-independent reactions, also called the Calvin cycle, which takes place in the stroma of chloroplasts. In this stage, the ATP and NADPH produced in the light-dependent reactions are used to drive the production of glucose and oxygen [8]. Chlorophyll, chlorophyllides, and phycobiliproteins such as phycoerythrin and phycocyanin, are all pigments that are involved in the process of photosynthesis. Chlorophyll is the primary pigment found in plants and algae, while chlorophyllides and phycobiliproteins are found in smaller quantities. The main difference between these pigments is their chemical structure, which results in different absorption spectra and therefore different functions in photosynthesis. The process of obtaining pheophorbides from chlorophyll is called chlorophyll degradation, which is a process that occurs naturally in plants and algae. This process can be triggered by different environmental factors such as light intensity, temperature, and water availability. During chlorophyll degradation, chlorophyll is broken down into different pigments, including pheophytin, which is a form of chlorophyll that lacks a magnesium ion, and pheophorbide, which is a form of chlorophyll that has been modified by the removal of the phytol tail [8]. Chlorophyllides are pigments that are closely related to chlorophyll, and they differ in the arrangement of atoms. Their chemical structure is similar to chlorophyll but they have a different central atom such as zinc, iron, or copper, and they have different absorption spectra. They are found in prokaryotic organisms such as cyanobacteria and they have a role in photosynthesis similar to chlorophyll [9], Figure 2.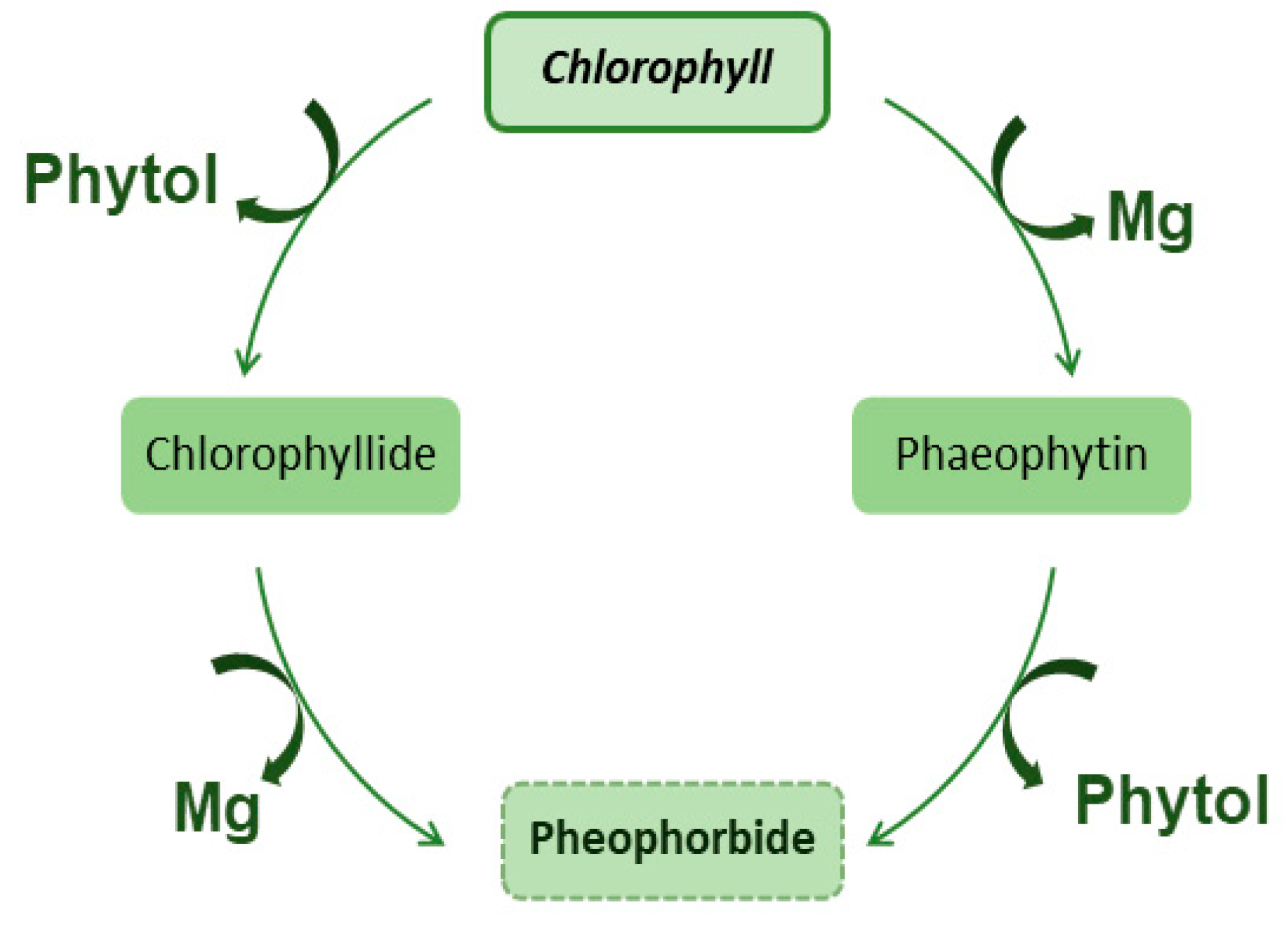
Figure 2.
Conversion of Chlorophyll in phaeophytin, chlorophyllide and pheophorbide.
3. Effects of Chlorophyll in Health
3.1. Historic Perspective/Herbal Ethnomedicines
The utilization of medicinal plants has a long history, dating back to ancient times, with its practice spanning across different regions worldwide [11,12][11][12]. Even in the present era, herbal ethnomedicines continue to be of fundamental importance in primary healthcare, particularly for impoverished populations in remote areas [13] and developing countries [14]. These medicinal plants are employed to address a wide array of illnesses, including cancer, skin diseases, cardiovascular disorders, endocrinal imbalances, gastrointestinal ailments, genitourinary conditions, respiratory system disorders, musculoskeletal disorders, liver diseases, and even treatment for poisonous bites, among others [12,13,15,16,17,18][12][13][15][16][17][18]. While the therapeutic effects of medicinal plants are often attributed to their secondary metabolites [18,19,20[18][19][20][21],21], it is worth noting that photosynthetic pigments may also play a significant role [5[5][6],6], with chlorophyll being the most well-known pigment associated with these plants. Among the various plant parts used for ethnomedicinal purposes, leaves stand out as the most utilized [22,23,24,25,26][22][23][24][25][26]. Leaves possess several advantages, such as easy accessibility, straightforward processing, prolonged availability, and therapeutic properties resulting from the accumulation of photosynthates and phytochemicals [18,20,24,27][18][20][24][27]. Notably, harvesting leaves does not pose a threat to the survival of the plant, unlike the harvesting of whole plants or roots, which can endanger the survival of medicinal plant species and contribute to a decline in plant biodiversity within specific regions [22,23][22][23].3.2. Chlorophyll Bioavailability
Chlorophylls, the most abundant pigments on Earth, are present in photosynthetic organisms such as bacteria, algae, and higher plants. In plants, chlorophyll a and b are the predominant pigments, with their ratio varying depending on the species, environmental conditions, and ripening stage [6]. However, chlorophylls are highly sensitive to physical and chemical changes and exhibit instability when isolated or consumed outside of their biological context. This has led to the development of chlorophyll derivatives [28]. Consequently, natural chlorophylls are not commonly used in experimental research, as their purification is challenging and expensive. Semi-synthetic sodium copper-chlorophyllins (SCC) have been commercially available as food colorants and supplements. SCC is produced through the saponification of natural chlorophylls, where the magnesium ion in the tetrapyrrole is substituted by copper and the hydrophobic side chain is eliminated. These modifications enhance the stability, solubility in water, and accessibility of SCC [29,30][29][30]. Commercial SCC products may vary in composition, although two primary components, Cu-chlorin e4 and Cu-chlorin e6, are typically present [31,32,33][31][32][33]. Due to these advantageous properties, SCC has found widespread use in biological experiments. However, it is important to note that the bioavailability of SCC may not match that of natural chlorophylls obtained from our diet. Chlorophylls can be obtained from the human diet through the consumption of green fruits and vegetables. However, their content varies significantly depending on factors such as cultivar, maturity stage, growing conditions, harvest time, plant parts used, storage conditions, food processing methods, and extraction and quantification techniques [6,28][6][28]. While a diet rich in vegetables and green fruits may provide a substantial amount of chlorophylls, their bioavailability, metabolism, and the effects of food processing influence their potential impact on human health. Early studies assumed that humans did not absorb chlorophylls, resulting in limited research on their absorption through the gastrointestinal tract. However, a few studies have demonstrated that native chlorophylls undergo significant transformation during the digestive process, and the absorption of different chlorophyll derivatives may differ based on their molecular structure. In vitro studies have provided valuable insights into the digestion, metabolism, and absorption of natural chlorophylls. Using an in vitro model simulating the gastric and small intestinal digestive processes, Ferruzi et al. [34] demonstrated that native chlorophylls obtained from fresh spinach puree undergo various transformations. The highly acidic gastric phase led to the conversion of chlorophylls into their metal-free pheophytin derivatives, while Zn-pheophytins treated with ZnCl2 remained stable. Furthermore, the micellarization of chlorophyll a series was found to be more efficient compared to the b series. The uptake of micellarized chlorophyll derivatives by Caco-2 human intestinal cells predominantly consisted of pheophytins and their epimers, comprising around 5–10% of the absorbed compounds. Another study utilizing the same in vitro approach revealed that Cu-chlorin e4 in SCC remained stable during digestion, whereas 90% of Cu-chlorin e6 underwent degradation. However, incorporating SCC into a food matrix reduced the degradation of Cu-chlorin e6 [32]. Additionally, SCC derivatives were taken up by Caco-2 cells and transported to the basolateral compartment, suggesting their potential absorption and transport to peripheral tissues [32,34][32][34]. Furthermore, a study using chlorophylls from pea puree demonstrated that native chlorophylls were completely transformed into their magnesium-free derivatives during gastric digestion in vitro, primarily due to the acidic conditions [35]. Pheophorbide a, the most micellarized chlorophyll derivative, exhibited the highest absorption by Caco-2 cells [35]. Another investigation by Gandul-Rojas et al. [36], using standard pigments such as chlorophyll a, chlorophyll b, pheophytin a, pheophytin b, pyropheophytin a, pheophorbide a, and pyropheophorbide a, indicated that the de-esterification of the phytol group enhanced the transfer efficiency of dephytylated derivatives (pheophorbide a and pyropheophorbide a) to the aqueous micellar fraction and their transport to Caco-2 intestinal cells, thereby increasing their bioaccessibility. Moreover, the cellular uptake of phytylated chlorophyll derivatives (pheophytin a and pyropheophytin a) by Caco-2 cells occurred through simple diffusion, whereas the uptake of dephytylated derivatives was mediated by facilitated diffusion at lower concentrations tested [36]. A recent study investigated the in vitro digestion of chlorophyll pigments derived from three types of edible dried seaweeds: Nori (red algae, containing only chlorophyll a series), Sea lettuce (green algae, containing a and b series), and Kombu (brown algae, presenting a and c series). The findings revealed that chlorophylls from a series were more susceptible to pheophytinization compared to the b and c series. The formation of pheophorbides during digestion occurred when the initial chlorophyll profile contained significant amounts of pheophytins, as observed in the Kombu algae. Furthermore, the digestive stability and recovery of chlorophyll derivatives after in vitro digestion appeared to depend on the chemical structure and the food matrix [37]. The same authors demonstrated that the percentage of micellization and the uptake by Caco-2 cells were higher for dephytylated chlorophylls compared to phytylated derivatives. Additionally, chlorophylls from Nori algae exhibited higher bioaccessibility than those from Sea lettuce and Kombu [38]. Regarding studies demonstrating in vivo absorption of chlorophyll derivatives, a chemoprevention trial in humans using SCC revealed that daily ingestion of SCC tablets (300 mg/day) resulted in the absorption of Cu-chlorin e4 ethyl ester into the bloodstream, and to a lesser extent, Cu-chlorin e4 [31]. This provided the initial evidence that chlorophyll derivatives can be absorbed by the human gastrointestinal tract. Another study conducted on human volunteers detected the presence of pheophytin and pheophorbide derivatives in the blood three hours after the ingestion of 1.2 kg of freshly boiled spinach [39]. In a study by Gomes et al. [33], rats fed a diet supplemented with 10 or 30 g/kg SCC showed absorption of Cu-chlorin e4, which was detected in the serum, liver, and kidneys. The absorption was macroscopically visible as a green color in these biological materials. However, Cu-chlorin e6 was not found in the serum or organs, suggesting degradation during passage through the gastrointestinal tract or interaction with other food components [33]. Regarding chlorophylls from natural sources, Fernandes et al. [40] studied the apparent absorption of chlorophylls in dogs by analyzing ingested and excreted chlorophylls. The results showed that after supplementation with 0.8% freeze-dried ground spinach leaves (18 mg chlorophyll/day) for 10 days, the average apparent absorption of chlorophyll derivatives was 3.4%. By analyzing the excreta, the authors inferred that pheophytinization, the major degradation process occurring in the acidic gastrointestinal tract of dogs, transformed chlorophylls a and b into their respective magnesium-free pheophytins a and b. However, they were unable to detect chlorophyll derivatives in the plasma of dogs after consuming a diet containing 10% freeze-dried spinach [40]. In a biodistribution study conducted in rabbits, where a single dose of 100 g of freeze-dried spinach powder (prepared from fresh spinach) was administered after 24 h of fasting, and rabbits were sacrificed after 2, 4, 8, 12, and 24 h, the major metabolites observed were chlorophyllides and pheophorbides [41]. The occurrence and levels of chlorophyll derivatives were organ-specific, found in the plasma, liver, gallbladder, and kidney. In the feces, the major metabolites detected were native chlorophylls and pheophytins [41]. More recently, Vieira et al. [42] analyzed the livers of mice fed a diet containing 15% spirulina powder, a blue-green microalgae primarily composed of chlorophyll a. The results demonstrated that the formation and absorption of pheophorbides, pyro-derivatives, and phytyl-chlorin e6 required first-pass metabolism. The absorption and accumulation of pheophorbide a in the liver may be partially protein-mediated through the scavenger receptor B type I, while the presence of phytol in the liver may occur due to the de-esterification of pheophytin a, leading to the formation of pheophorbide a and phytol [42]. In the feces, the percentage of pheophorbide derivatives and allomerized derivatives was similar to that in the supplemented feed, while pheophytin derivatives and pyro-derivatives exhibited increased content compared to the supplemented feed. Additionally, native chlorophyll a was detected in the feces of mice [42]. In summary, the collective findings from both in vitro and in vivo studies using native chlorophylls indicate that the potential health benefits associated with chlorophyll a and b are likely attributed to their metal-free derivatives. While these studies provide valuable insights into the bioavailability of chlorophylls, there is still a need for a comprehensive characterization of the chlorophyll derivatives formed in the gastrointestinal tract and a better understanding of their pharmacokinetic parameters. Unfortunately, as of now, there is a lack of published in vivo studies involving omnivorous species, which poses challenges in translating this information directly to humans. Further research in this area is warranted to bridge the gap in our understanding of chlorophyll metabolism and absorption in the human body.3.3. Bioactive Properties of Chlorophyll Compounds
The chemical structure of chlorophylls is a key determinant of their bioactivity, influencing their potential health benefits [5]. Understanding the relationship between chemical structure and bioactivity is crucial for unraveling the therapeutic properties of chlorophylls and their derivatives [43,44,45][43][44][45]. The chemical structure of chlorophylls consists of a porphyrin ring, which serves as the core framework, and a long hydrophobic side chain. This unique structure confers distinctive physicochemical and biological properties to chlorophylls. For instance, the presence of magnesium at the center of the porphyrin ring enables chlorophylls to efficiently capture light energy during photosynthesis. Moreover, the chemical structure of chlorophylls contributes to their bioactive properties. Studies have shown that chlorophyll derivatives, which undergo structural modifications, exhibit enhanced bioactivity compared to native chlorophylls. These modifications can involve alterations in the porphyrin ring, such as the addition of functional groups or substitutions. These structural changes lead to variations in the solubility, stability, and interaction capabilities of chlorophyll compounds [7,46][7][46]. The bioactivity of chlorophylls is attributed to their ability to act as antioxidants, antimutagens, and anticarcinogens. The unique chemical structure allows chlorophylls to scavenge harmful free radicals, mitigate DNA damage, and modulate cellular processes involved in disease development. Furthermore, their hydrophobic side chains facilitate interactions with biological membranes, influencing cellular uptake and signaling pathways [5,47][5][47]. Despite advances in understanding the relationship between the chemical structure of chlorophyll compounds and their bioactivity, further research is warranted. Investigations into the specific structural features responsible for different bioactive properties are necessary to unlock the full potential of chlorophylls as therapeutic agents. Moreover, exploring the interactions between chlorophylls and other bioactive compounds in a synergistic manner can provide insights into the holistic benefits of consuming chlorophyll-rich foods [48]. In conclusion, the bioactive properties of chlorophyll compounds are intricately linked to their chemical structure. By deciphering the structural determinants of their bioactivity, we can uncover new opportunities for utilizing chlorophylls and their derivatives in promoting human health [47]. Continued research in this field holds great promise for harnessing the full potential of chlorophylls as functional ingredients and contributing to the development of novel therapeutic approaches (Figure 3) [49,50,51][49][50][51].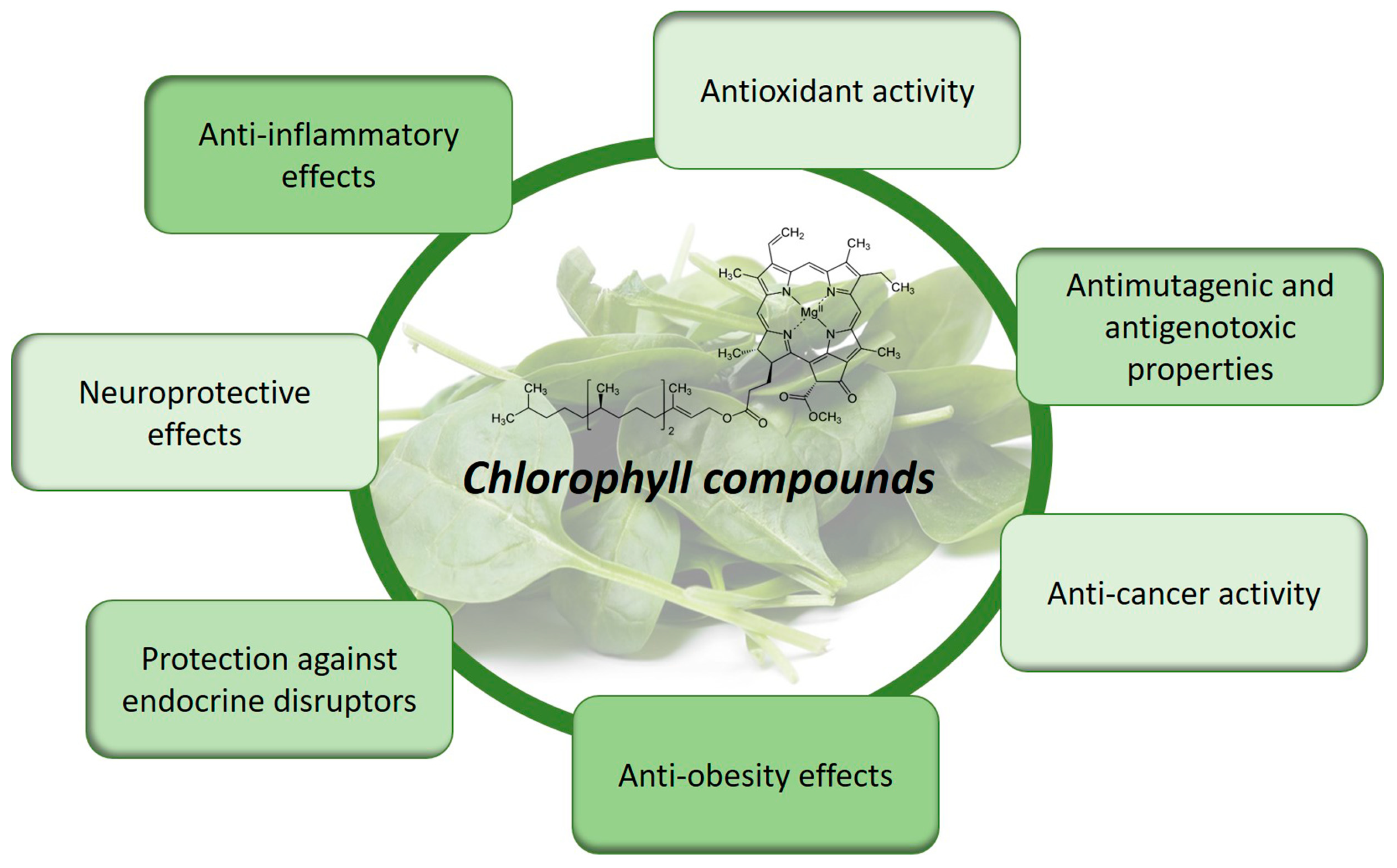
Figure 3.
Bioactive properties and health benefits of chlorophyll compounds.
4. Chlorophyll Content in Fruits and Vegetables
Modern societies are currently facing food waste problems that are increasing as the world’s population also increases, leading to economic and environmental issues. Food losses and waste occur at all stages of the food supply chain: agricultural production, post-harvest handling and storage, processing, distribution, and consumption stages [4]. Simultaneously with these difficulties, changes in eating habits, increased consumption of more processed foods, and less variety in diets have contributed to the increase in modern societies’ diseases such as obesity, diabetes, cardiovascular diseases, and atherosclerosis. The discarded material can be a valuable resource to answer these problems. For instance, leafy material or fruit peels, which are discarded in these first stages, are usually rich in bioactive compounds beneficial to health [107,108,109][52][53][54]. The use of these currently discarded products may represent a return to past eating habits, with the use of more diverse foods, sometimes not so appealing, but with less caloric concentrations and rich in a high variety of bioactive compounds. For example, broccoli is one of the most produced crops worldwide, where only the inflorescence part is used, while the stem and leaves are discarded. Nevertheless, this discarded material, in addition to glucosinolates, is also extremely rich in chlorophylls, especially the leaves. OuThe researchers' group evaluated the chlorophyll and carotenoid contents in the broccoli plant in two different harvest years (Supplementary Methods).
When it comes to green plants and vegetables, storage and processing conditions greatly impact the green color of these foods conferred by chlorophyll, whose degradation can be delayed or accelerated by these conditions [112,113,114,115,116][55][56][57][58][59]. This, in turn, has a great influence on the behavior of the final consumer, that is, not consuming them if the products do not have an attractive green color, thus further contributing to food waste.
Based on the provided information, here are some observations regarding the processing methods and conditions that can help retain higher chlorophyll content in certain fruits and vegetables: (i) Boiling: Boiling for a shorter duration appears to be more effective in retaining chlorophyll content. For example, in the case of green beans, boiling for 5 min resulted in higher chlorophyll content compared to longer boiling times; (ii) Steaming: Steaming for a moderate duration seems to be beneficial for maintaining chlorophyll levels. In the case of spinach, steaming for 7.5 min resulted in higher chlorophyll content compared to both shorter and longer steaming times; (iii) Microwaving: Microwaving for a shorter duration tends to preserve chlorophyll content. For instance, in the case of peas, microwaving for 1.5 min resulted in higher chlorophyll content compared to longer microwaving times; (iv) Storage conditions: Some vegetables, such as celery and leek, demonstrated a decrease in chlorophyll content after storage at low temperatures (0 °C) for an extended period. Therefore, it is advisable to minimize storage time at low temperatures to maintain higher chlorophyll levels.
It is important to note that the optimal method and conditions for preserving chlorophyll content may vary depending on the specific fruit or vegetable being processed. Additionally, other factors such as the desired texture, taste, and nutrient retention should also be considered when determining the best processing method for a particular food item. Further research and experimentation may be necessary to obtain more specific and comprehensive guidelines for maximizing chlorophyll retention during food processing.
References
- Björn, L.O.; Papageorgiou, G.C.; Blankenship, R.E.; Govindjee, A. Viewpoint: Why chlorophyll a? Photosynth. Res. 2009, 99, 85–98.
- Nagini, S.; Palitti, F.; Natarajan, A.T. Chemopreventive Potential of Chlorophyllin: A Review of the Mechanisms of Action and Molecular Targets. Nutr. Cancer. 2015, 67, 203–211.
- MacKeen, D. Influencers Are Drinking Chlorophyll Water. But Why? The New York Times: New York, NY, USA, 2 July 2021.
- FAO. Global Food Losses and Food Waste: Extent, Causes and Prevention; FAO: Rome, Italy, 2011.
- Queiroz Zepka, L.; Jacob-Lopes, E.; Roca, M. Catabolism and bioactive properties of chlorophylls. Curr. Opi. Food Sci. 2019, 26, 94–100.
- Roca, M.; Chen, K.; Pérez-Gálvez, A. 6-Chlorophylls. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 125–158.
- Durrett, T.P.; Welti, R. The tail of chlorophyll: Fates for phytol. J. Biol. Chem. 2021, 296, 100802.
- Pareek, S.; Sagar, N.A.; Sharma, S.; Kumar, V.; Agarwal, T.; González-Aguilar, G.A.; Yahia, E.M. Chlorophylls: Chemistry and Biological Functions. In Fruit and Vegetable Phytochemicals; Yahia, E.M., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 269–284.
- Wang, Y.-T.; Yang, C.-H.; Huang, K.-S.; Shaw, J.-F. Chlorophyllides: Preparation, Purification, and Application. Biomolecules 2021, 11, 1115.
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600.
- Halberstein, R.A. Medicinal Plants: Historical and Cross-Cultural Usage Patterns. Ann. Epidemiol. 2005, 15, 686–699.
- Soelberg, J.; Davis, O.; Jäger, A.K. Historical versus contemporary medicinal plant uses in the US Virgin Islands. J. Ethnopharmacol. 2016, 192, 74–89.
- Weckerle, C.S.; Ineichen, R.; Huber, F.K.; Yang, Y. Mao’s heritage: Medicinal plant knowledge among the Bai in Shaxi, China, at a crossroads between distinct local and common widespread practice. J. Ethnopharmacol. 2009, 123, 213–228.
- Calixto, J.B. Twenty-five years of research on medicinal plants in Latin America: A personal view. J. Ethnopharmacol. 2005, 100, 131–134.
- Ayyanar, M.; Ignacimuthu, S. Ethnobotanical survey of medicinal plants commonly used by Kani tribals in Tirunelveli hills of Western Ghats, India. J. Ethnopharmacol. 2011, 134, 851–864.
- Boy, H.I.A.; Rutilla, A.J.H.; Santos, K.A.; Ty, A.M.T.; Yu, A.I.; Mahboob, T.; Tangpoong, J.; Nissapatorn, V. Recommended Medicinal Plants as Source of Natural Products: A Review. Digit. Chin. Med. 2018, 1, 131–142.
- Menendez-Baceta, G.; Aceituno-Mata, L.; Molina, M.; Reyes-García, V.; Tardío, J.; Pardo-de-Santayana, M. Medicinal plants traditionally used in the northwest of the Basque Country (Biscay and Alava), Iberian Peninsula. J. Ethnopharmacol. 2014, 152, 113–134.
- Zorofchian Moghadamtousi, S.; Rouhollahi, E.; Karimian, H.; Fadaeinasab, M.; Firoozinia, M.; Ameen Abdulla, M.; Abdul Kadir, H. The Chemopotential Effect of Annona muricata Leaves against Azoxymethane-Induced Colonic Aberrant Crypt Foci in Rats and the Apoptotic Effect of Acetogenin Annomuricin E in HT-29 Cells: A Bioassay-Guided Approach. PLoS ONE 2015, 10, e0122288.
- Chassagne, F.; Huang, X.; Lyles, J.T.; Quave, C.L. Validation of a 16th Century Traditional Chinese Medicine Use of Ginkgo biloba as a Topical Antimicrobial. Front. Microbiol. 2019, 10, 775.
- Ibrahim, M.H.; Jaafar, H.Z.; Karimi, E.; Ghasemzadeh, A. Primary, secondary metabolites, photosynthetic capacity and antioxidant activity of the Malaysian Herb Kacip Fatimah (Labisia Pumila Benth) exposed to potassium fertilization under greenhouse conditions. Int. J. Mol. Sci. 2012, 13, 15321–15342.
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal plants of the genus Betula--traditional uses and a phytochemical-pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83.
- Alamgeer; Sharif, A.; Asif, H.; Younis, W.; Riaz, H.; Bukhari, I.A.; Assiri, A.M. Indigenous medicinal plants of Pakistan used to treat skin diseases: A review. Chin. Med. 2018, 13, 52.
- Bhat, J.A.; Kumar, M.; Bussmann, R.W. Ecological status and traditional knowledge of medicinal plants in Kedarnath Wildlife Sanctuary of Garhwal Himalaya, India. J. Ethnobiol. Ethnomed. 2013, 9, 1.
- Ghorbani, A. Studies on pharmaceutical ethnobotany in the region of Turkmen Sahra, north of Iran: (Part 1): General results. J. Ethnopharmacol. 2005, 102, 58–68.
- González, J.A.; García-Barriuso, M.; Amich, F. Ethnobotanical study of medicinal plants traditionally used in the Arribes del Duero, western Spain. J. Ethnopharmacol. 2010, 131, 343–355.
- Tariq, A.; Mussarat, S.; Adnan, M. Review on ethnomedicinal, phytochemical and pharmacological evidence of Himalayan anticancer plants. J. Ethnopharmacol. 2015, 164, 96–119.
- Tajidin, N.E.; Shaari, K.; Maulidiani, M.; Salleh, N.S.; Ketaren, B.R.; Mohamad, M. Metabolite profiling of Andrographis paniculata (Burm. f.) Nees. young and mature leaves at different harvest ages using 1H NMR-based metabolomics approach. Sci. Rep. 2019, 9, 16766.
- Yilmaz, C.; Gökmen, V. Chlorophyll. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 37–41.
- Lanfer-Marquez, U.M.; Sinnecker, P. Chlorophylls: Properties, Biosynthesis, Degradation and Functions. In Food Colorants: Chemical And Functional Properties; Socaciu, C., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 195–211.
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green Natural Colorants. Molecules 2019, 24, 154.
- Egner, P.A.; Stansbury, K.H.; Snyder, E.P.; Rogers, M.E.; Hintz, P.A.; Kensler, T.W. Identification and characterization of chlorin e(4) ethyl ester in sera of individuals participating in the chlorophyllin chemoprevention trial. Chem. Res. Toxicol. 2000, 13, 900–906.
- Ferruzzi, M.G.; Failla, M.L.; Schwartz, S.J. Sodium copper chlorophyllin: In vitro digestive stability and accumulation by Caco-2 human intestinal cells. J. Agric. Food Chem. 2002, 50, 2173–2179.
- Gomes, B.B.; Barros, S.B.; Andrade-Wartha, E.R.; Silva, A.M.; Silva, V.V.; Lanfer-Marquez, U.M. Bioavailability of dietary sodium copper chlorophyllin and its effect on antioxidant defence parameters of Wistar rats. J. Sci. Food Agric. 2009, 89, 2003–2010.
- Ferruzzi, M.G.; Failla, M.L.; Schwartz, S.J. Assessment of degradation and intestinal cell uptake of carotenoids and chlorophyll derivatives from spinach puree using an in vitro digestion and Caco-2 human cell model. J. Agric. Food Chem. 2001, 49, 2082–2089.
- Gallardo-Guerrero, L.; Gandul-Rojas, B.; Mínguez-Mosquera, M.I. Digestive stability, micellarization, and uptake by Caco-2 human intestinal cell of chlorophyll derivatives from different preparations of pea (Pisum sativum L.). J. Agric. Food Chem. 2008, 56, 8379–8386.
- Gandul-Rojas, B.; Gallardo-Guerrero, L.; Mínguez-Mosquera, M.I. Influence of the chlorophyll pigment structure on its transfer from an oily food matrix to intestinal epithelium cells. J. Agric. Food Chem. 2009, 57, 5306–5314.
- Chen, K.; Roca, M. In vitro digestion of chlorophyll pigments from edible seaweeds. J. Funct. Foods 2018, 40, 400–407.
- Chen, K.; Roca, M. In vitro bioavailability of chlorophyll pigments from edible seaweeds. J. Funct. Foods 2018, 41, 25–33.
- Chao, P.-Y.; Huang, M.-Y.; Huang, W.-D.; Lin, K.-H.R.; Chen, S.-Y.; Yang, C.-M. Study of Chlorophyll-related Compounds from Dietary Spinach in Human Blood. Not. Bot. Horti. Agrobo. 2018, 46, 309–316.
- Fernandes, T.M.; Gomes, B.B.; Lanfer-Marquez, U.M. Apparent absorption of chlorophyll from spinach in an assay with dogs. Innov. Food Sci. Emerg. Technol. 2007, 8, 426–432.
- Hsu, C.Y.; Yeh, T.H.; Huang, M.Y.; Hu, S.P.; Chao, P.Y.; Yang, C.M. Organ-specific distribution of chlorophyll-related compounds from dietary spinach in rabbits. Indian J. Biochem. Biophys. 2014, 51, 388–395.
- Viera, I.; Chen, K.; Ríos, J.J.; Benito, I.; Pérez-Gálvez, A.; Roca, M. First-Pass Metabolism of Chlorophylls in Mice. Mol. Nutr. Food Res. 2018, 62, e1800562.
- Fasakin, C.F.; Udenigwe, C.C.; Aluko, R.E. Antioxidant properties of chlorophyll-enriched and chlorophyll-depleted polyphenolic fractions from leaves of Vernonia amygdalina and Gongronema latifolium. Food Res. Int. 2011, 44, 2435–2441.
- Ferruzzi, M.G.; Böhm, V.; Courtney, P.D.; Schwartz, S.J. Antioxidant and Antimutagenic Activity of Dietary Chlorophyll Derivatives Determined by Radical Scavenging and Bacterial Reverse Mutagenesis Assays. J. Food Sci. 2002, 67, 2589–2595.
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891.
- Yahia, E.M. Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; Volume I.
- Perez-Galvez, A.; Viera, I.; Roca, M. Chemistry in the Bioactivity of Chlorophylls: An Overview. Curr. Med. Chem. 2017, 24, 4515–4536.
- Cai, J.-Q.; Liu, X.-M.; Gao, Z.-J.; Li, L.-L.; Wang, H. Chlorophylls derivatives: Photophysical properties, assemblies, nanostructures and biomedical applications. Mater. Today 2021, 45, 77–92.
- De Vogel, J.; Jonker-Termont, D.S.; van Lieshout, E.M.; Katan, M.B.; van der Meer, R. Green vegetables, red meat and colon cancer: Chlorophyll prevents the cytotoxic and hyperproliferative effects of haem in rat colon. Carcinogenesis 2005, 26, 387–393.
- Egner, P.A.; Wang, J.B.; Zhu, Y.R.; Zhang, B.C.; Wu, Y.; Zhang, Q.N.; Qian, G.S.; Kuang, S.Y.; Gange, S.J.; Jacobson, L.P.; et al. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14601–14606.
- Hayes, M.; Ferruzzi, M.G. Update on the bioavailability and chemopreventative mechanisms of dietary chlorophyll derivatives. Nutr. Res. 2020, 81, 19–37.
- Castelao-Baptista, J.P.; Barros, A.; Martins, T.; Rosa, E.; Sardao, V.A. Three in One: The Potential of Brassica By-Products against Economic Waste, Environmental Hazard, and Metabolic Disruption in Obesity. Nutrients 2021, 13, 4194.
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510.
- Martins, T.; Colaco, B.; Venancio, C.; Pires, M.J.; Oliveira, P.A.; Rosa, E.; Antunes, L.M. Potential effects of sulforaphane to fight obesity. J. Sci. Food Agric. 2018, 98, 2837–2844.
- Sánchez, C.; Baranda, A.B.; Martínez de Marañón, I. The effect of High Pressure and High Temperature processing on carotenoids and chlorophylls content in some vegetables. Food Chem. 2014, 163, 37–45.
- Schwartz, S.J.; Lorenzo, T.V. Chlorophylls in foods. Crit. Rev. Food Sci. Nutr. 1990, 29, 1–17.
- Teng, S.S.; Chen, B.H. Formation of pyrochlorophylls and their derivatives in spinach leaves during heating. Food Chem. 1999, 65, 367–373.
- Turkmen, N.; Poyrazoglu, E.S.; Sari, F.; Sedat Velioglu, Y. Effects of cooking methods on chlorophylls, pheophytins and colour of selected green vegetables. Int. J. Food Sci. Techn. 2006, 41, 281–288.
- Viña, S.Z.; Osornio, M.M.L.; Chaves, A.R. Quality changes in fresh-cut celery as affected by heat treatment and storage. J. Sci. Food Agric. 2007, 87, 1400–1407.
More
