Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Erick Paul Gutiérrez-Grijalva.
Polyphenols have attracted attention for their anti-inflammatory, antidiabetic, and anticancer properties. Due to the antioxidant and anti-inflammatory potential of these molecules, they are also proposed as a potential therapeutic tool to prevent complications of cancer and decrease the secondary effects of conventional chemotherapeutic drugs.
- polyphenols
- cancer
- encapsulation
- anticancer
1. Introduction
Phenolic compounds, known by the general audience as “polyphenols”, are secondary metabolites from plants produced mainly as a defense mechanism against abiotic and biotic factors. These constituents also contribute to pollination by granting color to flowers and plants, attracting pollinators [1]. For their study, polyphenols can be grouped according to their chemical structure, number of phenol rings, and number and distribution of their -OH groups. The most common groups are (1) flavonoids: anthocyanins, chalcones, dihydrochalcones, dihydroflavonols, flavanols, flavanones, flavones, flavonols, isoflavonoids; (2) lignans, (3) non-phenolic compounds, (4) phenolic acids: hydroxybenzoic acids, hydroxycinnamic acids, hydroxyphenylacetic acids, hydroxyphenylpropanoic acids, hydroxyphenylpentanoic acids, (5) stilbenes, and (6) other polyphenols (Figure 1) [2,3,4][2][3][4]. Polyphenols are of interest in the nutraceutical and pharmaceutical industries because of their antioxidant, anti-inflammatory, and anticancer properties.
Figure 1. Chemical structures of known polyphenols: (a) anthocyanins (cyanidin), (b) dihydrochalcones (phloretin), (c) flavanols (catechin), (d) flavanones (naringenin), (e) flavones (apigenin), (f) flavonols (quercetin).
Cancer is a group of diseases that begins when abnormal cells grow uncontrollably and can start in nearly all tissues and organs. Cancer is the second leading cause of death worldwide, with around 9.6 million deaths in 2018. According to the International Agency for Research on Cancer of the World Health Organization, the cancers that caused most deaths in 2020 were lung, colorectum, liver, stomach, breast, esophagus, pancreas, and prostate cancer (Figure 2).
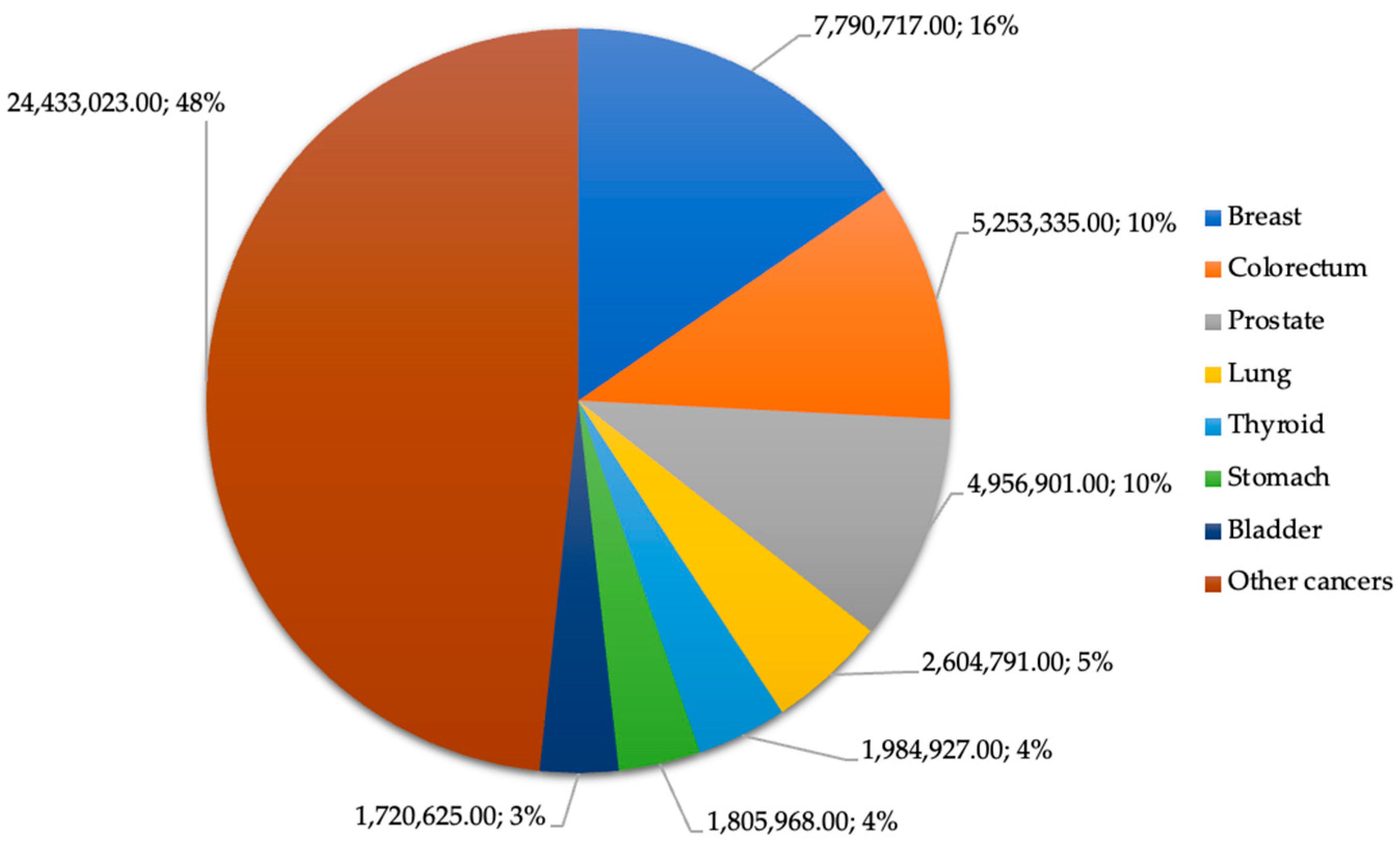
Figure 2. Estimated number of prevalent cases worldwide (5 years) in 2020 for both sexes of all ages.
Currently, there is evidence that a regular intake of food sources rich in polyphenols can decrease cancer incidence and prevent disease onset. In vitro studies have shown that many polyphenols have antiproliferative activity through several mechanisms of action involved in signaling cascades in the different cancer development stages [6,7][5][6]. Furthermore, in vivo studies have also shown that polyphenols can exert an anticancer potential also at this level. Nonetheless, one of the problems dealing with polyphenols is their low bioavailability because the xenobiotic metabolism highly metabolizes them. In this sense, encapsulation strategies using polymeric matrixes have been developed to increase the bioavailability of polyphenols.
2. Anticancer Properties of Polyphenols
2.1. In Vivo Studies
Different studies in mice and rats showed the potential anticancer properties of polyphenols (Table 1). They can act at different levels, including by apoptosis induction of tumor cells in different ways. In this sense, this type of cell death can be induced by ellagic acid and galangin via increased caspases-3 expression and activity in retinoblastoma tumors and lymphomas [8,9][7][8], as well as by an increase in Bad and a decrease in Bcl-2 gene expression by vanillic acid [10][9]. In addition, it was observed that polyphenols decrease metastasis by inhibiting the migration and invasion of cancer cells; among them, eriodictyol and luteolin showed this effect [11,12][10][11]. Furthermore, taxifolin decreased epithelial–mesenchymal transition, an early event of metastasis [13][12]. In addition, signaling pathways such as PI3K/Akt and MAPK/ERK are related to cell proliferation, which could be downregulated by caffeic acid and caffeic acid phenylpropyl ester in induced colon cancer [14][13]. On the other hand, inflammation and oxidative stress are two physiological processes which have been linked to cancer [15][14]; in this context, naringenin had an anticancer effect in the lungs by decreasing the expression of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (a bridge between inflammation and cancer), which decreased the expression of cytokines such as interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF-α); at the same time, this flavanone could increase the antioxidant enzymes and non-enzymatic antioxidants, exerting a cytoprotective function against induced lung cancer [16][15]. Moreover, taxifolin increased the antioxidant response by the upregulation of the nuclear factor erythroid 2-related factor 2 (Nrf2) and decreased inflammation by downregulation of the NF-κB and Wnt/β-catenin signaling pathways in induced colon cancer [17][16]; also, Nrf2 upregulation in induced hepatic cancer by vanillic acid was detected [10][9].Table 1. Effect of polyphenols supplementation against cancer in rats and mice models.
| Compound | Dose | Model | Effect | Reference |
|---|---|---|---|---|
| Caffeic acid | 50 nmol/kg | Mice with human colon cancer xenografts | Inhibition of tumor growth via downregulation of PI3K/Akt and MAPK/ERK signaling | [14][13] |
| Caffeic acid phenylpropyl ester | 50 nmol/kg | Mice with human colon cancer xenografts | Inhibition of tumor growth via downregulation of PI3K/Akt and MAPK/ERK signaling | [14][13] |
Table 2. Studies of the association between polyphenols intake and cancer risk.
| Compound(s) | Cancer Type |
|---|
Table 3. Clinical studies of polyphenols against different types of cancer.
| Cancer | Population | Subjects (Age) | Effect | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Dose | Subjects | Effect | Reference | |||||||||
| Flavonols and lignans | Bladder | 477,312 European subjects | 35–70 years | Inverse association between flavonols and lignans intake and bladder cancer risk | [26 | ||||||||
| Bladder | Genistein | 300 or 600 mg | 59 subjects with urothelial bladder cancer | ] | [25] | ||||||||
| Inhibition of bladder cancer growth by inhibiting the phosphorylation of the epidermal growth factor receptor | [ | 40 | ] | [ | 39] | Flavonols, isorhamnetin, kaempferol, flavanones and naringenin | Breast | 877 Chinese women with breast cancer, 792 control subjects |
25–70 years | The concentration of the flavonoids in the serum was associated with a lower breast cancer risk | [23] | ||
| Colorectal | Ginger (Zingiber officinale) extract with 5% of gingerols | [ | 22 | 2 g | 21 healthy subjects with a high risk of colorectal cancer | Decrease in the proliferation of crypts] | [39][38] | Chlorogenic acid | 20–40 mg/kg | Mice with breast cancer xenografts | Decrease in tumor growth and inhibition of metastasis via an increase in CD4+ and CD8+ cells in the spleen | [ | |
| 18 | ] | Epigallocatechin gallate | 780 mg | 32 subjects with rectal aberrant crypt foci[17] | |||||||||
| Anthocyanidins and flavan-3-ols | Breast | 233 Mexican women with breast cancer, 221 control subjects |
>18 (mean 53) years | Higher intake of flavonoids reduced the risk of breast cancer, synergistically working with butyl benzyl phthalate | [24][23] | No difference in the number of the rectal aberrant crypt foci | [37][36] | Ellagic acid | 40 mg/kg | Mice with human bladder cancer xenografts | Decrease in tumor growth rate, infiltrative behavior, and tumor-associated angiogenesis. | [19][18] | |
| Flavonols, flavones, flavanones, flavan-3-ols, and anthocyanins |
Colorectal | 51,528 US male health professionals and 121,701 US female nurses |
Men: 40–75 years Women: 30–55 years |
No decrease in colorectal cancer was detected | [28][27] | ||||||||
| Familial adenomatous polyposis | Curcumin | 3000 mg | 44 subjects with familial adenomatous polyposis | No difference in the mean number or size of polyps | [35][34] | 80 mg/kg | Mice with induced lymphoma | Induction of apoptosis via an increase in caspase-3 expression and activity and PKCs activity and a decrease in LDH-A activity and expression in ascites fluid | 35–70 years[8 | No association between flavonoids intake and colorectal cancer was found | |||
| Oral | Curcuma longa phenolic extract | ] | 100 or 200 mg | 12 oral cancer patients 13 normal subjects[7] |
|||||||||
| [ | 29 | ] | [ | 28 | ] | ||||||||
| Decrease in IL-1β, IL-6, and IL-8 content in the saliva. | |||||||||||||
| Flavonoids | Colorectal | Increased gene expression related to differentiation and T cell recruitment to the tumor microenvironment. | [ | 34][33] | Eriodictyol | 60 mg/kg | Mice with mammary cancer xenografts | Decrease in tumor growth and progression and in lung metastasis | [ | Phenolic acids, hydroxycinnamic acids, flavonols, and stilbenes | Colorectal and colorectal adenoma | 129 Iranian subjects with colorectal cancer, 130 with colorectal adenoma, and 240 controls11][10] | |
| 30–79 years | Higher intake of phenolic acids, hydroxycinnamic acids, and flavonols was associated with a decrease in colorectal cancer risk. | Higher intake of stilbenes was associated with a lower colorectal adenoma risk. | [ | 32][ | |||||||||
| Prostate | 31 | ] | |||||||||||
| Cranberry fruit powder | 1500 mg | 62 subjects with prostate cancer | Decrease in serum prostate-specific antigen | [ | 38][37] | Galangin | 25–50 mg/kg | Mice with human retinoblastoma xenografts | Decrease in tumor growth via a decrease in Akt signaling pathway and increase in caspase-3 level | [9 | Polyphenols][8] | ||
| Epithelial ovarian cancer | 309,129 European women | 35–70 years | No association between polyphenols intake and endothelial ovarian cancer was found | [ | 30][29] | Luteolin | |||||||
| Epigallocatechin gallate | 600 mg | 43 subjects with a prior negative biopsy, but suspicious | 1.2 mg/g | Mice with AOM/DMH-induced colon cancer | Decrease in LDH levels and in iNOS and COX-2 expression in colon tissue | [20] | |||||||
| 521,448 European subjects (with exceptions) | ] | [ | 19 | 100 mg/kg | Mice with human epithelial xenograft | Decrease in migration and invasion | [12][11] | ||||||
| Naringenin | 50 mg/kg | Mice with benzo(a)pyrene induced lung cancer | Downregulation of CYP1A1, PCNA, and NF-κB expression; decrease in lipid peroxidation, TNF-α, IL-6 and IL-1β; increase in antioxidant enzymes activity in lung tissue | [16][15] | |||||||||
| No difference in fatty acid synthase or antigen Ki-76 | [ | 36 | ] | [ | 35] | Naringenin, peonidin, and catechin | General | 14,029 US subjects | >18 | Inverse association between flavonoids intake and cancer mortality | [33][32] | ||
| Flavonoids and lignans | Pancreatic | 477,309 European subjects | 25–70 years |
No association between flavonoids and lignans intake and pancreatic cancer was found | [31][30] | ||||||||
| Caffeic acid and ferulic acid | Prostate | 118 Italian prostate cancer subjects, 22 controls |
Mean age: 69.13 years | High intake of phenolic acids may be associated with a decrease in prostate cancer risk | [25][24] | Quercetin | 30 mg/kg | Mice with AOM/dextran sodium sulfate-induced colorectal cancer | Decrease in tumor growth and proliferation via a decrease in inflammation and ROS | [ | |||
| Polyphenols and phenolic acids | Thyroid | 21 | ] | 476,108 European subjects[20 | 35–70 years] | ||||||||
| Inverse association between polyphenols and phenolic acids intake and thyroid cancer risk in patients with BMI ≥ 25 | [ | 27 | ] | [ | 26] | 25–50 mg/kg | Rats with DMH-induced colon cancer | Decrease in tumor incidence and multiplicity; downregulation of the Wnt signaling pathway in colon tissue | [22][21] | ||||
| Taxifolin | 4 µg/kg | Mice with DMH-induced colon cancer | Upregulation of the Nrf2 signaling pathway, downregulation of the NF-κB and Wnt signaling pathways in colon tissue | [17][16] | |||||||||
| 1 mg/kg | Mice with human lung cancer xenograft | Decrease in tumor size via inhibition of PI3K and TCF4 signaling and by decreasing epithelial–mesenchymal transition | [13][12] | ||||||||||
| Vanillic acid | 75 mg/kg | Rats with DMH-induced hepatic cancer | Upregulation of the Nrf2 signaling pathway; induction of apoptosis via an increase in Bad and Caspase-3 genes expression and decrease in Bcl-2 gene expression; decrease in proliferation via a decrease in Cyclin D1 gene expression in hepatic tissue | [10][9] |
2.2. Clinical Studies
The effect of polyphenols supplementation has been studied on various cancers, including bladder, colorectal, oral, and prostate cancer (Table 3). For example, the compound curcumin has been studied for both oral cancer and familial adenomatous polyps (a condition that leads to colorectal cancer); in this sense, curcumin decreased interleukin content in the saliva and induced the attraction of T cells to the tumor microenvironment [34][33]. In contrast, no effect was observed on the mean size and number of polyps in the lower intestinal tract [35][34]. On the other hand, studies indicated no beneficial effect of epigallocatechin gallate against colorectal and prostate cancer [36,37References
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621.
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024.
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070.
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordoñez, M.; Knox, C.; Llorach, R.; Eisner, R.; Cruz, J.; Neveu, V.; Wishart, D.; Manach, C.; et al. Phenol-Explorer 2.0: A major update of the Phenol-Explorer database integrating data on polyphenol metabolism and pharmacokinetics in humans and experimental animals. Database 2012, 2012, bas031.
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107.
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.M.; Hennink, W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35, 3365–3383.
- Mishra, S.; Vinayak, M. Ellagic Acid Induces Novel and Atypical PKC Isoforms and Promotes Caspase-3 Dependent Apoptosis by Blocking Energy Metabolism. Nutr. Cancer 2014, 66, 675–681.
- Zou, W.-W.; Xu, S.-P. Galangin inhibits the cell progression and induces cell apoptosis through activating PTEN and Caspase-3 pathways in retinoblastoma. Biomed. Pharmacother. 2018, 97, 851–863.
- Punvittayagul, C.; Chariyakornkul, A.; Jarukamjorn, K.; Wongpoomchai, R. Protective Role of Vanillic Acid against Diethylnitrosamine- and 1,2-Dimethylhydrazine-Induced Hepatocarcinogenesis in Rats. Molecules 2021, 26, 2718.
- Debnath, S.; Sarkar, A.; Mukherjee, D.D.; Ray, S.; Mahata, B.; Mahata, T.; Parida, P.K.; Das, T.; Mukhopadhyay, R.; Ghosh, Z.; et al. Eriodictyol mediated selective targeting of the TNFR1/FADD/TRADD axis in cancer cells induce apoptosis and inhibit tumor progression and metastasis. Transl. Oncol. 2022, 21, 101433.
- Yao, Y.; Rao, C.; Zheng, G.; Wang, S. Luteolin suppresses colorectal cancer cell metastasis via regulation of the miR-384/pleiotrophin axis. Oncol. Rep. 2019, 42, 131–141.
- Wang, R.; Zhu, X.; Wang, Q.; Li, X.; Wang, E.; Zhao, Q.; Wang, Q.; Cao, H. The anti-tumor effect of taxifolin on lung cancer via suppressing stemness and epithelial-mesenchymal transition in vitro and oncogenesis in nude mice. Ann. Transl. Med. 2020, 8, 590.
- Chiang, E.-P.I.; Tsai, S.-Y.; Kuo, Y.-H.; Pai, M.-H.; Chiu, H.-L.; Rodriguez, R.L.; Tang, F.-Y. Caffeic Acid Derivatives Inhibit the Growth of Colon Cancer: Involvement of the PI3-K/Akt and AMPK Signaling Pathways. PLoS ONE 2014, 9, e99631.
- Vaidya, F.U.; Chhipa, A.S.; Sagar, N.; Pathak, C. Oxidative Stress and Inflammation Can Fuel Cancer; Springer: Singapore, 2020; pp. 229–258.
- Bodduluru, L.N.; Kasala, E.R.; Madhana, R.M.; Barua, C.C.; Hussain, M.I.; Haloi, P.; Borah, P. Naringenin ameliorates inflammation and cell proliferation in benzo(a)pyrene induced pulmonary carcinogenesis by modulating CYP1A1, NFκB and PCNA expression. Int. Immunopharmacol. 2016, 30, 102–110.
- Manigandan, K.; Manimaran, D.; Jayaraj, R.L.; Elangovan, N.; Dhivya, V.; Kaphle, A. Taxifolin curbs NF-κB-mediated Wnt/β-catenin signaling via up-regulating Nrf2 pathway in experimental colon carcinogenesis. Biochimie 2015, 119, 103–112.
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol. Rep. 2020, 45, 717–727.
- Ceci, C.; Tentori, L.; Atzori, M.; Lacal, P.; Bonanno, E.; Scimeca, M.; Cicconi, R.; Mattei, M.; De Martino, M.; Vespasiani, G.; et al. Ellagic Acid Inhibits Bladder Cancer Invasiveness and In Vivo Tumor Growth. Nutrients 2016, 8, 744.
- Pandurangan, A.K.; Sadagopan, S.K.A.; Dharmalingam, P.; Ganapasam, S. Luteolin, a bioflavonoid inhibits azoxymethane-induced colon carcinogenesis: Involvement of iNOS and COX-2. Pharmacogn. Mag. 2014, 10, 306–310.
- Lin, R.; Piao, M.; Song, Y.; Liu, C. Quercetin Suppresses AOM/DSS-Induced Colon Carcinogenesis through Its Anti-Inflammation Effects in Mice. J. Immunol. Res. 2020, 2020, 9242601.
- Shree, A.; Islam, J.; Sultana, S. Quercetin ameliorates reactive oxygen species generation, inflammation, mucus depletion, goblet disintegration, and tumor multiplicity in colon cancer: Probable role of adenomatous polyposis coli, β-catenin. Phytother. Res. 2021, 35, 2171–2184.
- Feng, X.-L.; Zhan, X.-X.; Zuo, L.-S.-Y.; Mo, X.-F.; Zhang, X.; Liu, K.-Y.; Li, L.; Zhang, C.-X. Associations between serum concentration of flavonoids and breast cancer risk among Chinese women. Eur. J. Nutr. 2021, 60, 1347–1362.
- Mérida-Ortega, Á.; Hernández-Alcaraz, C.; Hernández-Ramírez, R.U.; García-Martínez, A.; Trejo-Valdivia, B.; Salinas-Rodríguez, A.; Svensson, K.; Cebrián, M.E.; Franco-Marina, F.; López-Carrillo, L. Phthalate exposure, flavonoid consumption and breast cancer risk among Mexican women. Environ. Int. 2016, 96, 167–172.
- Russo, G.; Campisi, D.; Di Mauro, M.; Regis, F.; Reale, G.; Marranzano, M.; Ragusa, R.; Solinas, T.; Madonia, M.; Cimino, S.; et al. Dietary Consumption of Phenolic Acids and Prostate Cancer: A Case-Control Study in Sicily, Southern Italy. Molecules 2017, 22, 2159.
- Zamora-Ros, R.; Sacerdote, C.; Ricceri, F.; Weiderpass, E.; Roswall, N.; Buckland, G.; St-Jules, D.E.; Overvad, K.; Kyrø, C.; Fagherazzi, G.; et al. Flavonoid and lignan intake in relation to bladder cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Cancer 2014, 111, 1870–1880.
- Zamora-Ros, R.; Cayssials, V.; Franceschi, S.; Kyrø, C.; Weiderpass, E.; Hennings, J.; Sandström, M.; Tjønneland, A.; Olsen, A.; Overvad, K.; et al. Polyphenol intake and differentiated thyroid cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int. J. Cancer 2020, 146, 1841–1850.
- Nimptsch, K.; Zhang, X.H.; Cassidy, A.; Song, M.Y.; O’Reilly, E.J.; Lin, J.H.; Pischon, T.; Rimm, E.B.; Willett, W.C.; Fuchs, C.S.; et al. Habitual intake of flavonoid subclasses and risk of colorectal cancer in 2 large prospective cohorts. Am. J. Clin. Nutr. 2016, 103, 184–191.
- Zamora-Ros, R.; Barupal, D.K.; Rothwell, J.A.; Jenab, M.; Fedirko, V.; Romieu, I.; Aleksandrova, K.; Overvad, K.; Kyrø, C.; Tjønneland, A.; et al. Dietary flavonoid intake and colorectal cancer risk in the European prospective investigation into cancer and nutrition (EPIC) cohort. Int. J. Cancer 2017, 140, 1836–1844.
- Londoño, C.; Cayssials, V.; De Villasante, I.; Crous-Bou, M.; Scalbert, A.; Weiderpass, E.; Agudo, A.; Tjønneland, A.; Olsen, A.; Overvad, K.; et al. Polyphenol Intake and Epithelial Ovarian Cancer Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Antioxidants 2021, 10, 1249.
- Molina-Montes, E.; Sánchez, M.J.; Zamora-Ros, R.; Bueno-De-Mesquita, H.B.; Wark, P.A.; Obon-Santacana, M.; Kühn, T.; Katzke, V.; Travis, R.C.; Ye, W.; et al. Flavonoid and lignan intake and pancreatic cancer risk in the European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2016, 139, 1480–1492.
- Bahrami, A.; Jafari, S.; Rafiei, P.; Beigrezaei, S.; Sadeghi, A.; Hekmatdoost, A.; Rashidkhani, B.; Hejazi, E. Dietary intake of polyphenols and risk of colorectal cancer and adenoma–A case-control study from Iran. Complement. Ther. Med. 2019, 45, 269–274.
- Zhou, Y.; Gu, K.; Zhou, F. Dietary Flavonoid Intake and Cancer Mortality: A Population-Based Cohort Study. Nutrients 2023, 15, 976.
- Basak, S.K.; Bera, A.; Yoon, A.J.; Morselli, M.; Jeong, C.; Tosevska, A.; Dong, T.S.; Eklund, M.; Russ, E.; Nasser, H.; et al. A randomized, phase 1, placebo-controlled trial of APG-157 in oral cancer demonstrates systemic absorption and an inhibitory effect on cytokines and tumor-associated microbes. Cancer 2020, 126, 1668–1682.
- Cruz-Correa, M.; Hylind, L.M.; Marrero, J.H.; Zahurak, M.L.; Murray-Stewart, T.; Casero, R.A.; Montgomery, E.A.; Iacobuzio-Donahue, C.; Brosens, L.A.; Offerhaus, G.J.; et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients With Familial Adenomatous Polyposis. Gastroenterology 2018, 155, 668–673.
- Zhang, Z.; Garzotto, M.; Beer, T.M.; Thuillier, P.; Lieberman, S.; Mori, M.; Stoller, W.A.; Farris, P.E.; Shannon, J. Effects of ω-3 Fatty Acids and Catechins on Fatty Acid Synthase in the Prostate: A Randomized Controlled Trial. Nutr. Cancer 2016, 68, 1309–1319.
- Sinicrope, F.A.; Viggiano, T.R.; Buttar, N.S.; Song, L.M.W.K.; Schroeder, K.W.; Kraichely, R.E.; Larson, M.V.; Sedlack, R.E.; Kisiel, J.B.; Gostout, C.J.; et al. Randomized Phase II Trial of Polyphenon E versus Placebo in Patients at High Risk of Recurrent Colonic Neoplasia. Cancer Prev. Res. 2021, 14, 573–580.
- Student, V.; Vidlar, A.; Bouchal, J.; Vrbkova, J.; Kolar, Z.; Kral, M.; Kosina, P.; Vostalova, J. Cranberry intervention in patients with prostate cancer prior to radical prostatectomy. Clinical, pathological and laboratory findings. Biomed. Pap. 2016, 160, 559–565.
- Citronberg, J.; Bostick, R.; Ahearn, T.; Turgeon, D.K.; Ruffin, M.T.; Djuric, Z.; Sen, A.; Brenner, D.E.; Zick, S.M. Effects of Ginger Supplementation on Cell-Cycle Biomarkers in the Normal-Appearing Colonic Mucosa of Patients at Increased Risk for Colorectal Cancer: Results from a Pilot, Randomized, and Controlled Trial. Cancer Prev. Res. 2013, 6, 271–281.
- Messing, E.; Gee, J.R.; Saltzstein, D.R.; Kim, K.; Disant’Agnese, A.; Kolesar, J.; Harris, L.; Faerber, A.; Havighurst, T.; Young, J.M.; et al. A Phase 2 Cancer Chemoprevention Biomarker Trial of Isoflavone G-2535 (Genistein) in Presurgical Bladder Cancer Patients. Cancer Prev. Res. 2012, 5, 621–630.
More
