Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Miruna Florina Stefan and Version 2 by Jessie Wu.
The number of patients diagnosed with breast cancer and cardiovascular disease is continuously rising. Treatment options for breast cancer have greatly evolved, but radiotherapy (RT) still has a key role in it. Despite many advances in RT techniques, cardiotoxicity is one of the most important side effects.
- cancer
- radiotherapy
- breast cancer
- cardiovascular toxicity
1. Introduction
Breast cancer survivors who undergo radiotherapy are at risk of developing a wide spectrum of cardiovascular pathologies, including cardiac ischemia, cardiac dysfunction, heart failure, valvular heart disease, arrhythmias, QT interval prolongation, and autonomic dysfunction [1] (Figure 1) [2].
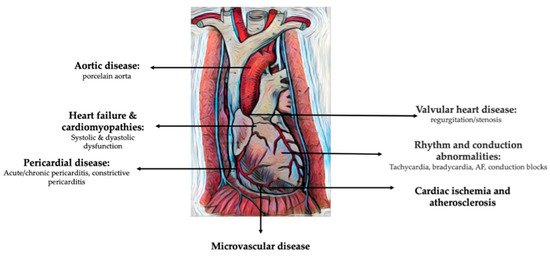
Figure 1. Cardiovascular pathologies induced by radiation therapy for breast cancer (modified and adapted from [35]).
2. Cardiac Ischemia and Atherosclerosis
Coronary artery disease (CAD) is by far the most frequent form of cardiovascular toxicity of radiotherapy (RT); some studies conducted in patients treated with thoracic RT for other kind of cancers reported an incidence as high as 85% [2][36]. A large meta-analysis that included 39 studies with breast cancer survivors revealed a higher risk of coronary disease and mortality during the first and second decade after RT. They found the dose of 5 Gy to the left ventricle as the most predictive for coronary events [3][37].
Any vascular bed exposed to RT is at increased risk of accelerated atherosclerosis [4][18]. This results in, among other effects, an early manifestation of coronary artery disease. The first signs of radiation-induced CAD were observed after the Hiroshima and Nagasaki atomic bombings. In this observed group, 10% died of CV disease [5][6][13,38].
In the case of the coronary arteries, the time between RT exposure and the development of CAD depends on the grade of atherosclerosis present before initiation of the treatment, exposure to classic CAD risk factors, as well as on the age of the patient at cancer diagnosis, the most affected being the younger ones [7][16]. Classic CV risk factors double the risk of a major coronary event [7][16]. CAD development also depends on the administered dose and the cardiac field that was exposed, many studies showing differences in CAD development between women that received RT for left versus right breast cancer. A 7.4–16.5% relative increase in rates of coronary events with each additional Gray has been observed [7][8][16,39]. Lai et al. evaluated coronary artery calcium (CAC), showing a higher risk CAC burden in the left-sided breast cancer patients compared to right-sided breast cancer patients exposed to RT [9][40]. Still, one recent study made on Asian cancer patients did not reveal a significant increase in the risk of ischemic heart disease-related mortality or overall mortality comparing left- vs. right-sided breast cancers in modern-era RT [10][41]. On the other hand, Tagami et al. showed that left-breast-cancer patients treated with RT have a significantly higher incidence of CAD compared with right-breast-cancer patients. Moreover, radiation doses are correlated with the incidence of CAD [11][42].
According to the BACCARAT study (breast cancer and cardiotoxicity induced by radiotherapy), the radiation doses administered to patients with right-sided breast cancer were significantly lower compared to those with left-sided breast cancer (p < 0.001), with the exception of the dose to the right coronary artery (RCA), which was higher in right-breast-cancer patients. The study also revealed that the mean dose to the heart and the mean dose to the left ventricle were significantly higher in patients who experienced CAC progression compared to those without CAC progression. Among the arteries assessed, the left anterior descending artery (LAD) was found to be the most exposed and affected. Non-zero CAC at baseline was identified as a major risk factor for CAC progression, along with traditional CAD risk factors and medication use. Therefore, patients with pre-existing coronary atherosclerosis and CAD risk factors are at a higher risk of accelerated CAD after exposure to radiotherapy. Additionally, the BACCARAT study demonstrated that subclinical left ventricle dysfunction, defined as a decrease in GLS greater than 10%, is associated with cardiac radiation doses. This suggests that left ventricle dosimetry could potentially assist in identifying high-risk populations susceptible to cardiac damage induced by radiotherapy [12][43]. The decrease in left ventricle strain was most significant in the endocardial layer. In other words, the endocardial layer-based longitudinal strain (LS) might be the most sensitive parameter to predict cardiac damage [13][44].
RT-induced atherosclerosis is characterized by long, diffuse and concentric lesions [14][45]. Optical coherence tomography (OCT) of these coronary lesions confirms high amounts of fibrin, lipids, and macrophages in these lesions [15][46]. Necropsy studies showed that the atherosclerotic plaques of people exposed to RT are more fibrous than of people not exposed to RT [16][47]. In addition to the accelerated atherosclerosis, the local inflammation provoked by RT increases intimal proliferation, platelet aggregation, activates the coagulation cascade and promotes clot formation. Moreover, free radicals cause DNA disruption, preventing replication and protein synthesis and thus contributing to fragile tissue formation. Therefore, when following-up their patients, clinicians should take into account that CAD could manifest as sudden cardiac death, and syncope, acute or chronic coronary syndromes.
In the development and localization of CAD, the radiation dose is an important predictive factor. One study showed that the dose–volume histogram was significantly higher in patients with left-sided breast cancer. Those patients had more perfusion abnormalities detected by perfusion SPECT, predominantly in the apical region and the anterolateral walls [17][48]. Scintigraphic defects can appear before electrocardiographic or classic echocardiographic changes; so, screening for silent ischemia should be taken into account.
A recent study showed that the dose received by the LAD correlated with adverse cardiac events. Minimizing the dose administered to the LAD should be of great importance in minimizing ischemic events in left-breast cancer survivors [18][49].
When treating thoracic RT-induced CAD, PCI should be performed using the implantation of a drug-eluting stent (studies showing no difference in cardiac mortality between patients with and without prior chest RT) [19][50], rather than using bare-metal stents or balloon angioplasty (increased risk of all-cause and CV mortality in patients exposed to RT) [20][4].
Surgical myocardial revascularization remains an option, but case selection is more difficult and should be applied only to particular cases. Among the difficulties encountered, there are the following: harder healing of the tissues exposed to RT, fragile native coronary arteries and the possibility of atherosclerosis or other inadequacy of the internal mammal arteries that have also been exposed to RT [21][51]. Studies show that restenosis of the grafts is more frequent than in the general population in people exposed to RT [22][52]. Another important aspect when considering surgical revascularization of the myocardium in thoracic cancer survivors is the fact that the thoracic aorta might be severely calcified, which would make aortic clamping for cardiopulmonary bypass difficult and dangerous (risk of aortic dissection or stroke) [22][52].
Hence, rwesearchers c consider that screening for CAD should be performed in all breast cancer survivors that have been exposed to RT. Functional stress testing (stress echocardiography, stress single-photon emission CT (SPECT)/positron emission tomography (PET), and stress CMRI) and CT imaging should be taken into account to detect CAD. Previous studies recommend functional imaging or CCTA, beginning at 5 years post RT [23][53].
One study that analyzed aortic distensibility after RT in breast cancer showed a significant decrease in aortic distensibility and increase in hs-CRP in the patients that were exposed to RT compared to the patients that were not. Importantly, the aortic distensibility was significantly related to age, systolic blood pressure, and RT dose, the aortic distensibility decreasing with higher radiation doses [24][54].
3. Valvular Heart Disease
Valvular heart disease is another frequently observed pathology after RT exposure, the incidence being as high as 26% after 10 years and 60% after 20 years [25][8]. Patients who have undergone RT display fibrotic changes in their heart valves that are similar to those found in other areas of the heart. This includes thickening and an increased concentration of collagen, which exceeds that of degenerated valves in other patient populations [25][8].
RT usually affects the left-sided valves, with aortic regurgitation being the most frequent valvular disease after radiation exposure, followed by aortic stenosis [5][13]. One trademark of patients exposed to mantle-RT is calcification of the aortomitral continuity, which can be observed at echocardiography [26][55]. In the case of the mitral valve, the base and middle portions of the anterior mitral valve leaflets are being affected, sparing the mitral valve tips and commissures, which makes it distinguishable from rheumatic disease [27][19].
Valvular disease is more likely to occur with greater radiation exposure, with the median interval between RT exposure and valvular disease being 23 years [25][8]. In thoracic cancer survivors treated with RT that develop radiation-induced severe aortic stenosis, TAVI should be taken into consideration even for those with intermediate surgical risk [28][56]. Still, Bouleti et al. found a nonsignificant increase in 5-year mortality in patients that developed radiation-induced aortic stenosis and were subsequently treated by TAVI [29][57]. Moreover, these patients have a higher risk of post-procedural atrial fibrillation and atrioventricular block requiring permanent cardiac pacing than the normal population [30][58]. Also, an echocardiographic study showed a higher incidence of paravalvular aortic regurgitation post-TAVI, probably due to the poor quality of the peri-valvular tissue that was affected by RT [31][59]. In patients with severe aortic stenosis undergoing SAVR, patients with prior mediastinal RT have significantly worse longer-term survival [32][60]. A study that was conducted at the Mayo Clinic and that compared TAVI and SAVR in patients previously exposed to RT showed a lower 30-day mortality with TAVI (1.8% versus 9.1%, p = 0.21) [33][61]. Surgical valve replacement of both the aortic and mitral valve of patients exposed to RT has been associated with dramatic decreases in 5-year postoperative survival [34][35][62,63]. To our knowledge, no large scale studies have been made to study solely the case of breast cancer survivors priorly exposed to RT.
4. Heart Failure and Cardiomyopathies
Breast cancer survivors who have undergone RT may experience heart failure with reduced or preserved ejection fraction due to various cardiomyopathies. In addition to ischemic etiologies, fibrosis of the myocardium can lead to decreased compliance and diastolic dysfunction, while hypertrophy and/or dilatation may result from valvular disease. Some patients may also develop restrictive cardiomyopathy as a result of constrictive pericarditis [36][64]. Interestingly, sometimes the right ventricle is more affected by the radiation, as it is located more anterior and closer to the chest wall [37][65]. Treatment of heart failure in those patients is guided the same way as in non-RT-exposed patients.
5. Disease
Pericardial damage after RT can take any form, from asymptomatic to acute pericarditis and cardiac tamponade or chronic constrictive pericarditis. A pathology post-mortem study showed that up to 70% of RT-exposed patients have some form of pericardial disease [38][66]. Currently, pericardial diseases are less common as a complication of chest radiotherapy, but are more likely to develop in patients treated with a radiation dose of at least 50 Gy. The incidence of pericarditis caused by radiation is closely linked to the dose received by the pericardium, with an increase from less than 5% to over 50% as the total radiation dose to the heart is increased from 40 to 50 Gy. Chronic pericarditis may occur from 6 months to 15 years after radiotherapy and can develop in up to 20% of patients treated with high radiation doses. The use of newer RT techniques that involve smaller total heart doses and conformational techniques has led to a decrease in these manifestations [39][40][67,68].
Pericardial effusion can develop as soon as a couple of days after radiation exposure, but cases have been reported when it appeared a month or even decades after initial treatment, and in some cases it remains chronic. The most serious form of pericardial involvement remains constrictive pericarditis, which usually develops 10 years after exposure [5][13]. Treatment is guided the same way as pericardial disease in non-RT-exposed patients, with the final solution being pericardiectomy in severe/recurrent forms. Cases of occult constrictive pericarditis have been reported 40 years after chest radiation exposure [41][69]. This highlights the importance of continuous monitorization of breast cancer survivors, even after long periods.
6. Rhythm and Conduction Abnormalities
Arrhythmias, conduction disease, and autonomic disease may appear. They may include AV block, bundle branch block, and sick sinus syndrome, and should be managed according to the 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy [1][2]. Any type of supraventricular arrhythmia may also arise acutely, of which AF is the most common [42][70].
One study that aimed to identify risk factors for arrhythmia occurrence after RT showed that whole heart dose, left ventricle, right ventricle and left atrium doses were not associated with an increased risk of arrhythmia (OR = 1.00, p > 0.90). In contrast, a non-significant trend toward a potentially higher risk of arrhythmia with increasing right atrium dose was observed (OR = 1.19, p = 0.60). This research atudy also showed that patients with arrhythmia were more likely to have right-sided breast cancer than patients without arrhythmia [43][71]. To gain a more comprehensive understanding of the relationship between RT and the development of arrhythmias in breast cancer survivors, future studies should examine the potential role of right atrium exposure to RT. The laterality of breast cancer is also a crucial factor in the incidence of rhythm disturbances. Furthermore, in evaluating the impact of radiation on the heart, it is important to consider not only the whole heart dose but also the individual doses delivered to each of the four heart chambers. While the whole heart dose may be relatively low, one chamber of the heart may still receive a significant amount of radiation, making it essential to analyze radiation exposure in a more detailed and nuanced manner. Of note, in the role of arrhythmia development, the position of different structures in the heart should be noted, such as the sino-atrial node, which is located in the wall of the right atrium and the atrioventricular node, which is also located in the wall of the right atrium. For example, right bundle branch blocks are present more frequently, as a result of both myocardial fibrosis, as well as of direct exposure to radiation, due to their anterior position in the interventricular septum, which makes them very exposed to the radiation field [44][72]. Infranodal blocks occur more often than nodal blocks [45][12].
Studies have shown that supraventricular and ventricular arrhythmias are more common in patients after thoracic RT, a possible mechanism being the larger amount of myocardial fibrosis [46][73]. Atrial fibrillation (AF) has an increased incidence in cancer patients. The proposed mechanisms for the pathogenesis of AF in cancer patients (other than classic risk factors) are cancer and cancer-treatment-related factors, such as inflammation, fibrosis and hypercoagulability [47][74]. One study that analyzed a large cancer population found that patients who received radiation therapy had a significantly higher prevalence of AF compared to patients who did not receive radiation therapy (5.9 vs. 4.2%; p = 0.046) [48][75]. Moreover, a recent study that investigated the appearance of AF after RT for pulmonary cancer found an increased AF incidence in the first 30 days following RT [49][76]. The maximal dose delivered to the sino-atrial node in RT for lung cancer was an independent factor associated with the occurrence of AF [50][77]. In the case of breast cancer patients, the highest risk of AF was present in women who did not have surgery. The incidence of new onset AF was reported to be as high as 4% in breast cancer patients. These women had a three-fold higher risk of dying from heart or blood vessel problems within one year. Interestingly, women who received brachytherapy, in which radioactive seeds are placed in or near the tumor, had half the risk of developing AF than women who received external beam radiation [51][78]. It remains unclear whether the risk of AF is increased solely due to cancer itself or as a side effect of cancer therapy, or if cancer populations are inherently at a higher risk due to certain population characteristics or an increased incidence of comorbidities. In fact, comprehensive data on the real prevalence of cancer-therapy-induced AF are lacking.
A very recent study by Zafar et al. found that left atrial appendage (LAA) volume rather than left atrium (LA) volume (measured by 3D-volume-rendered cardiac CT) has a poor prognosis in cancer survivors treated with prior thoracic RT [52][79]. To our knowledge, no large population studies have been made regarding the predictive capacities of LA strain in breast cancer patients treated with RT.
7. Effects on Cardiac Implantable Devices
The number of patients with cardiac implantable devices is rising, along with the number of cancer patients. It is therefore expected that a part of breast cancer patients that are being exposed to RT might have cardiac implantable devices. RT can lead to the malfunction of cardiac implantable electronic devices (CIEDs). The risk of CIED malfunction increases with higher radiation doses (with 2–5 Gy generally being considered the dose threshold) and with the use of high-energy photon RT. As a result, non-neutron-producing treatment is preferred for patients with a CIED. Malfunction of these devices can result in temporary or permanent damage to the device, leading to pacing issues. Patients with a CIED should be evaluated by their cardiologist or electrophysiologist according to group risk and task group paper recommendations. In some cases, relocating the CIED may be necessary, which carries additional risks [1][53][2,80].
Patients who require ventricular assist devices (VADs) are increasingly being diagnosed with neoplasia (7% of VAD patients) and may require radiotherapy. Therefore, close monitoring is essential. To detect VAD dysfunction, monitoring of vital signs is crucial. When treating these patients with radiotherapy, lower energies (<10 MV) and conformal radiation methods should be used, with the aim of minimizing the dose to the VAD components. However, there are currently no established protocols for these patients [54][81].
