Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Masoud Manjili.
The tumor microenvironment (TME) is a complex and dynamic system that plays a critical role in cancer development and progression. It consists of a variety of cell types, including cancer cells, immune cells, and stromal cells (fibroblasts and endothelial cells), as well as extracellular matrix components and signaling molecules.
- systems immunology
- hepatocellular carcinoma
- inflammation
1. From a Reductionistic Approach to a Systems Immunology Approach
The tumor microenvironment (TME) is a complex and dynamic system that plays a critical role in cancer development and progression. It consists of a variety of cell types, including cancer cells, immune cells, and stromal cells (fibroblasts and endothelial cells), as well as extracellular matrix components and signaling molecules. Solid tumors are generally considered to be more complex than blood cancers in terms of their TME.
Reductionistic approaches to the understanding of the TME involve breaking down the complex system into its individual components and studying them in isolation. This approach has been useful in identifying key molecular pathways and cellular interactions that drive cancer development and progression. However, it has also been criticized for oversimplifying the complex interactions within the TME and ignoring the dynamic nature of the system. In fact, it often fails to capture the full complexity of the TME. For example, studies that focus solely on cancer cells or specific immune cell types may miss important autocrine and paracrine multi-directional mutual interactions, which dynamically change cells of the TME. Another limitation of reductionistic approaches is that they can lead to a narrow focus on specific molecular targets or pathways, which may not fully capture the complexity of the system. For example, targeting a single immune checkpoint molecule may have limited efficacy if other components of the immune system are also contributing to tumor growth and progression. Finally, reductionistic approaches assume that the complex system of interactions in the TME can be understood by assessing the individual function of each cell type present through their cause–effect signaling outcomes. This has been shown by studies evaluating immunosuppressive cell types such as Tregs and MDSCs, which have been identified as one source hindering the effectivity of immune checkpoint therapies [1,2][1][2], yet other studies have found the presence of Tregs for modulating inflammation through co-stimulatory molecule inhibition [3]. The depletion of suppressive cell types or pathways may transiently restore the effector functionality of CD8+ T cells [4], whereas the efficacy of immune checkpoint therapies targeting T cells is still lackluster, which is particularly evident by RNA-seq methods discovering distinct expression states of immune cells throughout the course of tumorigenesis [5]. Thus, a major issue with research conducted with highly targeted approaches is the deficiency of dynamic assessments by snapshot studies at a single time point, as well as the evaluation of only a single cell type or signaling mechanism in an isolated state. Next-generation sequencing techniques focused on characterizing the TME of solid tumors have exemplified this, as they were unable to elucidate the transformation and generation of cancer [6]. To this end, other methods like live cell imaging can be utilized for the identification of dynamic features in real time. Even though they do not extract nearly as much information as snapshot studies using novel sequencing methods, transitions between phenotypes occurs in temporal fashions [7]. Temporal changes such as these are also seen in cytokine expression, which can further be influenced by dynamic changes in the resident microbial species at the same time during tumor progression [8].
An important finding from recent single-cell sequencing methods is the vast pools of data showing unique transcriptional states of individual cell types. Especially in regard to the canonical understanding of cellular subsets such as macrophages being termed M1 or M2, in which transcriptional data have shown that this classification scheme does not provide the best results for macrophages found in the TME [9[9][10],10], which is actually an oversimplification of their heterogeneity [11]. In fact, tumor-associated macrophages (TAMs) identified with high-throughput sequencing methods have been shown to have a much more complex phenotype than the classic dichotomy of M1 and M2, along with other immune cells such as T cells and dendritic cells exhibiting distinct gene expression states [12]. This has also been seen in cancer patients where TAMs that were considered M1 or M2 shared features of one another, such as M2 TAMs producing TNF-α [13[13][14],14], as well as macrophages not abiding by the prototypical polarization patterns [15]. This would suggest dynamic cellular states in the TME, which cannot be understood by classic cellular classifications. This is a key point because utilization of standard markers for these cell types may bias analyses within the TME that could be more comprehensively evaluated through scRNA-seq approaches, appreciating the plasticity of the cell subsets, along with the vast amount of transcriptomic data obtained in this approach to decipher their true phenotype. However, the use of sequencing techniques generating big data has put in a position where there is too much information to handle, in which there is a growing appreciation for the inadequacies of targeted studies to fully comprehend the diverse networks of signaling interactions between cell types in the microenvironment [20,21][16][17]. Therefore, the biological function and outcome of disease are multifaceted and too complex to understand through rather reductionistic perspectives, as the interactions between cells are constitutively changing in a dynamic manner.
2. Application of Systems Immunology Approach from Descriptive to Mechanistic Understanding of the TME
The immune response against cancer functions as a system where immune cells are connected to each other through a mutually interacting network that would be dynamically changing alongside the tumor [26][18]. Therefore, the outcome of immune responses encompassing the effector and suppressor cells dynamically interacting would depend on the collective immune function. This could only be understood by taking a systems immunology approach that has not been commonly used in the past, but with the advent of novel “omic” technologies, it has begun to induce a resurgence in more holistic research [27][19]. Systems immunology is becoming more tangible and achievable in its application to diseases like cancer and immune intolerance as a way to make sense of the complex interactions between immune cells and the development of novel therapeutics [28,29][20][21]. To this end, recent events have shown the capability of system-level approaches to understand immune responses in COVID-19 patients and discover molecules involved in disease pathogenesis [30,31][22][23]. In addition, big data has started to be used as a tool to comprehend the TME alongside biomarker identification, drug discovery, and molecular diagnostics [20,32,33][16][24][25]. Regardless, the lack of methods to efficiently comprehend high-throughput sequencing data has resulted in the generation of an immense amount of descriptive knowledge without information regarding the causative mechanisms of disease. Such descriptive information requires a method for shedding light on the mechanism of diseases. Advances in other “omic” techniques, such as proteomics via mass spectrometry and integrated technological methods, have also been able to comprehend communication networks and mechanisms of processes like host defense and tissue homeostasis [37][26]. Additionally, “omic” methods focused on understanding the tissue microbiome can provide insights as well, given the role of microbial species in both homeostatic or inflammatory events during dysbiosis [38[27][28],39], exemplifying yet another method that can be used to understand the TME at the systems level. Systems-level approaches certainly seem daunting, given the immense amount of information and knowledge required to fully understand the TME, but many of these novel “omic” techniques, like microbiome composition, represent avenues to further study the microenvironment. In conjunction with advances in big data, the optimization and availability of machine learning have become useful in certain complex cancers like HCC [40,41][29][30]. In addition to these novel methods, computational modeling has been used for understanding inflammatory diseases like multiple sclerosis to evaluate interactions between tumors and immune cells [42][31]. Together, these methods enabled to understand the immune system from this holistic perspective, which has been lost in the field of immunology over time and replaced by targeted approaches that fixate on a single cell type or mechanism. However, these approaches have helped generate an insurmountable amount of immunological insight; this represents an urgent need for change in the field, as pattern discovery and computational modeling have shown their usefulness in areas of neuroscience and physics to comprehend dynamic systems via top-down models [43][32].3. Pattern Discovery Approach to the Understanding of the TME
Ironically, these reductionist approaches miss actual mechanisms of tumor progression or inhibition by overlooking the collective immune function and, in fact, miss the forest for the trees [23][33]. Other groups have also used pattern recognition through the implementation of novel algorithms to identify immune cell patterns in cancer patients that were associated with different prognoses [44][34] and responsiveness to immunotherapy [45][35]. Therefore, pattern discovery methods should be utilized to evaluate the immune system and the network of interactions between the cell types present in the TME, as this can provide a means to modulate the immune patterns present in order to achieve effective anti-tumor immunity. Strategies to modulate immune patterns require more comprehensive data on the TME and are highly dependent on the perspective and level at which the pattern is to be modulated. This has recently been discussed even for the understanding of the pattern of breast cancer dormancy by settling at the mitochondria scale [24][36] because of its key role in cell death and cell cycle arrest during tumor response to cancer therapies [46][37]. Interestingly, red blood cells are the only human cells that lack mitochondria, and they do not become malignant. An excessive red blood cell production or polycythemia vera is, in fact, a disorder of the bone marrow or myeloproliferative neoplasm rather than a malignancy of red blood cells [47][38]. Exosome signaling is one component that cannot be detected without more specific techniques using microfluidics and biosensors [48[39][40][41],49,50], which could identify additional signaling mechanisms undetectable by single-cell sequencing technologies. In addition, the tissue-resident microbiome is another piece of the TME that can influence the inflammation status and frequency of carcinogenic events [51,52,53,54,55][42][43][44][45][46]. Microbial species even fluctuate during the development of cancer, in which these shifts can modulate immune cell phenotypes and facilitate inflammation [56[47][48],57], thereby representing another piece of the TME puzzle that can be exploited as a target to influence the overall immune pattern in the microenvironment. Therefore, manipulating the gut microbiome or breast microbiome could also be considered a potential immune-modulating therapy for cancer [58,59][49][50]. Interestingly, the underlying microbiome has already been targeted by less intrusive interventions like dietary alterations and supplements [60,61][51][52] and also serves as a potential preventative therapy [62][53]. Lastly, even patients given immunotherapies with suitable responses have detected beneficial microbial species that coincide with elevated immune function [63][54], further exemplifying the importance of studying the microbiome’s role in the system of immune cells in the TME. Of course, this necessitates additional studies focused on comprehending the key microbial species to be targeted, as well as their byproducts and subsequent effects on the immune pattern. Nevertheless, there are plenty of “omic” technologies that could be used to generate the insight required to devise these novel therapies, which could effectively and non-invasively modulate the immune cells in the TME toward an advantageous functionality.4. Collective Immune Function Emerging from Immune Pattern of Interactions: A Case for Artificial Intelligence
The immune response to the presence of a tumor is induced as a system comprising the innate and adaptive immune cell types interacting with each other and with non-immune cells of the TME to create a collective function that determines the progression or inhibition of cancer. Few studies attempt to understand the immune system in this manner, especially in regard to the immense amount of interactions occurring between cells in the TME, but characterizing the TME of cancer patients has shown both prognostic value and immunotherapy responsiveness based on the immune cell patterns and their biological function [64][55]. Assessments of circulating immune cell composition in patients given immunotherapies have also demonstrated dynamic features in peripheral immune cell signatures [65][56], as well as immune phenotyping of cancers that show different patterns of immune signatures corresponding to patient prognosis [65,66][56][57]. Even transcriptional signatures of mixed immune cell populations have identified components involved in disease pathogenesis, but more importantly, understanding how multiple signaling stimuli are integrated and influence immune cells in inflammatory microenvironments can provide a more comprehensive understanding of the TME [70][58]. The collective function of the immune response would also dynamically change in the TME through multiple signaling axes from the immune, structural, and tumor cells present and engaging in mutual signaling interactions. Such multi-directional mutual signaling interactions may change depending on the pattern of immune cells in the TME. To this end, dysregulation of the immune system and the proportion of individual immune cell types, along with their production of functional signaling molecules and the expression of activating or inhibitory receptors and ligands, are critical for understanding the development of cancer [71][59]. Even epigenetic studies using systems immunology via a pattern discovery approach in the TME have identified patterns of infiltrating immune cells in cancer patients that correlate to DNA methylation regulator genes, in which scoring systems could help predict patient survival [72][60]. This represents another level of understanding of the immune response, where epigenetic alterations may polarize cells toward a set of functional signaling that perturbs the immune patterns and facilitates the retention of functions fostering tumor survival. These reports help signify the fact that the immune system manifests variable functions when evaluating the individual cell types, but their mutual interactions together as a whole generate a collective immune response that dictates if a tumor will persist or be eliminated. This can be likened to the proportion of ingredients needed in a recipe and how significantly these components impact flavor, texture, and the appearance of a dish. Chefs experiment with the proportion of ingredients to generate new unique flavor combinations, as well as different textures and appearances that would be desirable to the consumer. In other words, the use of different ingredients in varying amounts can result in pleasant and unpleasant flavors in a dish, similar to the pattern of immune cells, where an excess or deficiency of a particular cell type in the immunological pattern, along with TME conditions lead to different outcomes of disease progression (Figure 1).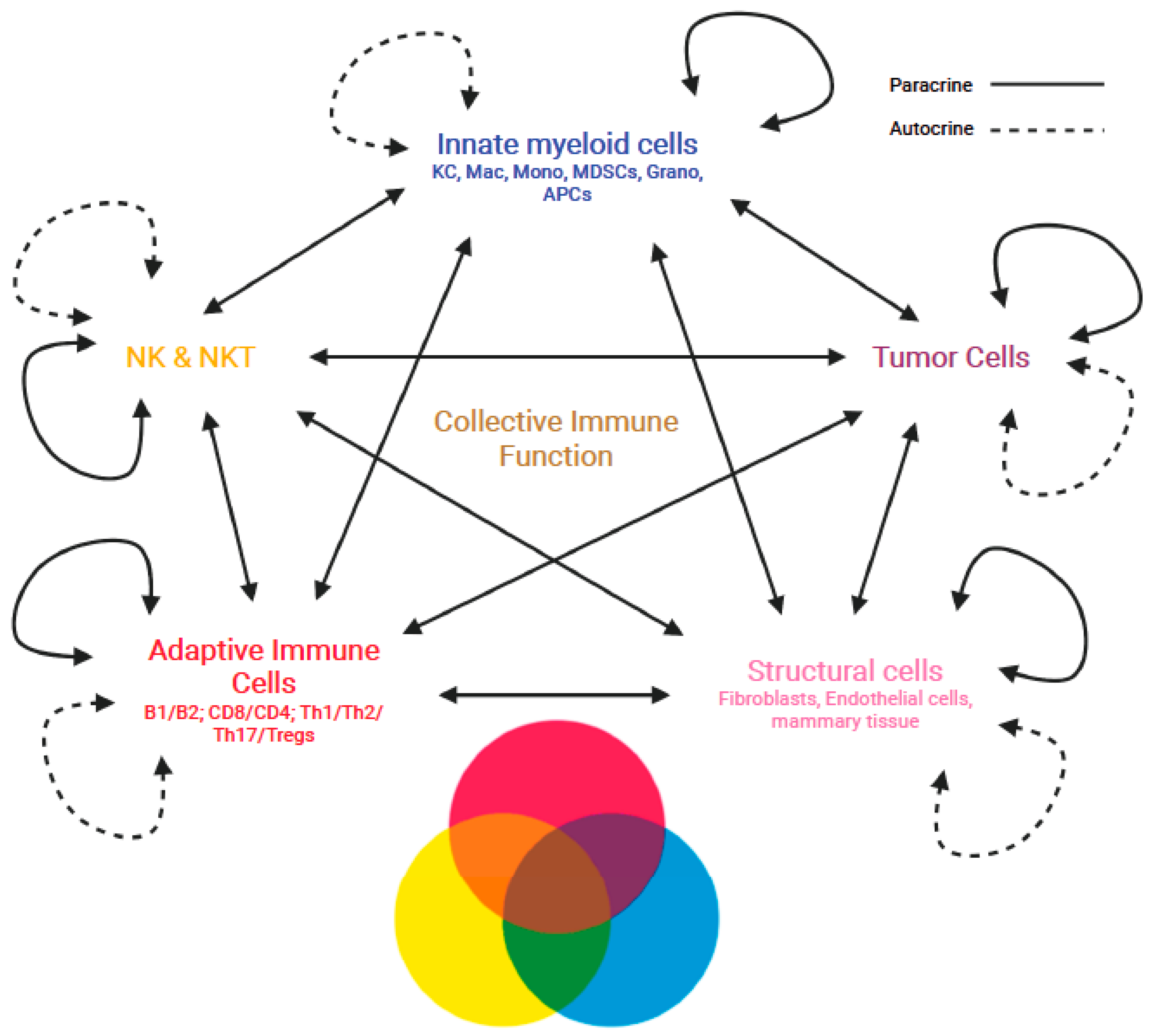
Figure 1. Manifestation of emergent collective immune functions through the multi-directional mutual interactions of immune cells in the TME. Multi-directional mutual interactions of the immune cells and non-immune cells in autocrine and paracrine fashions dynamically shaping the collective immune function in the TME.
5. Immunotherapy of Cancer: Immune Modulation or Immune Cell Induction?
Current immunotherapies are guided by reductionist approaches and offer a specific immune cell type, such as T cells, NK cells, or antibodies, for the treatment of breast cancer. Although decades of experience with these immunotherapeutics have proven to prolong patients’ survival, a cure for advanced-stage disease remains elusive. In fact, breaking down various therapies into a single cell type or signaling pathway like anti-CTLA4 immunotherapy [82][69], anti-PD-L1 immunotherapy [83][70], CAR-NK cell therapy [84][71], and antibody therapies [85][72] exemplify the shortcomings of this approach, given the fact that none of these therapies could offer a cure to cancer patients. One reason for the transient efficacy of such immunotherapies is that it would take time for an altered immune pattern within the TME to control new anti-tumor T cells by forcing them to adapt to the TME. Additional efforts in this reductionist route are expected not to go beyond incremental advances in cancer immunotherapy. Therefore, immune modulatory interventions that may change the immune patterns could serve as a better avenue for therapeutic development, as so many immunotherapies targeting a single cell type solely focus on augmenting individual immune cell responses. For example, CAR T cell therapies have been quite promising for hematological cancers because of the less complexity of the TME, but they are still appraised as rather exploratory and require much more research, despite repeated failures to become effective for solid tumors, perhaps because of the complexity of the TME [86,87][73][74]. This makes CAR T cell therapy ineffective against breast cancer [88][75]. On the other hand, advances in systems immunology could result in breakthroughs in understanding the TME and, in turn, the development of immune modulatory approaches for targeting and fixing an impaired pattern of immune cell interactions or their collective immune function [89][76]. Artificial intelligence has been applied to the prediction of responses to immunotherapy based on immune signatures as well as neoantigen prediction based on the MHC background of patients for designing personalized cancer vaccines. Future application of artificial intelligence in systems immunology is expected to be focused on the discovery and modulation of immunological patterns as a therapeutic means for breast cancer patients. Major differences between reductionistic and systems immunology approaches in the understanding of the TME are summarized in Table 1.Table 1.
Reductionistic vs. Systems Immunology approach to TME.
| Reductionistic | Systems | |
|---|---|---|
| Mechanism | Cause-Effect | Pattern of mutual interactions |
| Function | Cellular or humoral immunity | Collective immune function |
| Outcome | Effector or suppressor cells | Dominant-subdominant interactions |
| Immunotherapy | Inducing killer T cells and inhibiting suppressor cells | Modulation of immunological pattern |
References
- Langhans, B.; Nischalke, H.D.; Krämer, B.; Dold, L.; Lutz, P.; Mohr, R.; Vogt, A.; Toma, M.; Eis-Hübinger, A.M.; Nattermann, J.; et al. Role of regulatory T cells and checkpoint inhibition in hepatocellular carcinoma. Cancer Immunol. Immunother. 2019, 68, 2055–2066.
- Iwata, T.; Kondo, Y.; Kimura, O.; Morosawa, T.; Fujisaka, Y.; Umetsu, T.; Kogure, T.; Inoue, J.; Nakagome, Y.; Shimosegawa, T. PD-L1+MDSCs are increased in HCC patients and induced by soluble factor in the tumor microenvironment. Sci. Rep. 2016, 6, 39296.
- Chatzigeorgiou, A.; Chung, K.-J.; Garcia-Martin, R.; Alexaki, V.-I.; Klotzsche-von Ameln, A.; Phieler, J.; Sprott, D.; Kanczkowski, W.; Tzanavari, T.; Bdeir, M.; et al. Dual role of B7 costimulation in obesity-related nonalcoholic steatohepatitis and metabolic dysregulation. Hepatology 2014, 60, 1196–1210.
- Kalathil, S.; Lugade, A.A.; Miller, A.; Iyer, R.; Thanavala, Y. Higher frequencies of GARP+CTLA-4+Foxp3+ T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013, 73, 2435–2444.
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8+ T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232.
- Kim, E.; Viatour, P. Hepatocellular carcinoma: Old friends and new tricks. Exp. Mol. Med. 2020, 52, 1898–1907.
- Wang, W.; Douglas, D.; Zhang, J.; Kumari, S.; Enuameh, M.S.; Dai, Y.; Wallace, C.T.; Watkins, S.C.; Shu, W.; Xing, J. Live-cell imaging and analysis reveal cell phenotypic transition dynamics inherently missing in snapshot data. Sci. Adv. 2020, 6, eaba9319.
- Keane, J.M.; Walsh, C.J.; Cronin, P.; Baker, K.; Melgar, S.; Cotter, P.D.; Joyce, S.A.; Gahan, C.G.M.; Houston, A.; Hyland, N.P. Investigation of the gut microbiome, bile acid composition and host immunoinflammatory response in a model of azoxymethane-induced colon cancer at discrete timepoints. Br. J. Cancer 2023, 128, 528–536.
- Kohlhepp, M.S.; Liu, H.; Tacke, F.; Guillot, A. The contradictory roles of macrophages in non-alcoholic fatty liver disease and primary liver cancer-Challenges and opportunities. Front. Mol. Biosci. 2023, 10, 1129831.
- Degroote, H.; Lefere, S.; Vandierendonck, A.; Vanderborght, B.; Meese, T.; Van Nieuwerburgh, F.; Verhelst, X.; Geerts, A.; Van Vlierberghe, H.; Devisscher, L. Characterization of the inflammatory microenvironment and hepatic macrophage subsets in experimental hepatocellular carcinoma models. Oncotarget 2021, 12, 562–577.
- Remmerie, A.; Martens, L.; Scott, C.L. Macrophage Subsets in Obesity, Aligning the Liver and Adipose Tissue. Front. Endocrinol. 2020, 11, 259.
- Zhang, Q.; He, Y.; Luo, N.; Patel, S.J.; Han, Y.; Gao, R.; Modak, M.; Carotta, S.; Haslinger, C.; Kind, D.; et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell 2019, 179, 829–845.e20.
- Cheng, K.; Cai, N.; Zhu, J.; Yang, X.; Liang, H.; Zhang, W. Tumor-associated macrophages in liver cancer: From mechanisms to therapy. Cancer Commun. 2022, 42, 1112–1140.
- Chen, Y.; Wen, H.; Zhou, C.; Su, Q.; Lin, Y.; Xie, Y.; Huang, Y.; Qiu, Q.; Lin, J.; Huang, X.; et al. TNF-α derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/β-catenin pathway in SMMC-7721 hepatocellular carcinoma cells. Exp. Cell Res. 2019, 378, 41–50.
- Song, G.; Shi, Y.; Zhang, M.; Goswami, S.; Afridi, S.; Meng, L.; Ma, J.; Chen, Y.; Lin, Y.; Zhang, J.; et al. Global immune characterization of HBV/HCV-related hepatocellular carcinoma identifies macrophage and T-cell subsets associated with disease progression. Cell Discov. 2020, 6, 90.
- Reticker-Flynn, N.E.; Engleman, E.G. Cancer systems immunology. eLife 2020, 9, e53839.
- Flynn, J.L.; Chan, J. Immune cell interactions in tuberculosis. Cell 2022, 185, 4682–4702.
- Mirshahi, F.; Aqbi, H.F.; Cresswell, K.; Saneshaw, M.; Coleman, C.; Jacobs, T.; Idowu, M.O.; Dozmorov, M.; Sanyal, A.J.; Manjili, M.H. Longitudinal studies can identify distinct inflammatory cytokines associated with the inhibition or progression of liver cancer. Liver Int. 2020, 40, 468–472.
- Delker, R.K.; Mann, R.S. From Reductionism to Holism: Toward a More Complete View of Development Through Genome Engineering. Adv. Exp. Med. Biol. 2017, 1016, 45–74.
- Davis, M.M.; Tato, C.M.; Furman, D. Systems immunology: Just getting started. Nat. Immunol. 2017, 18, 725–732.
- Bonaguro, L.; Schulte-Schrepping, J.; Ulas, T.; Aschenbrenner, A.C.; Beyer, M.; Schultze, J.L. A guide to systems-level immunomics. Nat. Immunol. 2022, 23, 1412–1423.
- Dhillon, B.K.; Smith, M.; Baghela, A.; Lee, A.H.Y.; Hancock, R.E.W. Systems Biology Approaches to Understanding the Human Immune System. Front. Immunol. 2020, 11, 1683.
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.A.P.M.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tak-Yin Tsang, O.; et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 2020, 369, 1210–1220.
- Wooden, B.; Goossens, N.; Hoshida, Y.; Friedman, S.L. Using Big Data to Discover Diagnostics and Therapeutics for Gastrointestinal and Liver Diseases. Gastroenterology 2017, 152, 53–67.e3.
- Lazar, J.; Antal-Szalmas, P.; Kurucz, I.; Ferenczi, A.; Jozsi, M.; Tornyi, I.; Muller, M.; Fekete, J.T.; Lamont, J.; FitzGerald, P.; et al. Large scale plasma proteome epitome profiling is an efficient tool for the discovery of cancer biomarkers. Mol. Cell. Proteom. 2023, 22, 100580.
- Sukumaran, A.; Coish, J.M.; Yeung, J.; Muselius, B.; Gadjeva, M.; MacNeil, A.J.; Geddes-McAlister, J. Decoding communication patterns of the innate immune system by quantitative proteomics. J. Leukoc. Biol. 2019, 106, 1221–1232.
- Potrykus, M.; Czaja-Stolc, S.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Intestinal Microbiota as a Contributor to Chronic Inflammation and Its Potential Modifications. Nutrients 2021, 13, 1189.
- Chen, F.; Yang, J.; Guo, Y.; Su, D.; Sheng, Y.; Wu, Y. Integrating bulk and single-cell RNA sequencing data reveals the relationship between intratumor microbiome signature and host metabolic heterogeneity in breast cancer. Front. Immunol. 2023, 14, 1140995.
- Zou, Z.-M.; Chang, D.-H.; Liu, H.; Xiao, Y.-D. Current updates in machine learning in the prediction of therapeutic outcome of hepatocellular carcinoma: What should we know? Insights Imaging 2021, 12, 31.
- Chen, D.; Liu, J.; Zang, L.; Xiao, T.; Zhang, X.; Li, Z.; Zhu, H.; Gao, W.; Yu, X. Integrated Machine Learning and Bioinformatic Analyses Constructed a Novel Stemness-Related Classifier to Predict Prognosis and Immunotherapy Responses for Hepatocellular Carcinoma Patients. Int. J. Biol. Sci. 2022, 18, 360–373.
- Coggan, J.S.; Bittner, S.; Stiefel, K.M.; Meuth, S.G.; Prescott, S.A. Physiological Dynamics in Demyelinating Diseases: Unraveling Complex Relationships through Computer Modeling. Int. J. Mol. Sci. 2015, 16, 21215–21236.
- Pezzulo, G.; Levin, M. Top-down models in biology: Explanation and control of complex living systems above the molecular level. J. R. Soc. Interface 2016, 13, 20160555.
- Mirshahi, F.; Aqbi, H.F.; Isbell, M.; Manjili, S.H.; Guo, C.; Saneshaw, M.; Bandyopadhyay, D.; Dozmorov, M.; Khosla, A.; Wack, K.; et al. Distinct hepatic immunological patterns are associated with the progression or inhibition of hepatocellular carcinoma. Cell Rep. 2022, 38, 110454.
- Huang, J.; Zhang, L.; Chen, J.; Wan, D.; Zhou, L.; Zheng, S.; Qiao, Y. The Landscape of Immune Cells Indicates Prognosis and Applicability of Checkpoint Therapy in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 744951.
- Guo, Y.; Yang, J.; Ren, K.; Tian, X.; Gao, H.; Tian, X.; Zhang, X.; Kan, Q. The Heterogeneity of Immune Cell Infiltration Landscape and Its Immunotherapeutic Implications in Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 861525.
- Manjili, M.H.; Khazaie, K. Pattern recognition of tumor dormancy and relapse beyond cell-intrinsic and cell-extrinsic pathways. Semin. Cancer Biol. 2022, 78, 1–4.
- Klein, K.; He, K.; Younes, A.I.; Barsoumian, H.B.; Chen, D.; Ozgen, T.; Mosaffa, S.; Patel, R.R.; Gu, M.; Novaes, J.; et al. Role of Mitochondria in Cancer Immune Evasion and Potential Therapeutic Approaches. Front. Immunol. 2020, 11, 573326.
- Ronner, L.; Venugopal, S.; Moshier, E.; Mascarenhas, J. Improving the investigative approach to polycythaemia vera: A critical assessment of current evidence and vision for the future. Lancet Haematol. 2021, 8, e605–e612.
- Liu, C.; Xu, X.; Li, B.; Situ, B.; Pan, W.; Hu, Y.; An, T.; Yao, S.; Zheng, L. Single-Exosome-Counting Immunoassays for Cancer Diagnostics. Nano Lett. 2018, 18, 4226–4232.
- Fan, C.; Zhao, N.; Cui, K.; Chen, G.; Chen, Y.; Wu, W.; Li, Q.; Cui, Y.; Li, R.; Xiao, Z. Ultrasensitive Exosome Detection by Modularized SERS Labeling for Postoperative Recurrence Surveillance. ACS Sens. 2021, 6, 3234–3241.
- Li, J.; Xie, S.; Qu, F.; Tan, W. Aptasensors for Cancerous Exosome Detection. Methods Mol. Biol. 2022, 2504, 3–20.
- Song, Q.; Zhang, X. The Role of Gut-Liver Axis in Gut Microbiome Dysbiosis Associated NAFLD and NAFLD-HCC. Biomedicines 2022, 10, 524.
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandonà, A.; Paci, P.; Capuani, G.; et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65, 451–464.
- Soderborg, T.K.; Clark, S.E.; Mulligan, C.E.; Janssen, R.C.; Babcock, L.; Ir, D.; Young, B.; Krebs, N.; Lemas, D.J.; Johnson, L.K.; et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat. Commun. 2018, 9, 4462.
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; McGilvray, I.D.; Allard, J.P. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 2013, 58, 120–127.
- Niccolai, E.; Baldi, S.; Nannini, G.; Gensini, F.; Papi, L.; Vezzosi, V.; Bianchi, S.; Orzalesi, L.; Ramazzotti, M.; Amedei, A. Breast cancer: The first comparative evaluation of oncobiome composition between males and females. Biol. Sex Differ. 2023, 14, 37.
- Behary, J.; Amorim, N.; Jiang, X.-T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187.
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules 2021, 12, 56.
- Chen, J.; Douglass, J.; Prasath, V.; Neace, M.; Atrchian, S.; Manjili, M.H.M.H.; Shokouhi, S.; Habibi, M. The microbiome and breast cancer: A review. Breast Cancer Res. Treat. 2019, 178, 493–496.
- Soto-Pantoja, D.R.; Gaber, M.; Arnone, A.A.; Bronson, S.M.; Cruz-Diaz, N.; Wilson, A.S.; Clear, K.Y.J.; Ramirez, M.U.; Kucera, G.L.; Levine, E.A.; et al. Diet Alters Entero-Mammary Signaling to Regulate the Breast Microbiome and Tumorigenesis. Cancer Res. 2021, 81, 3890–3904.
- Pan, Y.; Zhang, X. Diet and gut microbiome in fatty liver and its associated liver cancer. J. Gastroenterol. Hepatol. 2022, 37, 7–14.
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14.
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.-X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774.
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103.
- Liu, Z.; Zhang, Y.; Shi, C.; Zhou, X.; Xu, K.; Jiao, D.; Sun, Z.; Han, X. A novel immune classification reveals distinct immune escape mechanism and genomic alterations: Implications for immunotherapy in hepatocellular carcinoma. J. Transl. Med. 2021, 19, 5.
- Brechbuhl, H.M.; Vinod-Paul, K.; Gillen, A.E.; Kopin, E.G.; Gibney, K.; Elias, A.D.; Hayashi, M.; Sartorius, C.A.; Kabos, P. Analysis of circulating breast cancer cell heterogeneity and interactions with peripheral blood mononuclear cells. Mol. Carcinog. 2020, 59, 1129–1139.
- Shimada, S.; Mogushi, K.; Akiyama, Y.; Furuyama, T.; Watanabe, S.; Ogura, T.; Ogawa, K.; Ono, H.; Mitsunori, Y.; Ban, D.; et al. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine 2019, 40, 457–470.
- Touzot, M.; Dahirel, A.; Cappuccio, A.; Segura, E.; Hupé, P.; Soumelis, V. Using Transcriptional Signatures to Assess Immune Cell Function: From Basic Mechanisms to Immune-Related Disease. J. Mol. Biol. 2015, 427, 3356–3367.
- Sachdeva, M.; Chawla, Y.K.; Arora, S.K. Immunology of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 2080–2090.
- Song, D.; Zhou, Z.; Wu, J.; Wei, T.; Zhao, G.; Ren, H.; Zhang, B. DNA methylation regulators-related molecular patterns and tumor immune landscape in hepatocellular carcinoma. Front. Oncol. 2022, 12, 877817.
- Li, H.; Zhang, J.; Loh, Y.H.; Billadeau, D.D. Editorial: Decoding immunologic complex systems in cancer: The cutting-edge technologies revolutionizing cancer immunology and immunotherapy. Front. Immunol. 2023, 14, 1208589.
- Mantrala, S.; Ginter, P.S.; Mitkari, A.; Joshi, S.; Prabhala, H.; Ramachandra, V.; Kini, L.; Idress, R.; D’Alfonso, T.M.; Fineberg, S.; et al. Concordance in Breast Cancer Grading by Artificial Intelligence on Whole Slide Images Compares With a Multi-Institutional Cohort of Breast Pathologists. Arch. Pathol. Lab. Med. 2022, 146, 1369–1377.
- Polónia, A.; Campelos, S.; Ribeiro, A.; Aymore, I.; Pinto, D.; Biskup-Fruzynska, M.; Veiga, R.S.; Canas-Marques, R.; Aresta, G.; Araújo, T.; et al. Artificial Intelligence Improves the Accuracy in Histologic Classification of Breast Lesions. Am. J. Clin. Pathol. 2021, 155, 527–536.
- Miao, Y.-R.; Xia, M.; Luo, M.; Luo, T.; Yang, M.; Guo, A.-Y. ImmuCellAI-mouse: A tool for comprehensive prediction of mouse immune cell abundance and immune microenvironment depiction. Bioinformatics 2022, 38, 785–791.
- Peng, L.; Chen, W.; Zhou, W.; Li, F.; Yang, J.; Zhang, J. An immune-inspired semi-supervised algorithm for breast cancer diagnosis. Comput. Methods Programs Biomed. 2016, 134, 259–265.
- Zhao, W.; Davis, C.E. A modified artificial immune system based pattern recognition approach—An application to clinical diagnostics. Artif. Intell. Med. 2011, 52, 1–9.
- Albaradei, S.; Thafar, M.; Alsaedi, A.; Van Neste, C.; Gojobori, T.; Essack, M.; Gao, X. Machine learning and deep learning methods that use omics data for metastasis prediction. Comput. Struct. Biotechnol. J. 2021, 19, 5008–5018.
- Sapoval, N.; Aghazadeh, A.; Nute, M.G.; Antunes, D.A.; Balaji, A.; Baraniuk, R.; Barberan, C.J.; Dannenfelser, R.; Dun, C.; Edrisi, M.; et al. Current progress and open challenges for applying deep learning across the biosciences. Nat. Commun. 2022, 13, 1728.
- Blomberg, O.S.; Kos, K.; Spagnuolo, L.; Isaeva, O.I.; Garner, H.; Wellenstein, M.D.; Bakker, N.; Duits, D.E.M.; Kersten, K.; Klarenbeek, S.; et al. Neoadjuvant immune checkpoint blockade triggers persistent and systemic T(reg) activation which blunts therapeutic efficacy against metastatic spread of breast tumors. Oncoimmunology 2023, 12, 2201147.
- Liang, X.; Chen, X.; Li, H.; Li, Y. Immune checkpoint inhibitors in first-line therapies of metastatic or early triple-negative breast cancer: A systematic review and network meta-analysis. Front. Endocrinol. 2023, 14, 1137464.
- Raftery, M.J.; Franzén, A.S.; Radecke, C.; Boulifa, A.; Schönrich, G.; Stintzing, S.; Blohmer, J.-U.; Pecher, G. Next Generation CD44v6-Specific CAR-NK Cells Effective against Triple Negative Breast Cancer. Int. J. Mol. Sci. 2023, 24, 9038.
- Dogan, I.; Aydin, E.; Khanmammadov, N.; Paksoy, N.; Saip, P.; Aydiner, A. Termination of trastuzumab in HER2-positive metastatic breast cancer patients who received trastuzumab beyond progression. Sci. Rep. 2023, 13, 8779.
- Efficace, F.; Vignetti, M. Quality of life and CAR-T cell therapy in children, adolescents, and young adults with haematological malignancies. Lancet Oncol. 2019, 20, 1625–1626.
- Guo, J.; Tang, Q. Recent updates on chimeric antigen receptor T cell therapy for hepatocellular carcinoma. Cancer Gene Ther. 2021, 28, 1075–1087.
- Schepisi, G.; Gianni, C.; Palleschi, M.; Bleve, S.; Casadei, C.; Lolli, C.; Ridolfi, L.; Martinelli, G.; De Giorgi, U. The New Frontier of Immunotherapy: Chimeric Antigen Receptor T (CAR-T) Cell and Macrophage (CAR-M) Therapy against Breast Cancer. Cancers 2023, 15, 1597.
- Lu, F.; Ma, X.-J.-N.; Jin, W.-L.; Luo, Y.; Li, X. Neoantigen Specific T Cells Derived From T Cell-Derived Induced Pluripotent Stem Cells for the Treatment of Hepatocellular Carcinoma: Potential and Challenges. Front. Immunol. 2021, 12, 690565.
More
