2. Phenolic Compounds in Human Health
Phenolic compounds are a heterogeneous group of molecules whose central structure can be only a hydroxybenzene or phenol ring (monophenols, e.g., gallic acid, p-coumaric), or composed of two (e.g., stilbenes, dimeric acids), three (e.g., quercetin, genistein), or more joined rings (e.g., proanthocyanins, tannins), the so-called polyphenols. Up to date, more than 50,000 different phenolic compounds are known, and they are classified according to their central structure and radical substituents, being differentiated between two main groups, which are, non-flavonoids and flavonoids
[11][13]. Non-flavonoids compounds are composed of phenolic acids such as hydroxybenzoic and hydroxycinnamic acids, lignans, coumarins and stilbenes, highlighting among them gallic acid, caffeic acid, p-coumaric acid and resveratrol. On the other hand, flavonoids are the most abundant phenolic compounds in plants and, contrary to the previous ones, they are characterized by being a broader set of molecules, being subdivided into flavonols, flavan-3-ols, flavones, flavanones, isoflavones, flavonols and anthocyanins. Among them, kaempferol, catechin, epicatechin, apigenin, hesperetin, naringenin, genistein, cyanidin and delphinidin are the predominant ones
[12][13][14][14,15,16]. All of them have in common the characteristic of being synthesized as plant secondary metabolites where they fulfill a wide range of biological functions. Phenolics can confer protection against ionizing radiation, respond to biological aggressions by secreting phytoalexins, act as antibacterial and antifungal agents, as well as attractants for pollinators, in addition to having capacity to accumulate certain molecules capable to modify the coloration of different organs
[15][17].
Like humans and other animals (Metazoa) are not able to synthetize phenolics, their obtainment comes from the uptake of fruits, vegetables, medicinal plants, food supplements, among others. Their consumption is extremely important and beneficial given their notable antioxidant and anti-inflammatory properties
[16][18]. However, their acquisition is limited owing to their bioavailability, which depends on multiple factors, such as the molecule itself, the intestinal microbiota, pH values and the consumption with other compounds. Furthermore, it is also important to take into account the inter-variability between individuals. All of these factors contribute to the different kinetic characteristics shown by each compound
[17][19]. Currently, the polyphenols with the highest bioavailability are phenolic acids, followed by isoflavones, flavonols, catechins, flavanones, proanthocyanidins and lastly anthocyanins. However, recent studies revealed that, probably, flavanones and anthocyanins can be more bioavailable than previously reported once they suffer an extensive metabolization in intestinal microbiota
[18][19][20,21]. Particularly, and focusing on anthocyanins, Ludwig et al.
[20][22] and Mueller et al.
[21][23] reported that cyanidin glycosides, after metabolization, can originate around 35 different metabolites, where the main ones are 2,4,6-trihydroxybenzaldehyde, p-coumaric, protocatechuic and vanillic acids, and phenolic conjugates (e.g., hippuric, phenylacetic, and phenylpropenoic acids).
Over the last few decades, the vision about phenolic compounds has changed drastically. If before they were considered xenobiotics with the ability to reduce the absorption of proteins and other bioactive compounds, today, their presence in food is increasingly important, as many depth studies have shown their ability to counteract oxidative stress, and therefore, preventing, or attenuating the symptoms of many chronic diseases, such as diabetes and cardiovascular pathologies, and also to control weight
[22][23][24,25]. These compounds already showed ability to regulate the appetite and lipid metabolism, inhibit the differentiation of adipocytes and serve as beneficial gut microbiota prebiotics
[24][26]. Furthermore, they are able to reduce the activity of disaccharidases (i.e.,
α-glucosidase and
α-amylase) and the absorption of sugars, and improve the use of monosaccharides by muscle cells
[25][27]. These capacities are essentially due to their chemical, standing out the presence of multiple hydroxyl groups, which can easily interact with gastrointestinal enzymes involved in carbohydrate metabolism, and hence, interfering with their functions
[26][28]. In the same way, other enzymatic activities have been described regarding phenolics, such as the ability to inhibit the DNA polymerases α and δ (which are involved in cells proliferation), as well as to interact with zinc metalloproteinases, including those involved in ACE system
[27][29]. Lastly, their consumption also shows to have a positive effect on the incidence of cardiovascular diseases, observing a direct relationship between the consumption of these compounds and the reduction of the risk of hypertension, dyslipidemia, coronary and arterial diseases events
[28][29][30,31]. In this way, phenolic compound consumption has been related to a vasodilator effect at the peripheral level, such as in the endothelium, relating this effect to the management of oxidative stress and the blocking of reactive oxygen species
[17][30][31][19,32,33]. Specifically, quercetin and resveratrol, have been shown to be efficient inhibitors of the signaling pathway of the protein Mammalian Target of Rapamycin, which is related to problems of arteriosclerosis, cardiac muscle degeneration and vascular integrity
[32][34]. Besides, both compounds can also regulate the concentration of low-density lipoprotein cholesterol in the blood, improve the oxidative balance due to their high antioxidant capacities and reduce the degenerative effects associated with this metabolic state
[29][31]. In addition, resveratrol, cherries’ cinnamic acids and anthocyanins have been shown capacity to regulate the basal levels of the control systems of circadian rhythms, through the modulation of the expression of CLOCK-BMAL1 genes. Since these genes are involved in the determination of liver sensitivity to insulin and are affected by dark cycles, the action of anthocyanins will allow restoring the correct metabolization of fatty acids
[33][35].
Besides, phenolics also possess antimicrobial activities. They already showed capacity to interfere with the growth of
Escherichia coli H157: H7,
Salmonella sp.,
Listeria monocytogenes and
Citrobacter, with the benefit that the appearance of resistance is a less common event than in the use of conventional antibiotics
[14][34][16,36].
Furthermore, they also play a relevant feature in the control of viral infections of Dengue virus
[35][37], human immunodeficiency virus
[36][38], severe fever with thrombocytopenia syndrome virus
[37][39], hepatitis B virus and influenza virus
[38][39][40][40,41,42], through inhibitory mechanisms of interaction, binding and replication of the virus in the host cells.
Therefore, several works already indicated that the consumption of phenolic compounds through the daily diet offers a wide range of benefits. Even so, it is believed that some of them are still to be discovered, and in this aspect, bioinformatics tools, as the use of molecular docking that allows extensive potentials studies using the information indexed in databases, such as Phenol-Explorer (
http://www.phenol-explorer.eu, accessed on 10 June 2021) or the USDA Nutrient Data Laboratory Flavonoid Database (
https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/methods-and-application-of-food-composition-laboratory/mafcl-site-pages/database-resources/, accessed on 10 June 2021), play an important role. In fact, these tools are considered effective for the search for new treatments against various diseases, once they permit modulating the molecular dynamics of these compounds with different target proteins
[10][11][41][42][43][44][45][10,13,43,44,45,46,47].
Taking into account the described above and knowing that, generally, obese and/or diabetic individuals and people who suffer some morbidities are more susceptible to develop the most serious symptoms of COVID-19, it is not surprising that phenolics have really some anti-SARS-CoV-2 actions, as already documented by these modern tools.
3. Phenolic Compounds in COVID-19
In accordance with the mentioned above, namely in the ability of phenolics to interact in viruses, multiple studies revealed phenolics can also inhibit infection by previous coronaviruses, which are, the SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV). Both viruses present an identic mechanism of infection when compared to that of SARS-CoV-2
[46][47][48][48,49,50].
Focusing on the SARS-CoV-2, its infection and continuous replication into cells can cause an excessive and non-specific immune response in some patients, originating an exacerbated production of pro-inflammatory markers (e.g., interleukins (IL), namely the IL-6, tumor necrosis factor α, macrophage inflammatory protein 1a, monocyte chemoattractant protein 1, and interferon-gamma inducible protein-10)
[49][50][51,52]. Consequently, severe attacks can occur in many organs, including lungs and kidneys, leading to eventual cell death, sepsis, organs failure, and sometimes, patient death
[50][52]. In this context, some phenolic compounds have been shown to have a determining effect on the modulation of interleukin synthesis induction routes, allowing them to be promising tools in the management of this response
[51][53]. These can act at different levels, modulating gene expression and inhibiting the activity of certain receptors related to the initiation of chronic inflammatory response cascades, such as nuclear factor kappa-B, or mitogen-activated protein kinase and cyclooxygenase-2
[52][54].
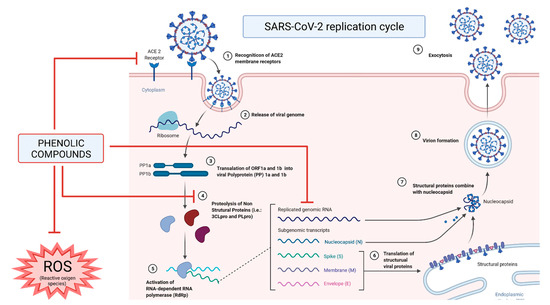
In addition to their anti-inflammatory properties, phenolics already proved to be able to prevent the entry or fusion of this virus in cells by binding to the S protein, modifying its binding site, and thus, avoiding the recognition process with the host, as described in
Figure 2 [42][53][54][44,55,56]. Additionally, phenolics can also interact with membrane-binding receptors, mainly ACE2 proteins, which are the main point of recognition and entry into the host cell by the SARS-CoV-2 virus and block them
[8]. Among phenolics, hesperetin shows a notable ability to binds with ACE2 protein, preventing the ligation of the virus S protein
[42][44].
Figure 2. Mechanisms of action and inhibition of phenolic compounds against severe acute respiratory syndrome of coronavirus 2 (SARS-CoV-2) on its replication cycle and in the host’s immune response.
Phenolics can also interact with the amino acids present in the active sites of M
pro, 3CL
pro and PL
pro proteases, interfering with their activity and blocking the synthesis of several proteins necessary for the correct replication of the virus
[47][55][49,57]. Similarly, it has been observed that phenolics can also stop the activity of RdRp in an analogous way exerted by the antiviral remdesivir, and hence, prevent the replication of the virus
[56][57][58][58,59,60].
Table 1 summarizes the main anti-SARS-CoV-2 effects attributed to phenolic compounds.
Another aspect by which phenolic compounds can act against SARS-CoV-2 infection is due to their ability to control and manage the oxidative stress state once patients with COVID-19 presented higher total oxidative and reduced glutathione levels
[59][61]. For this reason, it is not surprising that the daily intake of phenolics can, through a multifactorial approach at several metabolic and regulatory levels, mitigate this situation of oxidative stress and attenuate the severity of the symptoms
[60][61][62,63].
Table 1.
Anti-SARS-CoV-2 effects attributed to phenolic compounds.
|
Phenolic Compounds
|
Main Outcome
|
Reference
|
|
Cyanidin
|
Mpro inhibitor
|
[62][63][64][65][64,65,66,67]
|
|
Daidzein
|
|
Dieckol
|
|
Genistein
|
|
Mearnsitrin
|
|
Myricitrin
|
|
Psoralidin
|
|
Quercetin 3-O-β-D-glucoside
|
|
Rutin
|
|
Xanthoangelol E
|
|
Benzoic acid
|
RdRp inhibitor
|
[66][68[67],69]
|
|
Cyanidin
|
|
Daidzein
|
|
Ellagic acid
|
|
Gallic acid
|
|
Genistein
|
|
Kaempferol 3-O-rutinoside
|
|
Naringenin
|
|
Oleuropein
|
|
Quercetin
|
|
Quercetin 3-O-rutinoside
|
|
Resveratrol
|
|
Myrcetin
|
Non-structural SARS-CoV-2 helicases inhibitor
|
[68][70]
|
|
Scutellarein
|
|
Cyanidin 3-O-glucoside
|
PLpro inhibitor
|
[55][69][70][57,71,72]
|
|
Epigallocatechin
|
|
Epigallocatechin gallate
|
|
Hypericin
|
|
Kaempferol
|
|
Quercetin
|
|
Cryptotanshinone
|
TMPRSS2 inhibitor
|
[10][68][71][10,70,73]
|
|
Ellagic acid
|
|
Gallic acid
|
|
Kaempferol
|
|
Luteolin
|
|
Quercetin
|
|
Afzelin
|
ACE2 inhibitor
|
[42][68][71][72][73][44,70,73,74,75]
|
|
Apigenin
|
|
Baicalin
|
|
Biorobin
|
|
Caffeic acid
|
|
Catechin
|
|
Chlorogenic acid
|
|
Chrysin
|
|
Ellagic acid
|
|
Curcumin
|
|
Cyanidin
|
|
Delphinidin
|
|
Epigallocatechin
|
|
Epigallocatechin gallate
|
|
Ferulic acid
|
|
Galangin
|
|
Gallic acid
|
|
Hesperetin
|
|
Isoferulic acid
|
|
Kaempferol
|
|
Luteolin
|
|
Myricitrin
|
|
Naringenin
|
|
Nobiletin
|
|
Nympholide A
|
|
Pinocembrin
|
|
Quercetin
|
|
Rhoifolin
|
|
Rutin
|
|
Scutellarein
|
|
Taiwanhomoflavone A
|
|
Tangeretin
|
|
ε-Viniferin
|
|
Chrysin
|
Interact with Spike protein
|
[42][68]][44[71,70][72][73,73,74,75]
|
|
Ellagic acid
|
|
Gallic acid
|
|
Hesperetin
|
|
Pinocembrin
|
|
Artepillin C
|
Inhibit p21-activated kinase 1
|
[74][76]
|
|
Ellagic acid
|
Inhibit furin
|
[75][77]
|
|
Gallic acid
|
Abbreviations: Mpro, Main Protease; PLpro, papain-like protease; RdRp, RNA-dependent RNA polymerase; TMPRSS2, transmembrane protease serine 2; ACE2, Angiotensin-Converting Enzyme II.