2. Proteomics Breakthroughs in Protein Identification
In recent years, the application of multi-omics analysis in translational research on patient samples has proven to be a robust technique. Multiple -omics approaches, including genomics, proteomics, metabolomics, and transcriptomics, have contributed to extensive insights in integrated study of patient data
[17]. Proteomics is the integration of powerful bioanalytical protein studies
[18] on specific types of cells (e.g., tumour, blood, or tissue) in a wide range of diseases. Alteration of protein expression levels and/or post-translational modification allows for the investigation of potential protein biomarkers for diagnostic purposes and new therapeutic discoveries
[19][20][19,20]. To date, the outcome of a combination of proteomics and genomics assays has shown promise in translational disease research and could lead to future advances in next-generation diagnostic and therapeutic techniques. The invention of a robust protein separation technology, two-dimensional gel electrophoresis (2D-PAGE)
[21], and advancements in mass spectrometry techniques have enabled accurate mass and chemical structure analysis-propelled proteomics research
[22]. The proteomics workflow includes protein extraction from the biological sample, quantification of protein content via Bradford assay, protein separation via first-dimension electrophoresis (isoelectric focusing) based on its isoelectric points, and second-dimension electrophoresis, which separates proteins based on their molecular weight. The gel is then stained (e.g., silver staining). Protein spots with significant differences in expression are proteolytically cleaved using the trypsin enzyme, followed by mass spectrometry and software analysis to identify the target proteins. Subsequently, the rapid expansion of shotgun proteomics has contributed to the hypersensitivity of mass spectrometry by supplanting the laborious components of 2D gel-based proteomics
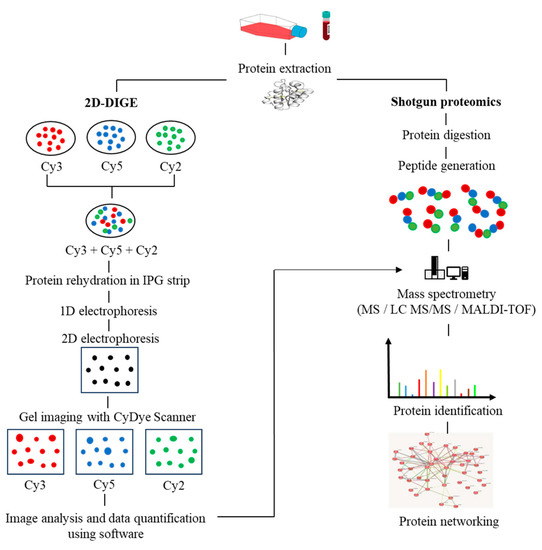
[23]. Moreover, the fluorescence-based two-dimensional difference gel electrophoresis (2D-DIGE) technique represents a significant advancement over the conventional 2D-PAGE method, as it enables advanced quantitative analysis of proteomic samples. The 2D-DIGE technique is characterized by its robust sensitivity, improved accuracy, and resolution, which are achieved through the pre-labelling of individual protein samples with fluorescent cyanine dyes (Cy2, Cy3, and Cy5). Following this, the labelled samples are mixed together and subjected to 2-dimensional electrophoresis (2DE), after which the gel is visualized using fluorescence imaging to identify variations in protein expression across the samples
[24], as illustrated in
Figure 1.
Figure 1.
The workflow in proteomics experiments incorporates protein 2D-DIGE electrophoresis and shotgun mass spectrometry.
3. Post-Translational Modifications in MM
Proteins interact with one another to execute a variety of biological mechanisms
[25][44]. As a result of the dynamic interaction between cellular homeostasis mechanisms and protein interactions that enable speed, adaptability, and compartmentalization, specific proteins are synthesized in the meticulous subcellular compartment and activated in response to stimulation by specific signals
[26][45]. Proteins undergo PTMs, which involve phosphorylation, with a focus on serine, tyrosine, and threonine residues; glycosylation; and ubiquitination. Titanium dioxide enrichment of phosphopeptides and LC-MS/MS analysis revealed 530 phosphorylation sites in primary MM cells from patients. These phosphorylation sites are spread across 325 different phosphopeptides and 260 proteins
[27][46]. Ge et al. studied the cytotoxic effect in MM after bortezomib therapy using quantitative serine/threonine phosphoproteomics. A total of 233 phosphorylated proteins were found, 72 of which were associated with significant upregulation of 1.5-fold or more after bortezomib treatment. Bortezomib induces Ser38 phosphorylation on stathmin, resulting in unstable microtubules and the eventual death of MM cells
[28][47]. Another study found that a fibroblast growth factor receptor 3 (FGFR3) inhibitor reduced the phosphorylation of 52 proteins with 61 pY-phosphotyrosine (pY) sites in KMS11-MM cell lines. Since FGFR3 overexpression in patients with t(4;14) translocation is associated with chemoresistance and a poor prognosis, they might be advantageous as downstream FGFR3 targets
[29][48]. M-proteins have a greater rate of glycosylation, notably in the fragment antigen-binding (Fab) and fragment crystallizable (Fc) domains
[30][49]. Glycosylation modulates several events in the Fab region, including antigen attachment, immune complex formation, and immunoglobulin half-life extension
[31][50]. In contrast, N-glycosylation of the Fc region occurs at asparagine 297, strengthens MM with a stable structure to improve receptor binding on effector cells, and regulates immunoglobulin activity overall
[32][51]. As a result of these outstanding outcomes, the glycoproteomic profile of immunoglobulin has the potential to be used as a possible biomarker for MM.
Ubiquitination maintains the functional state of proteins in homeostasis through an enzymatic cascade comprising ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin-protein ligases (E3)
[33][52]. Due to the high amount of monoclonal antibodies synthesized in MM, the delicate balance between protein synthesis, folding, and degradation is essential for the survival of MM cells
[34][53]. Covalent interactions between E3 ligase and ubiquitin promote monoubiquitination or polyubiquitination. K48 linked polyubiquitination is the most well-studied polyubiquitination pathway related to proteasomal degradation via the ATP-dependent 26 S-proteasome complex
[33][52]. K-63 is associated with endocytotoxic migration, inflammation, and DNA damage repair. Another study has found that K11-linked proteins are involved in mitotic regulation and endoplasmic reticulum-associated degradation.
Monoubiquitination enhances protein activity and translocation errors
[35][54]. Monoubiquitination occurring at histones H2A and H2B derived from E3-ligases of the polycomb repression complex (BMI1 (B cell-specific Moloney murine leukemia virus integration site 1), RING1A/RING1B (Really Interesting New Gene 1A/1B), PCGF2 (Polycomb group RING finger protein 2), RNF8, and 2A-HUB/hRUL138 inhibited RNA polymerase 2 elongation activity and eventually suppressed gene expression
[36][37][38][39][55,56,57,58]. Concurrently, higher BMI1 expression has been observed in the plasma cells of patients with MM
[40][41][59,60], as well as increased MM cell proliferation in vitro and in vivo
[42][61]. In MM, aberrant ubiquitination is predominantly described in the NFB pathway
[43][62], which leads to more aggressive MM by obstructing the deubiquitinating enzyme CYLD
[44][63]. Activation of the NFB pathway regulates the apoptotic mechanism and produces a genetic deletion in MM, such as homozygous deletions of TRAF3, which is induced by the ubiquitin ligases BIRC2/BIRC3
[45][46][64,65].
An extensive effort has been made to formulate certain antibodies for the purpose of post-translational modification analysis. Nevertheless, this effort is challenged by the low binding affinity of available antibodies due to the small size of post-translational modification (PTM) motifs. Additionally, chemical structure similarities among certain PTMs, inadequate antigenicity, and other obstacles in antibody production
[47][66] are also credited with the limitation. Antibodies that are specific to pan-PTM are frequently utilized for immunoaffinity enrichment before conducting LC-MS/MS, Western blotting, protein microarrays, immunohistochemistry, and flow cytometry. The aforementioned methodology has been efficaciously employed for the comprehensive evaluation of protein lysine acetylation
[48][49][67,68], arginine methylation
[50][69], tyrosine nitration
[51][70], and tyrosine phosphorylation
[52][53][71,72]. Additionally, peptide PTM motif-specific antibodies have been utilized to recognize kinase substrates and measure their PTM alterations
[54][73]. The approach for detecting new substrates can be extended to cases where a PTM regulatory enzyme of interest possesses a consensus PTM motif. However, several other post-translational modifications (PTMs) encounter challenges in generating site-specific antibodies, either because of the PTM’s size or swiftness (such as ubiquitination), or due to inadequate evidence of site occupancy, which discourages investing in antibody generation
[55][74].
In numerous proteomics investigations, post-translational modifications (PTMs) are labelled through chemical derivatization, which involves the incorporation of affinity tags to enhance the abundance of PTM peptides that are of interest. For example, azide was utilized as a chemical label for post-translational modification (PTM) proteins and then linked to an affinity linker, such as biotin
[56][57][75,76]. This technique was employed in the detection of protein farnesylation
[58][77], O-GlcNAc modifications
[59][78], palmitoylation
[60][79], and myristoylation
[61][80].
Although various components of the proteomics approach have progressed in the meantime, the process of protein digestion remains predominantly reliant on a single enzyme, typically trypsin. Other proteases, including chymotrypsin, LysC, LysN, AspN, GluC, and ArgC, are utilized to a certain extent
[62][81]. Due to their unique specificities, these proteases produce distinct sets of peptides. Consequently, subjecting the same proteome to parallel digestion with multiple proteases can cover complementary regions of the protein sequence space and the proteome itself
[63][64][82,83]. The protease-digested lysate is an ideal strategy to increase the sequence coverage, thereby facilitating the differentiation of closely associated protein isoforms. Additionally, it enables accurate and reliable protein identification and quantification, encompassing diverse post-translational modification (PTM) sites
[65][66][84,85].
4. Proteomics Alteration in MM
4.1. Monoclonal Gammopathy of Undetermined Significance (MGUS)
MGUS is an early precursor of clonal plasma cell proliferation that forecasts the development of MM
[67][86]. MGUS is an asymptomatic, incidentally diagnosed disorder that is evaluated for progression to MM using a serum protein electrophoresis profile or immunofixation (SPEP or IFX). MGUS is diagnosed when there are less than 30 g/L of monoclonal protein (M-protein) in the blood or less than 10% plasma cells in the bone marrow
[68][87]. In MGUS, 11 proteins were identified as core matrisome proteins, including bone marrow proteoglycan 2 (PRG2) and bone marrow proteoglycan 3 (PRG3), and the remaining 9 proteins were associated with the matrisome, including the ECM-affiliated protein ficolin 1 (FCN1), the ECM-remodeling enzymes CTSG and Serpins, and the secreted factors HRNR, S100A8, and S100. In contrast, 32 proteins were discovered in MM patients, including 10 core matrisome proteins and 22 matrisome-associated proteins. Curiously, Annexin A2 (ANXA2) and Galectin-1 (LGALS1) are potential biomarkers in MM, since both were not observed in bone marrow from control donors or MGUS patients
[69][88]. ANXA2 is also involved in cell development as well as promoting osteoclast synthesis and bone erosion, resulting in an immunosuppressive microenvironment in MM
[70][89]. It also helps MM cells grow and escape from the apoptotic pathway in MM cell lines
[71][90].
The proteomic analysis of proteins obtained from circulating exosomes in 5 MGUS and 10 MM patients and 45 healthy subjects was investigated by Manier et al. using mass spectrometry. They identified a total of 272 proteins in the circulating exosomes. These proteins include annexins, CD9, HSP70, and Rab proteins (Rab7a, Rab5, and Rab27b), which are among those strongly linked with exosomes. The peptide counts for fibronectin, AMBP protein, and Ig gamma-1 chain C region were found to be markedly different. Interestingly, they also reported that elevated expression of fibronection in the microenvironment of multiple myeloma has been associated with both tumor progression and resistance to drug therapies
[72][91].
Proteomic evaluation of all MGUS, SMM, and MM patients revealed a linear trend in vascular endothelial growth factor A (VEGF-A), vascular endothelial growth factor 2 (VEGFR2), and interleukin-6 (IL-6) levels across MM stages. VEGFR2 downregulation was observed with the courses of MGUS, SMM, and MM
[73][92]. Furthermore, the VEGFR2-604TT genotype is more common and associated with MM patients with a higher disease burden. Vascular endothelial growth factor (VEGF) was found to be more prominent in both MM cell lines and primary MM cells from the bone marrow during cancer angiogenesis
[74][93].
4.2. Smoldering Multiple Myeloma (SMM)
MGUS precursors underwent extensive mutational events at a magnitude of 0.1 to 10 per megabase, along with genetic alterations that affected one or more genes, including aneuploidy, chromosomal translocations, single nucleotide variants, small insertions and deletions, and copy number variants in the state transition to SMM
[75][76][77][94,95,96]. It was reported that the higher progression risk of SMM to MM was 10% per year, whereas the progression risk of MGUS was only 1%
[78][97].
4.3. Multiple Myeloma (MM)
Integrative approaches using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry coupled with the ClinProt system revealed ten significantly different expressed proteins. Six and four of them were upregulated and downregulated, respectively, in MM patients compared to healthy controls. Four downregulated proteins were identified: alpha-fetoprotein (AFP), component 3f (C3f), fibronectin 1 (FN1), and glutathione S-transferase π 1 (GSTP1)
[79][102]. AFP is a glycoprotein and member of the serum albumin gene family, elevated levels of which are frequently observed in patients with 47 various malignancies and non-cancerous disorders
[80][103]. Yang et al. published a case study demonstrating an increased AFP level in a 66-year-old male patient diagnosed with IgD-light chain (Stage III B) where the patient also revealed extensive bone destruction of the skull
[80][103].
C3f is an essential component for activating the human innate immune system. It is also responsible for the elimination of potential pathogens
[81][104] and plays a crucial role in the complement cascade
[82][105]. The presence of an elevated C3f level is linked to an increased risk of developing renal illnesses, metabolic syndrome, and rheumatological diseases
[83][84][106,107]. Due to the limited number of publications, additional study is required in the case of MM to define the association between C3f and MM pathogenesis. Upregulation of FN1 is associated with a poor prognosis in certain cancers, including thyroid cancer and nasopharyngeal cancer
[85][86][108,109], because of greater cell motility, cytoskeletal structure, and oncogenic transformation, as previously described in multiple types of cell lines
[87][88][110,111]. Following FN1 overexpression, apoptotic mechanisms via the NF-B pathway are inhibited, which promotes tumor cell migration and invasion
[89][112]. FN1 is recommended as a strong biomarker for drug resistance and radiotherapy in head and neck squamous cell cancer
[90][113]. As a result of its identically elevated profile in MM, FN1 could be employed as a possible biomarker in diagnosing MM
[91][114]. Glutathione S-transferases (GSTs) are phase-II metabolic enzymes
[92][115] that regulate the antioxidant response system
[93][116], tumorigenesis, and detoxification
[94][117] and have been found to be increased in a variety of cancers, including drug-resistant cell lines
[95][118]. GSTs regulate many kinase pathways to promote tumorigenesis
[92][96][115,119]. In MM, GST regulates the BM environment by causing aberrant redox, which results in the initiation of myeloproliferative processes
[97][120]. GST upregulation may serve as a possible prognostic indication in MM and as a robust indicator for assessing patients’ MRD
[79][102].
The extracellular matrix of the bone marrow in patients with MM has been intriguingly characterized using proteomic profiling. ANXA2 and LGALS1 were shown to be elevated in NDMM patients, and the same trend was observed in MM cell lines
[71][98][90,123]. These proteins are thought to be crucial for regulating survival in MM. ANXA2 has been long reported to be significantly upregulated in multiple myeloma in comparison to normal plasma cells
[99][124]. Moreover, the ANXA2 receptor, also known as AX2R
[100][125], exhibits augmented expression in primary myeloma cells as well as in cell lines, thereby promoting the growth of myeloma cells and their adherence to stromal cells
[101][126]. ANXA2 has been found to facilitate multiple pathways, such as angiogenesis
[102][127], osteoblastic mineralization
[103][128], and the growth and differentiation of osteoclast precursors
[103][128]. ANXA2 expression is elevated in all phases of MGUS, SMM, NDMM, and MM, as well as relapsed MM
[69][88].
The LGALs1 gene exhibits Increased mRNA and protein expression that is uniform across inter-patient and inter-human myeloma cell lines (HMCLs)
[104][105][129,130]. Subsequent analysis of primary CD138+ cells revealed that elevated LGALS1 expression is exclusively observed in NDMM patients as opposed to MGUS, SMM, MM relapsed MM, and healthy control groups
[103][105][128,130]. According to Andersen et al., there was a marginally significant increase in LGALS1 protein in MM patients’ peripheral blood in comparison to healthy subjects. Nevertheless, the authors observed no significant association between the increased LGALS1 level and overall survival, treatment response, and clinical pathological parameters
[106][131]. Moreover, the inhibition of LGALS1 in mouse models in vivo results in a reduction in tumor sizes, angiogenesis, and the emergence of bone lesions
[105][130]. However, in 2017 Glavey et al. performed a proteomic characterization of the human multiple myeloma bone marrow extracellular matrix that revealed that MM patients who have elevated levels of ANXA2 and LGALS1 are associated with poor overall survival (OS)
[103][128].
4.4. Myeloma Bone Disease
Myeloma bone disease is a typical occurrence among myeloma patients and can be diagnosed in 70 percent of NDMM patients. Patients with MM who have bone disease suffer from multiple complications, the most significant of which are bone pain (80% of cases), bone fractures (60%), hypercalcemia (15%), and spinal cord compression (3%), all of which contribute significantly to the morbidity and mortality associated with the disease
[107][151]. Previous research employing SELDI-TOF MS revealed a set of four unique biomarkers that could be used to monitor myeloma bone disease in the future
[108][152]. Within a few years, proteome profiling using label-free mass spectrometry revealed a considerable increase in C4 and serum paraoxonase/arylesterase 1 concentrations from MGUS/SMM to severe bone disease
[109][153]. Significantly different serum proteome profiles were studied in MM patients with various degrees of bone disease, describing the interplay between binding and inhibition. Multiple proteins associated with MM bone disease have been identified using a label-free mass spectrometry technique, including enzymes, extracellular matrix glycoproteins, and complement system components. The central protein network displays the most downregulated expression in alpha-1-antitrypsin (SERPINA1), albumin (ALB), α-2-macroglobulin (A2M), coagulation factor V (F5), fibronectin (FN1), plasminogen (PLG), kininogen 1 (KNG1), and platelet factor 4 (PF4), with only fibronectin showing downregulation (FN1)
[109][153].
4.5. Relapse/Refractory MM
A distinct extracellular matrix (ECM) profile was discovered in relapsed MM patients, with 25 significantly elevated proteins including 10 matrisome core proteins and 15 matrisome associate proteins. MGUS and MM patients exhibit a reduction in collagen and other fibrillar ECM glycoproteins, such as fibronectin, when compared to healthy individuals. The presence of specific collagens and matrix metalloproteinases (MMPs) (COL1A1, COL1A2, COL3A1, COL5A1, MMP8 and MMP9) in both healthy donors and MM patients
[69][88] indicates early ECM remodeling leading to MM progression
[110][154]. Alterations in ECM contributed to several stages of metastasis. Furthermore, interactions with the bone marrow microenvironment led to the progression of MM and enhanced a tumorigenic microenvironment
[111][155].
Proteinase inhibitor 9 (SERPINB9) and 34 additional proteins with differential expression in patients with bortezomib-resistant RRMM compared to NDMM patients were discovered using tandem mass tag-mass spectrometry (TMT-MS). Overall, the discovered proteins demonstrated roles in regulating cellular metabolism, apoptosis, programmed cell death, lymphocyte-mediated immunity, and defensive response pathways in RRMM
[112][156]. SERPINB9 is the most established serine protease inhibitor (serpin), and inhibits the serine protease granzyme B (GrB)
[113][157] in the granules of cytotoxic T lymphocytes (CTLs) via the perforin pathway
[114][115][158,159], antigen-presenting cells, and natural killing cells
[116][117][160,161], thereby manipulating the apoptotic cascade
[118][162]. Additionally, SERPINB9 induces resistance to CTL-mediated cell death and cancer cell immune response escape
[119][163].
Numerous upregulated protein trends were observed in refractory MM patients, including proteasome activator complex subunit 1 (PSME1), PSME2, heat shock protein 90 (HSP90), HSPA9, stress-induced-phosphoprotein 1, nucleophosmin, and protein disulfide-isomerase, which significantly differentiated them from patients with very good partial or complete responses. Furthermore, differences in expression were found in proteins involved in oxidative stress and redox homeostasis (thioredoxin (TXN)). Protein downregulation in apoptosis and inflammation-related proteins was seen in bortezomib therapy failure patients. Likewise, MM patients who were resistant to bortezomib had changes in their proteome profile affecting apolipoprotein C1, complement components, and sulfhydryl oxidase 1, all of which play key roles in modulating hydrolase activity and cellular reactions to stimuli
[120][164].
4.6. Extramedullary MM
The aggressive stage of MM is characterized by extramedullary MM (EMM), which was reported in roughly 7% of NDMM and increased to 30% of patients with relapse
[121][165]. After a series of molecular alterations, the clonal cells of the MM disease were able to escape from the bone marrow and migrate via the circulatory system to access and grow independently in other organs and tissues
[122][166]. Zatula et al. employed super-SILAC quantitative proteome profiling to study alterations in proteome architecture in MM and secondary plasma cell leukemia (sPCL) patients and discovered that a few potential protein markers could be targeted by currently offered small molecule therapies. Interestingly, proteome change was distinguished by the vast number of proteins affected (
n = 795). Patients with plasma cell leukemia (PCL) had metabolic changes in aerobic glycolysis, and downregulation of enzymes responsible for glycan production led to changes in surface receptor glycosylation pathways. They also discovered that no substantial change in genomic 5-methylcytosine or 5-hydroxymethylcytosine was observed at either stage, indicating that epigenetic dysregulation is not the primary driver in the progression from MM to PCL
[123][167].
A label-free liquid chromatography mass spectrometric approach identified 21 proteins with significantly different expression in EMM patients compared to MM patients. Antibody-based ELISAs were used to validate six proteins (vascular cell adhesion molecule 1 (VCAM1), hepatocyte growth factor activator (HGFA), pigment epithelial-derived factor (PEDF), α-2-macroglobulin (A2M), cholinesterase (BCHE), and aminopeptidase N (CD13)). Consequently, ROC studies indicate that VCAM1, HGFA, and PEDF have excellent discriminatory power for predicting EMM
[124][168]. Another quantitative MS-based proteomic method revealed that 275 and 271 proteins in EMM were significantly upregulated and downregulated, respectively. Rho-associated protein kinase 2 (ROCK2), Ras-related C3 botulinum toxin substrate 1 (Rac1), and platelet endothelial cell adhesion molecule (PECAM-1) were among the proteins that were upregulated. Interestingly, overexpression of proteins associated with leukocyte transendothelial invasion was also discovered
[125][169].
5. Investigating Biomarkers in Drug-Resistant MM
The most challenging part of treating MM patients is overcoming treatment resistance, which is most likely caused by a complex interaction between clonal heterogeneity and therapeutic pressure. The inevitable emergence of multi-drug resistance in MM contributes to the battle in drug research to identify the protein phenotype responsible for clone resistance
[126][170]. Proteomics technology makes it possible to find out which proteins directly affect the biological processes that lead to the formation of resistant sub-clones.
Quantitative proteomics demonstrate that myristoylated alanine-rich C-kinase substrate (MARCKS) overexpression and phosphorylation are consistent with bortezomib-resistant MM cell lines
[127][171]. LC-MS/MS analysis has also revealed that clusterin (CLU), angiogenin (ANG), and complement 1Q (C1Q) biomarkers are associated with the bortezomib response
[128][172]. Another study found higher expression of apolipoprotein C-I and apolipoprotein C-I’ in bortezomib-resistant MM patients using mass spectrometry
[129][173]. iTRAQ and label-free quantitative mass spectrometry detected 118 proteins (35 up-regulated and 83 down-regulated) that have significantly different expression in a patient with relapsed refractory MM who had a relatively low response to bortezomib, doxorubicin, and dexamethasone (PAD) chemotherapy and achieved a very good partial response (VGPR). The proteins that were discovered are those that are responsible for proteasome activity, responsiveness to oxidative stress, the defensive response, and the regulation of apoptosis
[130][174]. The Frassanito group reported that the oxidative stress response and pro-survival autophagy pathways were activated in bortezomib-resistant MM patients, based on a proteomic study of bone marrow stromal cells (BMSCs)
[131][175]. Metabolomic analysis of ANBL-6 cell lines resistant to bortezomib revealed a significant change in purines and pyrimidines anabolism and catabolism, as well as numerous forms of coenzyme A (CoAs)
[132][176]. In addition, the same analysis demonstrated that hypoxia-inducible factor 1 and its target gene lactate dehydrogenase A are responsible for hypoxia-induced bortezomib resistance in MM
[133][177]. Integrated quantitative tandem mass tag (TMT)-based proteome and phosphoproteomic profiling revealed that CDK6 protein is upregulated in in vitro-generated lenalidomide-resistant MM.1S and LP-1 cells, suggesting that it is a potential target for IMiD-resistant MM.1S
[134][178].
Proteomics analysis and bioinformatics tools proved that the exportin-1 (XPO1) protein and its network contributed to emerging bortezomib resistance in the RPMI 8226 human MM cell line. The cluster of interaction proteins includes structural maintenance of chromosomes protein 1A (SMC1A), regulator of chromosome condensation 2 (RCC2), CSE1, nuclear pore complex protein Nup88 (NUP88), nuclear pore complex protein Nup50 (NUP50), nucleoprotein TPR (TPR), heat shock protein 70 kDa 14 (HSPA14), dynein light chain 1 (DYNLL1), double-strand-break repair protein rad21 homolog (RAD21), and E3 SUMO-protein ligase RanBP2 (RANBP2)
[135][179]. XPO1 is a member of the exportin protein family, also known as the (chromosome region maintenance 1) protein, which has been linked to a variety of cancers, including hematological malignancies
[136][137][138][139][180,181,182,183]. XPO1 is recognized as a nuclear transporter of various RNAs and protein loads from the nucleus to the cytoplasm
[140][141][184,185], and disturbance in this transport channel is typically associated with cancer emergence
[142][186], where extreme nuclear export promotes cancer progression and treatment resistance
[143][187].
6. Therapeutic Agents to Target Protein Signatures
Numerous therapeutic agents have been studied that target protein signatures identified through proteomic analysis in the context of multiple myeloma
[144][197]. The following therapeutic agents are currently available. Bortezomib (Velcade; Takeda Pharmaceuticals, Cambridge, MA, USA) was granted approval by the U.S. Food and Drug Administration (FDA) in 2003, marking the first proteasome inhibitor authorized for use as a third-line treatment of multiple myeloma (MM)
[145][198]. The advent of bortezomib has brought about an important advancement in the management of MM. Presently, bortezomib is sanctioned as a primary therapeutic option for MM and mantle cell lymphoma
[146][199]. Bortezomib is a pharmacological agent classified as a proteasome inhibitor that selectively targets the 26S proteasome complex. The aforementioned phenomenon hinders the processes of protein degradation, transcription factor activation, and cell-cycle regulation and triggers programmed cell death in cells affected by multiple myeloma
[147][148][200,201]. Bortezomib has obtained regulatory approval for the therapeutic management of multiple myeloma and is frequently administered in conjunction with other drugs. The utilization and efficacy of bortezomib in the management of multiple myeloma were investigated by Loke et al. The study involved patients who were administered with standard regimens such as Bortezomib, Melphalan and Prednisolone (VMP), Bortezomib, Cyclophosphamide, and Dexamethasone (CVD), and Bortezomib and Dexamethasone VD, and received Bortezomib either once weekly or twice weekly. It was reported that the overall response rate was 81%. Approximately 53% of the patients were unable to complete their intended treatment regimen due to various reasons, such as toxicity (30%), suboptimal response or disease progression (15%), or mortality during treatment (8%). The study reported a median overall survival of 40.7 months and a median progression-free survival of 17.7 months
[149][202].
Another proteasome inhibitor (PI), Carfilzomib (formerly PR-171), belongs to the epoxyketone class of proteasome inhibitors and exhibits structural and functional variations from BTZ
[150][203]. CFZ has been observed to exhibit strong potential for binding to the i20S proteasome through its highly specific inhibition of CT-L activity. As a consequence, CFZ induces antiproliferative and proapoptotic outcomes in multiple myeloma cell lines
[151][204]. It is noteworthy that CFZ demonstrates an enhanced and prolonged response by means of irreversible proteasome inhibition, in comparison to BTZ, which functions as a reversible proteasome inhibitor
[150][203]. Furthermore, the pharmacophore of epoxyketone exhibits a remarkable capacity for precise targeting of the NH2-terminal threonine residue, thereby enhancing specificity towards the proteasome and ultimately resulting in the irreversible inhibition of enzyme activity
[151][152][204,205]. The utilization of carfilzomib was observed in combination with lenalidomide and dexamethasone, as well as independently, in patients with relapsed and/or refractory multiple myeloma and diverse levels of renal capacity
[152][153][205,206]. In July 2012, the FDA granted approval for the use of Carfilzomib (Kyprolis
®) as a monotherapy for the management of MM in patients with refractory disease
[154][207]. This approval was reserved for patients who had undergone at least two prior lines of therapy and had exhibited disease progression within 60 days of completing their most recent therapy
[155][208].
Ixazomib is a second-generation PI and an orally administered PI that specifically targets the 20S proteasome
[156][212]. Minarik et al. provide evidence that the addition of ixazomib to the lenalidomide–dexamethasone (RD) treatment regimen results in significant enhancements in critical survival outcomes among patients diagnosed with relapsed and refractory multiple myeloma (RRMM) in a standard clinical environment. Patients who received IRD treatment exhibited enhanced PFS and OS outcomes, with a median duration of 36.6 months and 26.0 months, respectively. Nonetheless, the administration of IRD treatment did not confer any advantages to patients exhibiting extramedullary disease, as evidenced by a median PFS of 6.5 months
[157][213].
Additionally, daratumumab is a monoclonal antibody that specifically targets CD38, a protein that is prominently expressed on the surface of multiple myeloma cells
[158][159][214,215]. It facilitates diverse immune-mediated mechanisms to elicit apoptosis of myeloma cells
[160][216]. Daratumumab has received regulatory approval for the management of multiple myeloma, both as a monotherapy and in conjunction with other therapeutic modalities. Clinical trial data evidenced that the administration of daratumumab in patients with multiple myeloma resulted in enhanced survival rates among specific demographic groups. In the CASTOR study, the efficacy and safety of daratumumab in combination with bortezomib and dexamethasone were evaluated in comparison to bortezomib and dexamethasone alone, for patients suffering from relapsed MM. The findings indicate that the incorporation of daratumumab resulted in a noteworthy enhancement in the overall survival rate. The daratumumab group exhibited an indeterminate median overall survival, while the control group demonstrated a median overall survival of 40.0 months
[161][217]. Additionally, the POLLUX study was conducted to assess the effectiveness and safety of daratumumab when used in combination with lenalidomide and dexamethasone, as compared to the use of lenalidomide and dexamethasone alone, in patients suffering from relapsed or refractory multiple myeloma. This clinical trial indicates a noteworthy enhancement in the survival rate as a result of daratumumab administration. The daratumumab group exhibited an indeterminate median overall survival, whereas the control group demonstrated a median overall survival of 44.3 months
[162][218]. Another clinical trial, the MAIA study, conducted a clinical evaluation of the effectiveness and safety of daratumumab in conjunction with lenalidomide and dexamethasone, as compared to the administration of lenalidomide and dexamethasone alone, among individuals diagnosed with multiple myeloma who were deemed ineligible for transplant. The incorporation of daratumumab led to a notable enhancement in overall survival. The daratumumab group demonstrated an indeterminate median overall survival, while the control group exhibited a median overall survival of 68.3 months
[163][219].
Elotuzumab is a monoclonal antibody that specifically targets SLAMF7 (CS1), a cell surface protein that is expressed on myeloma cells
[164][220]. The drug has been sanctioned for the management of multiple myeloma in conjunction with other agents, as it amplifies immune-mediated cytotoxicity against myeloma cells
[165][221].
Venetoclax is a diminutive molecule inhibitor that specifically targets BCL-2, an anti-apoptotic protein that is frequently overexpressed in multiple myeloma cells
[166][222]. The medication has been authorized for the management of relapsed or refractory multiple myeloma in conjunction with other drugs by inducing apoptosis
[167][223]. It is noteworthy that the domain of multiple myeloma is subject to persistent research and development, with the emergence of novel therapeutic agents that target specific protein signatures.