Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 3 by Jessie Wu.
Microalgae are abundant components of the biosphere rich in low molecular weight carbohydrate-containing natural products (glycoconjugates). Glycoconjugates take part in the processes of photosynthesis, provide producers with important biological molecules, influence other organisms and are known by their biological activities. Some of them, for example, glycosylated toxins and arsenicals, are detrimental and can be transferred via food chains into higher organisms, including humans.
- microalgae
- diatoms
- glycerol
diatoms
1. Introduction
The low molecular weight natural products such as glycerol- and sphingoglycolipids, glycosylated fatty alcohols derivatives, carbohydrate-containing toxins, steroid and other glycosides, and some ribose-containing arsenicals belong to the group of molecules called glycoconjugates. Being key cellular components and participating in both inter- and intracellular communications, metabolites of this type are ubiquitous in nature and influence important biologic properties such as fluidity and permeability of biomembranes, stimulation of apoptosis, and defense against predators. Microalgae are one of the primary producers of glycoconjugates, which along with nutritionally important polyunsaturated fatty acids of ω-3 (n-3) series, pigments, antioxidants, and other bioregulators, are accumulated via food chains in many marine organisms, including edible species of fish and mollusks. Glycoconjugates are a portion of many various chemicals produced by microalgae.
Microalgae represent widespread eukaryotic microorganisms that populate fresh, brackish sea waters and bottom sediments and have economic significance that grows year to year [1]. A part of microalgal species are symbionts living in host organisms, for example, in corals. Microalgae are photosynthesizing organisms that provide about half of the atmospheric oxygen and the main part of organic substances on our planet and support more than 60% of the total primary production in marine ecosystems [2]. Diatoms, dinoflagellates, coccolithophores, microscopic green and red microalgae, and representatives of some smaller taxa are related to different groups of microalgae, in total belonging to more than 50,000 species that provide a significant biochemical diversity.
2. Glycosylated Arsenicals
After the discovery of arsenic-containing compounds in fishes in the 1920s [3], a variety of close related natural products of this type were found in macro- and microphytes as well as in different marine animals. Arsenic specification, toxicity and metabolism of these compounds in microalgae were discussed in the review by Wang et al. [4]. Typically, arsenic is present in seawater in a concentration of 1–2 μg per liter and is incorporated from the environment into living systems as a result of metabolic processes, leading to more bioavailable products. A variety of water-soluble arsenic species in marine microalgae includes not only arsenosugars but also inorganic species such as arsenate (V) and its methylated derivatives. Chlorophytes produce glycerol- and phosphate arsenosugars, whereas glycerol-, phosphate-, and sulfate-containing arsenoribosides, as well as dimethyl arsenates, are more common in heterokontophytes, for example, diatoms. Some arsenic-containing metabolites such as glycerol arsenosugars (1), phosphate arsenosugars (2), sulfate arsenosugars (3), and sulfonate arsenosugars (4) (Figure 1) along with glycosylated lipid-soluble arsenolipids from microalgae belong to glycoconjugates and together make up a part of a relatively large class of natural compounds, so-called arsenicals.
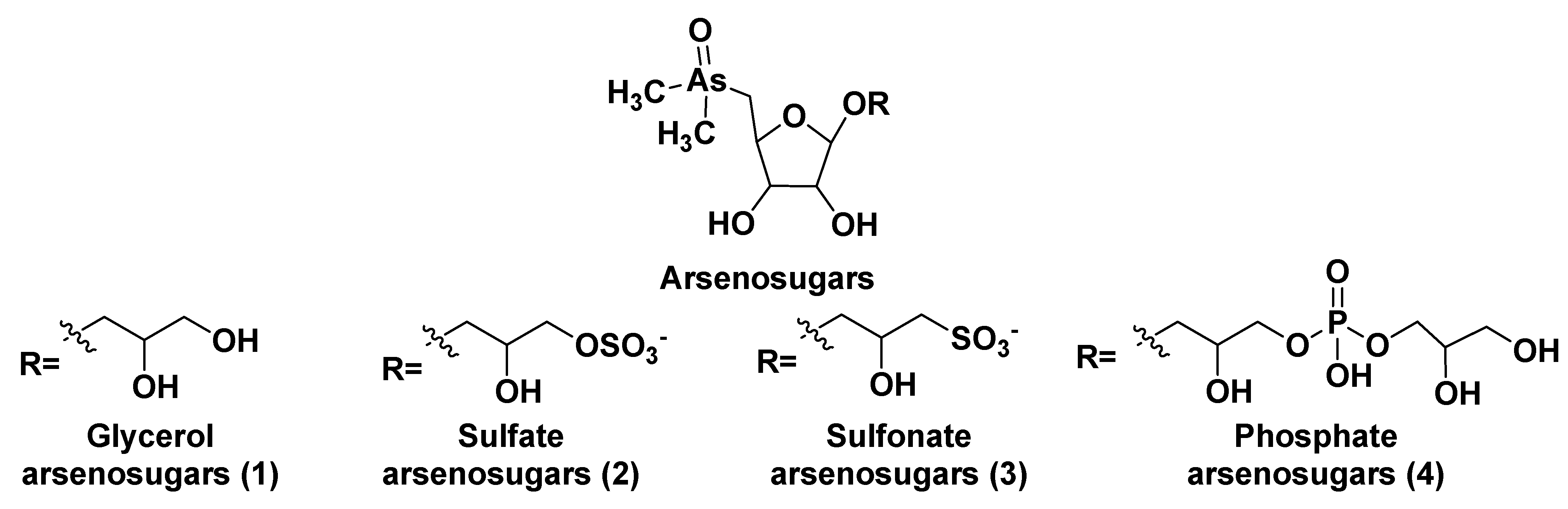
Figure 1. Main types of arsenosugar derivatives (1–4).
In this class, more than 70 arsenosugar compounds with established structures are known. Although their inorganic moieties can include either As (III) or As (V), the latter forms predominate. Contrary to arsenosugars, arsenolipids rarely contain monosaccharide residues and represent lipid-soluble and frequently volatile natural products. Both arsenosugars and arsenolipids can be transferred via the food chain into invertebrates and higher animals, including humans. These compounds are more or less detrimental due to their toxicity and cytotoxicity.
Arsenic toxicity is a global problem. Millions of people are exposed to As-containing substances through drinking water and food. High arsenic content can induce chronic toxicity and, in some cases, cancer. In some individuals, acute, subacute, and chronic poisonings, characterized by skin lesions, cardiovascular dysfunction, and multi-organ failure, can be developed [5].
Historically, As-containing preparations such as Salvarsan (arsphenamine) for the treatment of syphilis and arsenic trioxide for cancer were among the drugs that found widespread application in medicine. The useful properties of arsenic trioxide were recently rediscovered [6][7].
Arsenosugars (1–4) were found in different diatom and green microalgae such as Phaeodactylum tricornutum, Thalassiosira pseudonana, Ostreococcus tauri, Dunaliella tertiolecta, and others [8][9][10][11]. Generally, arsenosugars contain ribose or its derivatives, and marine microorganisms produce arsenoribosides in higher concentrations in comparison to fresh-water microorganisms. Total arsenic concentrations in algae (0.1–10 µg/g of dry mass) is 10–100 times higher than in terrestrial plants [12].
Lipid-soluble fractions of arsenic-containing compounds are characterized by a great diversity of metabolites. Frequently, arsenicals are analogous to common phospholipids with the change of phosphorus for arsenic; more than 100 different lipid-soluble arsenicals were isolated [13]. Very recently, Chinese scientists reported that over 300 species of naturally occurring organoarsenicals were identified by modern analytical methods, particularly HPLC/MS, as a promising option [14].
The unicellular green alga Dunaliella tertiolecta is one of the most known and capable of photosynthesizing marine microorganisms belonging to the order Chlamydomonadales. This species, like other lower plants of the genus Dunaliella, survives in hypersaline environments. Along with several previously known arsenosugars, the novel lipid-soluble arsenical phytyl 5-dimethylarsinoyl-2-O-methyl-ribofuranoside (AsSugPytol546) (5) (Figure 2) was detected by high-resolution electrospray mass spectrometry (HR ESI MS) in cultured microalgae D. tertiolecta as well as in extracts from oceanic phytoplankton.
In order to obtain compound 5 in quantities sufficient for use in NMR spectroscopy and to determine its structure, this microalga was cultured in an arsenate-enriched medium. About 2 g of cultured dried cells were used for solvent partitioning, column chromatography on silica gel and preparative reverse-phase HPLC. As a result, it was possible to obtain only a trace amount of the target compound (about 100 micrograms). Nevertheless, subsequent NMR analysis and comparison of the tandem mass spectrum (MS/MS) of the obtained arsenolipid with those of the synthesized model methyl-5-dimethylarsinoyl-2-O-methylriboside allowed the establishment of the structure of 5 and showed that it contains phytol as an aglycone. The Glycon moiety of this arsenolipid consisted of 5-dimethylarsinoyl 2-O-methylribose, and, therefore, a methoxy group replaces a sugar hydroxyl in this unusual monosaccharide. This structural feature, characteristic of some RNAs, was not previously found outside the RNAs world [15].
A possible pathway of the biosynthesis of AsSugPhytol546 was proposed to include the introduction of an arsenic group into the ribosyl moiety of S-adenosylmethionine (6→7) followed by 2-O-methylation by the corresponding methylase to yield 8. The loss of an adenosyl residue at the catalysis by adenosyl nucleosidase to give the ribose derivative (9), and finally, the interaction of the latter with phytyl diphosphate, catalyzed by riboside phytolsynthase, led to 5 (Figure 2) [16].
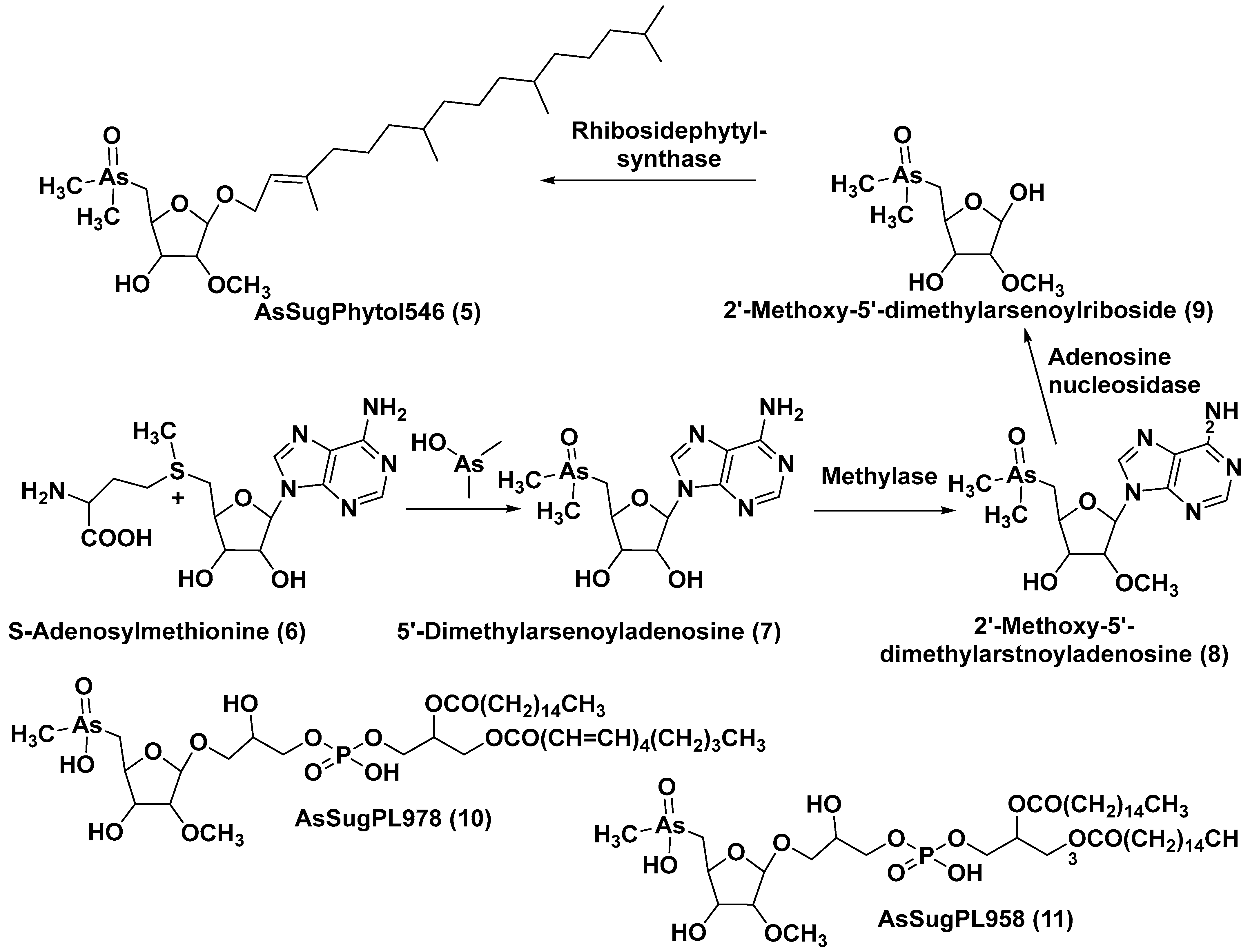
Figure 2. Biosynthesis of 5 and structures of other arsenolipids found in D. tertiolecta (6–11) [16].
In addition to the major arsenolipid 5, a new metabolite, designated as AsSugPL978 (10), and related AsSugPL958 (11) were identified in the same culture. It is of particular interest that more than half of the total arsenic is present in D. tertiolecta as lipid-soluble forms. Although the relative amount of total arsenolipids remained constant in different cultivation conditions, an increase in the content of hydrophilic arsenosugars was discovered under various As/P regimes when As concentrations were increased in the medium.
The accumulation of arsenic species from the environment into the above-mentioned microalgae stimulates the biosynthesis of various arsenic-containing glycoconjugates. Some arsenic-containing natural products of this type can be found in many popular sea foods. Further progress in the search for this type of compound is connected with the use of HPLC/MS after chemical derivatization [17]. It should be noted that some oleaginous microalgae, such as Scenedesmus sp. IITRIND2 are super accumulators of arsenic. Being stressed by arsenic intake, arsenic-tolerant species modulate their own cellular processes, enhance lipid production and accumulate large lipid droplets. The biodiesel obtained from such microalgae was comparable to plant oil methyl esters and had a high cetane number and oxidative stability [18].
References
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96.
- Downing, J.A. Marine nitrogen: Phosphorus stoichiometry and the global N:P cycle. Biogeochemistry 1997, 37, 237–252.
- Sadoli, E. Investigation into the occurrence of arsenic in the organism of fish. Biochem. Z. 1928, 201, 323–331.
- Wang, Y.; Wang, S.; Xu, P.; Liu, C.; Wang, Y.; Zhang, C.; Ge, Y. Review of arsenic specification, toxicity and metabolism in microalgae. Rev. Environ. Sci. Biotechnol. 2015, 14, 427–451.
- Nurchi, V.M.; Djordjevic, A.B.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic toxicity: Molecular targets and therapeutic agents. Biomolecules 2020, 10, 235.
- Zaffiri, L.; Gardner, J.; Toledo-Pereyra, L.H. History of antibiotics. From salvarsan to cephalosporins. J. Investig. Surg. 2012, 25, 67–77.
- Emadi, A.; Gore, S.D. Arsenic trioxide—An old drug rediscovered. Blood Rev. 2010, 24, 191–199.
- Duncan, E.G.; Maher, W.A.; Foster, S.D.; Krikowa, F. Influence of culture regime on arsenic cycling by the marine phytoplankton Dunaliella tertiolecta and Thallassiosira pseudonana. Environ. Chem. 2013, 10, 99–101.
- Foster, S.D.; Thomson, D.; Maher, W.A. Uptake and metabolism of arsenate by anexic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum. Mar. Chem. 2008, 108, 172–183.
- Zhang, S.-Y.; Sun, G.X.; Yin, X.X.; Rensing, C.; Zhu, Y.-G. Biomethylation and volatilization of arsenic by the marine microalgae Ostreococcus tauri. Chemosphere 2013, 93, 47–53.
- Duncan, E.G.; Maher, W.A.; Foster, S.D.; Krikowa, F. The influence of arsenate and phosphate exposure on arsenic uptake, metabolism and species formation in the marine phytoplankton Dunaliella tertiolecta. Mar. Chem. 2013, 157, 78–85.
- Duncan, E.G.; Mather, W.A.; Foster, S.D. Contribution of arsenic species in unicellular algae to cycling of arsenic in marine ecosystem. Environ. Sci. Technol. 2015, 49, 33–50.
- Dembitsky, V.M.; Levitsky, D.O. Arsenolipids. Prog. Lipid Res. 2004, 43, 403–448.
- Xue, X.M.; Xiong, C.; Yoshinaga, M.; Rosen, B.; Zhu, Y.G. The enigma of environmental organoarsenicals: Insights and implications. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3835–3862.
- Glabonjat, R.A.; Raber, G.; Jensen, K.B.; Guttenberger, N.; Zangger, K.; Francesconi, K.A. A 2-O-methylriboside unknown outside the RNAs world contains arsenic. Angew. Chem. Int. Ed. 2017, 56, 11963–11965.
- Glabonjat, R.A.; Raber, G.; Jensen, K.B.; Guttenberg, N.; Zangger, K.; Francesconi, K.A. Arsenolipid biosynthesis by the unicellular alga Dunalliella tertiolecta is influenced by As/P ratio in culture experiments. Metallomics 2018, 10, 145–153.
- Glabonjat, R.A.; Raber, G.; Jensen, K.B.; Ehgartner, J.; Francesconi, K.A. Quantification of arsenolipids in the certified reference material NMIJ 7405-a (Hijiki) using HPLC/mass spectrometry after chemical derivatization. Anal. Chem. 2018, 6, 10282–10287.
- Arora, N.; Gulati, K.; Patel, A.; Pruthi, P.A.; Poluri, K.M.; Pruthi, V. A hybrid approach integrating arsenic detoxification with biodiesel production using oleaginous microalgae. Algal Res. 2017, 24, 29–39.
More
