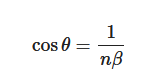Photodynamic therapy (PDT) is a promising therapeutic strategy for cancers where surgery and radiotherapy cannot be effective. PDT relies on the photoactivation of photosensitizers, most of the time by lasers to produced reactive oxygen species and notably singlet oxygen. The major drawback of this strategy is the weak light penetration in the tissues. To overcome this issue, recent studies proposed to generate visible light in situ with radioactive isotopes emitting charged particles able to produce Cerenkov radiation. In vitro and preclinical results are appealing, but the existence of a true, lethal phototherapeutic effect is still controversial.
- Photodynamic Therapy
- Cerenkov Light
- radioactive isotopes
1. Introduction
Photodynamic therapy (PDT) uses specific molecules, namely photosensitizing agents, photo-agents or photosensitizers (PS), along with light illumination, in the presence of oxygen, to kill cancer cells, ultimately leading to tumor eradication. PDT is also known as photoradiation therapy or photochemotherapy. PDT involves the presence of PS, light and endogenous molecular oxygen (3O2) to generate photochemical reactions. Over the past few decades, diverse synthesized PS activation by visible and near-infrared (NIR) light has been widely investigated [1]. The mechanism of PDT is based on type I and II photo-oxidation reactions. At a specific excitation wavelength (light photon absorption), the PS produces ROS causing oxidative cell damage which is highly dependent on the 3O2 content within the tissue [2].
Unfortunately, due to shallow visible light penetration depth into tissues, the photodynamic therapeutic strategy currently has largely been restricted to the treatment of surface localized tumors. Additional invasive strategies, i.e., interstitial PDT, through optical fibers are currently used for getting the visible light into the intended deep-seated targets [3][4]. Indeed, compared to ionizing-radiations, the light could not penetrate deeply because most tissue chromophores absorb in the range of the visible light spectrum commonly used in clinical practice. Moreover, the optimization of PDT modalities must consider numerous phenomena, regarding one or several main factors (photosensitizer, light, oxygen) involved in the treatment efficacy. A specific dosimetry remains challenging owing to their nonlinear interactions. Light penetration in the target tissue depends on its specific optical properties. If the tissue is hypoxic or becomes hypoxic because of the PDT treatment which consumes 3O2, the yield of singlet oxygen 1O2 will be lower than expected. Furthermore, making things tricky, PS concentration, light penetration and tissue oxygenation can vary during treatments and one parameter can influence the others.
To knock down the biotechnological barriers limiting the effectiveness of radiotherapy (i.e., curative X-ray dose to the tumor tissue without increasing it in the healthy adjoining tissue) and PDT (low penetration of the light), it has been proposed to use a bimodal therapy using biocompatible high-Z nanoparticles (NPs). This concept could combine both radiotherapy and PDT strategies, two clinical proven modalities, while maintaining the main benefits of each therapeutic strategy. Only PDT can generate 1O2, but unfortunately, the low penetration of light remains a limiting factor. To treat deep lesions without an invasive approach, X-ray could be used as an excitation source instead of normal light. This therapeutic approach is known as X-ray-induced PDT (X-PDT) [5][6][7][8][9]. The light penetration limitation in the tumor tissue will be overcome and PS activation within the tumor cells will be performed by radiotherapy. This methodology requires a material composition exhibiting appropriate physical properties: high density for a good ionizing radiation interaction, high scintillation quantum yield and efficient energy transfer toward the PS as well as a biocompatibility and adapted in vivo bio-distribution. However, most of the X-PDT studies were mainly obtained with cancer cell lines or animal models bearing subcutaneous grafted cancer cells, limiting therefore clinical relevance [10]. The potential toxicity of nanoscintillators, in fact, mostly relies on nanoparticles stability, which can be enhanced by an inorganic shell among other things. As an example, AGuIX-designed nanoplatforms which originally chelate gadolinium have been assessed in phase I and phase II clinical trials without evidence of any toxicity [11][12]. AGuIX nanoparticle being a chelator, Gd3+ cations can be easily replaced by another 3+ lanthanide.
Recently, another therapeutic strategy has been proposed, direct PS activation through Cerenkov radiation, referred as Cerenkov-induced PDT (CR-PDT) [13][14]. Ran et al., in their proof-of principle article, postulated that such a treatment could be a synergy between the radiotherapeutic and phototherapeutic effect, the latter involving solely PSs activation [15]. However, despite the various recent studies demonstrating a real benefit in terms of tumor growth decrease, there is still a debate about the presence of a cytotoxic phototherapeutic effect [16]. Indeed, the number of photons absorbed by the PS being lower than those involved in PDT, the amount of 1O2 produced is dramatically low.
2. Cerenkov Light
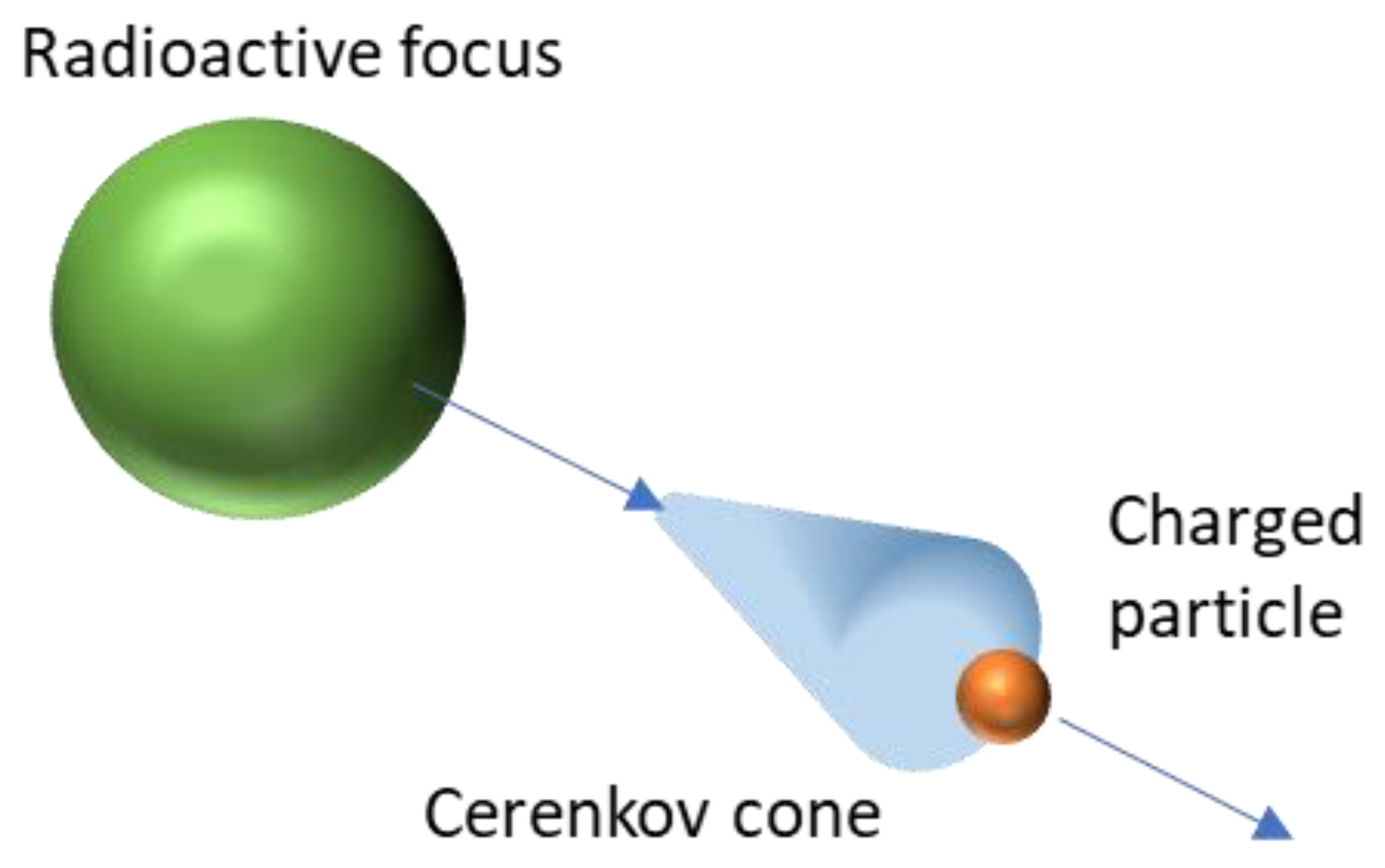
Cerenkov light is a luminescence signal produced by charged particles. Two conditions must be present to enable such an effect: the medium must be dielectric and the charged particle must travel faster than the phase velocity of light in that medium. Cerenkov photons are produced by successive polarization/depolarization of the medium along the particle path, yielding constructive interferences [17]. To make it clearer, Cerenkov radiation can be compared with sound barrier crossing, but for light.
The Cerenkov photon yield can be computed with the Frank–Tamm formula [18]:
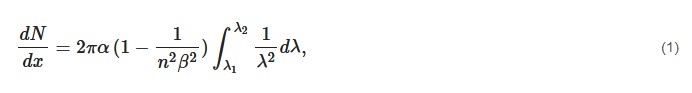

In biological tissues, where the refractive index is considered around 1.4, this energy threshold is around 250 keV. This threshold is much lower than most of the radionuclides emitting β+ or β− particles.
The Cerenkov spectrum is a continuous spectrum from UV to infra-red light where the number of photons per wavelength is proportional to 1/λ2. Cerenkov photons are not emitted isotropically from the particle, but within a cone aligned with the direction of the travelling particle. The cone aperture of the distribution (Figure 1) is related to the particle velocity as
Gill et al. highlighted the luminescence yield of several isotopes commonly used in nuclear medicine and biology [19]. They used simulations (including the true resolution of Franck–Tamm formula at each length step) to determine the quantity and spatial distribution of Cerenkov light for each assessed element. Moreover, they validated their simulation kernel by true experiments for five radionuclides covering energies from 0.611 up to 2.3 MeV. TableTable 1 1 summarizes the photon yield of the most common radionuclides used in nuclear medicine and Figure 2 presents the corresponding Cerenkov spectra for the same isotopes. It is noteworthy that most of them are dedicated to positron emission tomography. Monte Carlo simulations assessed the spatial distribution of Cerenkov photons in biological medium. Mitchell et al. computed the volume where the charged particles energy was still higher than the Cerenkov threshold, allowing for the conclusion that many β emitters produced Cerenkov light in a 2 mm-diameter sphere [18].
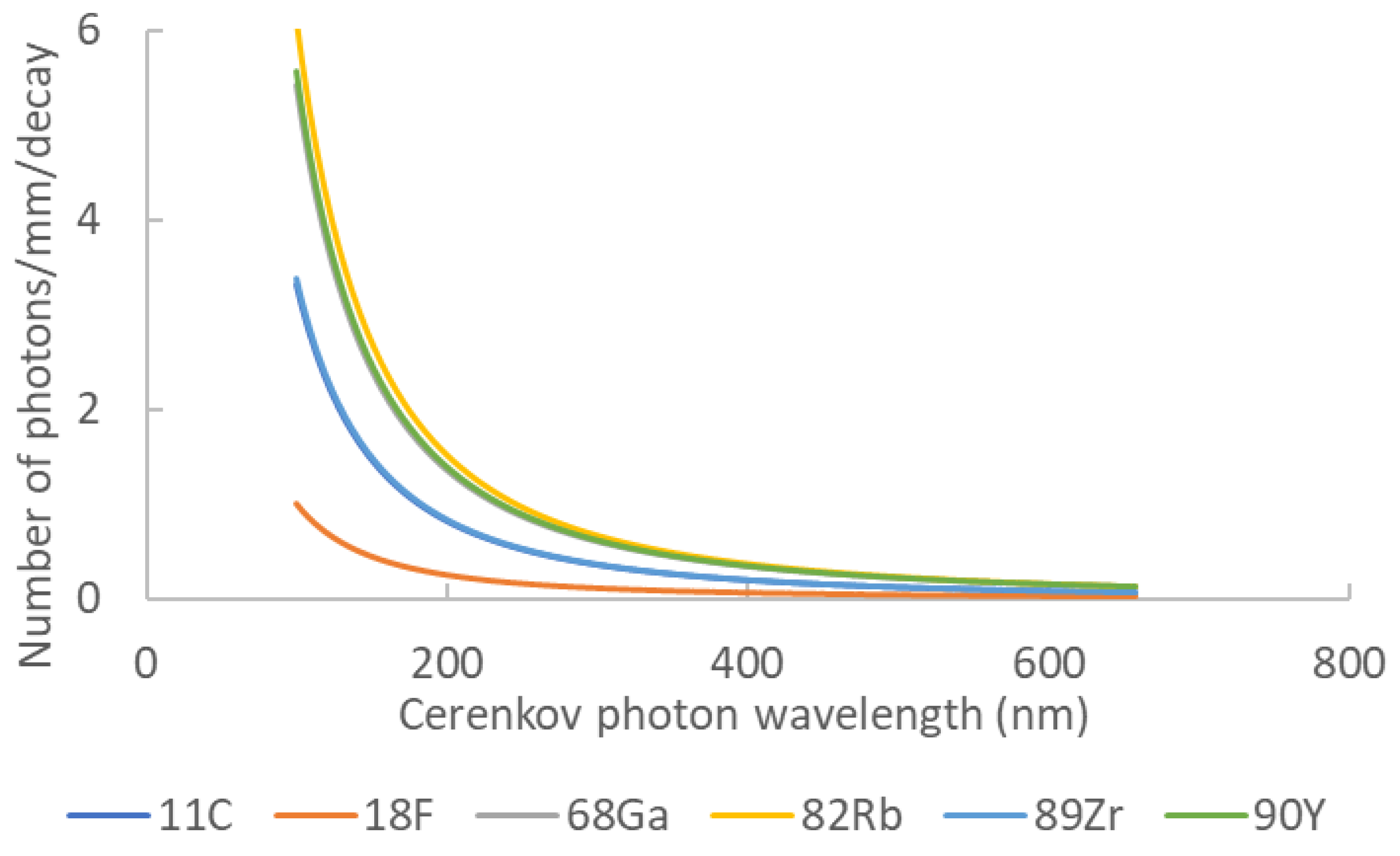
Table 1. Cerenkov photon yield for main nuclear medicine elements. Data extracted from [16].
| Element | Z | Halflife | Main Emission Type | Photon Yield | Emax (MeV) | Emean (Mev) |
|---|---|---|---|---|---|---|
| 11C | 6 | 20.4 min | b+ | 6.87 | 0.970 | 0.390 |
| 18F | 9 | 110 min | b+ | 1.32 | 0.63 | 0.252 |
| 68Ga | 31 | 67.7 min | b+ | 33.9 | 1.92 | 0.844 |
| 82Rb | 37 | 1.27 min | b+ | 80.8 | 3.378 | 1.551 |
| 89Zr | 40 | 78.4 h | b+ | 2.29 | 0.909 | 0.396 |
| 90Y | 39 | 64.1 h | b− | 47.3 | 2.28 | 0.935 |
References
- Niedre, M.; Patterson, M.S.; Wilson, B.C. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem. Photobiol. 2002, 75, 382–391.
- Hirschberg, H.; Berg, K.; Peng, Q. Photodynamic therapy mediated immune therapy of brain tumors. Neuroimmunol. Neuroinflamm. 2018, 5, 27.
- Gill, I.S.; Azzouzi, A.-R.; Emberton, M.; Coleman, J.A.; Coeytaux, E.; Scherz, A.; Scardino, P.T.; PCM301 Study Group. Randomized Trial of Partial Gland Ablation with Vascular Targeted Phototherapy versus Active Surveillance for Low Risk Prostate Cancer: Extended Followup and Analyses of Effectiveness. J. Urol. 2018, 200, 786–793.
- Bechet, D.; Mordon, S.R.; Guillemin, F.; Barberi-Heyob, M.A. Photodynamic therapy of malignant brain tumours: A complementary approach to conventional therapies. Cancer Treat. Rev. 2014, 40, 229–241.
- Chen, W.; Zhang, J. Using Nanoparticles to Enable Simultaneous Radiation and Photodynamic Therapies for Cancer Treatment. J. Nanosci. Nanotechnol. 2006, 6, 1159–1166.
- Popovich, K.; Tomanova, K.; Čuba, V.; Procházková, L.; Pelikánová, I.T.; Jakubec, I.; Mihóková, E.; Nikl, M. LuAG:Pr3+-porphyrin based nanohybrid system for singlet oxygen production: Toward the next generation of PDTX drugs. J. Photochem. Photobiol. B Biol. 2018, 179, 149–155.
- Chen, M.-H.; Jenh, Y.-J.; Wu, S.-K.; Chen, Y.-S.; Hanagata, N.; Lin, F.-H. Non-invasive Photodynamic Therapy in Brain Cancer by Use of Tb3+-Doped LaF3 Nanoparticles in Combination with Photosensitizer Through X-ray Irradiation: A Proof-of-Concept Study. Nanoscale Res. Lett. 2017, 12, 1–6.
- Bulin, A.-L.; Truillet, C.; Chouikrat, R.; Lux, F.; Frochot, C.; Amans, D.; LeDoux, G.; Tillement, O.; Perriat, P.; Barberi-Heyob, M.; et al. X-ray-Induced Singlet Oxygen Activation with Nanoscintillator-Coupled Porphyrins. J. Phys. Chem. C 2013, 117, 21583–21589.
- LaRue, L.; Ben Mihoub, A.; Youssef, Z.; Colombeau, L.; Acherar, S.; André, J.-C.; Arnoux, P.; Baros, F.; Vermandel, M.; Frochot, C. Using X-rays in photodynamic therapy: An overview. Photochem. Photobiol. Sci. 2018, 17, 1612–1650.
- Sun, W.; Zhou, Z.; Pratx, G.; Chen, X.; Chen, H. Nanoscintillator-Mediated X-Ray Induced Photodynamic Therapy for Deep-Seated Tumors: From Concept to Biomedical Applications. Theranostics 2020, 10, 1296–1318.
- Verry, C.; Sancey, L.; Dufort, S.; Le Duc, G.; Mendoza, C.; Lux, F.; Grand, S.; Arnaud, J.; Quesada, J.L.; Villa, J.; et al. Treatment of multiple brain metastases using gadolinium nanoparticles and radiotherapy: NANO-RAD, a phase I study protocol. BMJ Open 2019, 9, e023591.
- Verry, C.; Dufort, S.; Lemasson, B.; Grand, S.; Pietras, J.; Troprès, I.; Crémillieux, Y.; Lux, F.; Mériaux, S.; Larrat, B.; et al. Targeting brain metastases with ultrasmall theranostic nanoparticles, a first-in-human trial from an MRI perspective. Sci. Adv. 2020, 6, eaay5279.
- Dothager, R.S.; Goiffon, R.J.; Jackson, E.; Harpstrite, S.; Piwnica-Worms, D. Cerenkov Radiation Energy Transfer (CRET) Imaging: A Novel Method for Optical Imaging of PET Isotopes in Biological Systems. PLoS ONE 2010, 5, e13300.
- Liu, H.; Zhang, X.; Xing, B.; Han, P.; Gambhir, S.S.; Cheng, Z. Radiation-Luminescence-Excited Quantum Dots for in vivo Multiplexed Optical Imaging. Small 2010, 6, 1087–1091.
- Ran, C.; Zhang, Z.; Hooker, J.M.; Moore, A. In Vivo Photoactivation Without “Light”: Use of Cherenkov Radiation to Overcome the Penetration Limit of Light. Mol. Imaging Biol. 2012, 14, 156–162.
- Pratx, G.; Kapp, D.S. Is Cherenkov luminescence bright enough for photodynamic therapy? Nat. Nanotechnol. 2018, 13, 354.
- Jelley, J.V. Cherenkov Radiation and Its Applications; Pergamon Press: London, UK, 1958; p. 314.
- Mitchell, G.S.; Gill, R.K.; Boucher, D.L.; Li, C.; Cherry, S.R. In vivo Cerenkov luminescence imaging: A new tool for molecular imaging. Philos. Trans. R. Soc. A. 2011, 369, 4605–4619.
- Gill, R.K.; Mitchell, G.S.; Cherry, S.R. Computed Cerenkov luminescence yields for radionuclides used in biology and medicine. Phys. Med. Biol. 2015, 60, 4263–4280.