Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ana Paiva and Version 2 by Camila Xu.
The strategic importance of platinum and palladium, two platinum-group metals (PGMs), is particularly supported by their technological applications, one of the most relevant being the role they perform as catalysts for several sorts of chemical reactions.
- spent catalysts
- recycling
- hydrometallurgy
- solvometallurgy
- solvent extraction
1. Introduction
Platinum and palladium are the best-known elements of platinum-group metals (PGMs). The former has had an important use in jewellery for many years, but the interest in the utilization of the latter in the same business has recently increased. The third most important PGM element, considering its demand and applications, is rhodium. The total gross request for ruthenium was of the same order of magnitude as rhodium in 2021 (31.6 and 32.2 tonnes, respectively), whereas iridium is the fifth least demanded PGM (7.2 tonnes in 2021) [1]. Osmium is not taken into consideration in these figures, as it does not possess relevant technological applications.
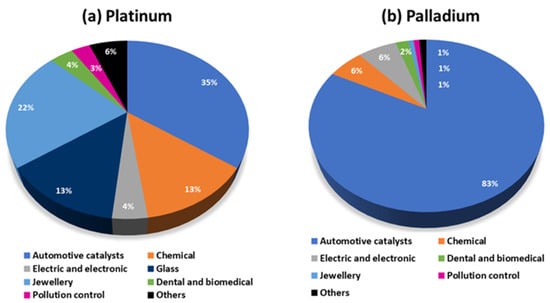
The extensive technological applications of platinum and palladium make these PGM metals the most widely used in the group (gross demand of 210 and 314 tonnes in 2021, respectively). An illustration of the main business lines relying on the use of these two PGMs can be seen in Figure 1 [1].
The major application of platinum is in the manufacture of automotive catalysts (35%), followed by its utilization in jewellery (22%). For palladium, 83% is used for the production of automotive catalysts, but its involvement in the chemical, and electric and electronic industries, cannot be neglected (data from 2021) [1].
Automotive catalysts are mandatory devices used in vehicles, since the mid-1970s, to minimize the emission of toxic exhaust gases from combustion engines. Initially, platinum and rhodium were the selected PGMs, but gradually palladium has been advantageously replacing platinum in modern three-way catalytic converters [2]. Furthermore, the utilization of platinum and palladium as industrial catalysts for several inorganic and organic reactions, particularly in nitric acid manufacture, and in the petroleum refining and petrochemical and pharmaceutical industries, is very well known [2].
The brand image of PGMs is their exclusive catalytic properties, justifying their leading technological applications. Other properties that PGMs exhibit, such as their high melting points, excellent resistance to elevated temperatures and corrosion, and good electrical and chemical stabilities, complement their high-tech interest and applications [3], as can be seen in Figure 1. Moreover, several end-of-life devices possess higher PGMs contents than primary ores: for instance, computer motherboards and mobile phone handsets contain around 80 g and up to 130 g per tonne, respectively, of palladium, while automotive catalysts may contain up to 2000 g per tonne of PGMs in the ceramic catalyst bricks (the active part of these apparatus). These contents are significantly higher than those existing in primary ores (on average less than 10 g per tonne) [2].
The difficulty in replacing PGMs in most of their applications, together with their rarity as primary resources and high economic value, have been used to justify the efforts of scientists and industries to develop recycling practices supported by sustainable processes, as the preservation of the Earth requires [4]. Accordingly, the responsible commissions of the European Union (EU) have always classified PGMs as critical raw materials in reports released every three years since 2011, with the last published report being from March 2023 [5]. The recycling of several materials has been found to be determinant for the maintenance and progression of mankind at a technological level, justifying increased financial investment in EU projects involving academia and industrial consortia, through Horizon 2020 and the current Horizon Europe [6]. Moreover, PGM recycling helps to reduce the environmental impact of removing these metals directly from the mines, whose exploitation causes significant effects on climate change [2].
The current PGM recycling levels are still far from the desirable figures, but an increasing tendency is noticeable in recent years [1]. In fact, the high economic value of PGMs provides justification for their better recycling profiles compared to those of most metals, although some non-profitable issues may inhibit industries from investing more in recycling than is strictly necessary, only obeying the rules imposed by the government.
With the growing development of hybrid, electric, and fuel-cell vehicles, a decrease in platinum and palladium demand for automotive catalysts manufacture may be expected in the next few years [6], but huge changes are usually slower than expected, and other potential applications for these metals will surely appear in the meantime. Moreover, even when conventional combustion engines end, spent catalytic converters will exist for several years after. Hence, the recycling practices will certainly proceed in the near- and medium-term future, possibly with unpredictable variations in the matrices where PGMs are included. Therefore, the most robust recycling practices of today, revealing the potential to be applied to variable substrate compositions, will be those better able to succeed in the future.
The established industrial routes to recycle PGMs from spent catalysts are pyrometallurgy and hydrometallurgy [7]. Pyrometallurgy can act alone to recover PGMs sponges, but hydrometallurgy is subsequently required if PGMs must be separated from one another. The major PGMs suppliers and refiners, as far as known, use both approaches to produce isolate PGMs from spent devices [7], thickening the supply coming from the harsh and polluting extractive facilities that dig PGMs from the Earth’s crust. The hydrometallurgical route can be easier to install by small and medium-sized recycler enterprises, and it is potentially more benign to the environment than pyrometallurgy; these are probably some of the reasons justifying the recent development of hydrometallurgy for PGMs recovery, e.g., [7][8][9][7,8,9]. Additionally, alternative liquid media to water have also been deeply investigated, particularly those involving ionic liquids (ILs) and deep eutectic solvents (DES) [10][11][12][10,11,12].
The chemical inertness of PGMs requires leaching agents with a high oxidation potential and complexation ability [13], which is why chloride reagents are typically chosen as aqueous media to dissolve these metal elements. Accordingly, high concentrations of hydrochloric acid in the presence of chlorine, chloride salts, or hydrogen peroxide [14] are the most common chemical agents utilized to leach PGMs from solid matrices.
To concentrate and/or purify the leaching solutions loaded with PGMs, solvent extraction (SX) (or liquid–liquid extraction) is one of the unit operations mostly applied in hydrometallurgy to recover palladium and platinum from leaching solutions of spent catalysts [15]. Accordingly, SX is the chosen unit operation to be explored. A scheme of the SX operating mode when applied in hydrometallurgy can be observed in Figure 2.
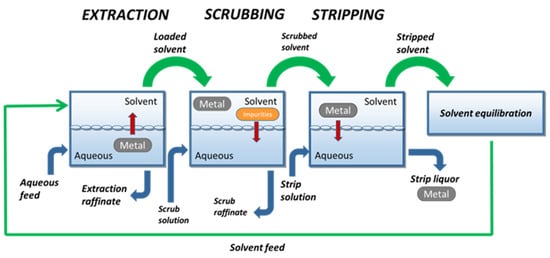 Industrial SX in hydrometallurgy includes extraction, scrubbing, and stripping stages in sequence. In extraction, the leaching solution (aqueous feed in Figure 2) is stirred with an adequate organic solvent to transfer the metal of interest to the organic medium. Undesired contaminants often accumulate in the solvent, which is why the metal-loaded solvent is often agitated with an aqueous scrubbing solution. The scrubbed solvent is then equilibrated with a stripping aqueous medium, causing the transfer of the metal to a new aqueous solution, the final strip liquor [15][16][15,16]. Successful SX schemes allow the efficient and selective transfer of the metal of interest from the aqueous feed to the strip liquor, leaving impurities in the extraction and scrub raffinates. The reutilization of the organic solvent in successive extraction–stripping cycles should therefore be guaranteed, justifying SX’s cost-effectiveness. Additionally, all aqueous phases are usually reutilized as well, contributing to a more sustainable and economically viable process. The concept of “non-aqueous SX”, involving two non-aqueous immiscible phases [11], will be presented in detail in the section “Solvometallurgy”.
In recent years, there have been extensive research efforts on the development of hydrometallurgy and/or solvometallurgy for PGM recovery from primary and secondary resources; the latter includes end-of-life devices such as spent catalysts and e-waste. Accordingly, reviews on this subject are regularly emerging in the literature, e.g., [7][8][9][12][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][7,8,9,12,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34], and the chosen sample of references cited herein corresponds only to those from 2018 onwards. A major part of these reviews describes pyrometallurgical methods as well [8][9][18][20][22][23][24][26][27][29][30][32][8,9,18,20,22,23,24,26,27,29,30,32], therefore, offering a detailed overview of the state-of-the-art of the most common industrial methodologies to process PGMs. Additionally, some of them are specifically devoted to providing a thorough outline of PGM recovery from spent automotive catalysts (SACs) [7][8][9][22][24][27][29][33][7,8,9,22,24,27,29,33], thus, informing about the general composition of SACs and how they are primarily processed for pyro-and/or hydrometallurgical treatments. It is worthwhile to mention that the use of ILs [12][30][31][12,30,31] and biometallurgical [23][24][26][27][28][29][32][23,24,26,27,28,29,32] recent advances to process PGMs have also been comprehensively summarised.
Industrial SX in hydrometallurgy includes extraction, scrubbing, and stripping stages in sequence. In extraction, the leaching solution (aqueous feed in Figure 2) is stirred with an adequate organic solvent to transfer the metal of interest to the organic medium. Undesired contaminants often accumulate in the solvent, which is why the metal-loaded solvent is often agitated with an aqueous scrubbing solution. The scrubbed solvent is then equilibrated with a stripping aqueous medium, causing the transfer of the metal to a new aqueous solution, the final strip liquor [15][16][15,16]. Successful SX schemes allow the efficient and selective transfer of the metal of interest from the aqueous feed to the strip liquor, leaving impurities in the extraction and scrub raffinates. The reutilization of the organic solvent in successive extraction–stripping cycles should therefore be guaranteed, justifying SX’s cost-effectiveness. Additionally, all aqueous phases are usually reutilized as well, contributing to a more sustainable and economically viable process. The concept of “non-aqueous SX”, involving two non-aqueous immiscible phases [11], will be presented in detail in the section “Solvometallurgy”.
In recent years, there have been extensive research efforts on the development of hydrometallurgy and/or solvometallurgy for PGM recovery from primary and secondary resources; the latter includes end-of-life devices such as spent catalysts and e-waste. Accordingly, reviews on this subject are regularly emerging in the literature, e.g., [7][8][9][12][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][7,8,9,12,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34], and the chosen sample of references cited herein corresponds only to those from 2018 onwards. A major part of these reviews describes pyrometallurgical methods as well [8][9][18][20][22][23][24][26][27][29][30][32][8,9,18,20,22,23,24,26,27,29,30,32], therefore, offering a detailed overview of the state-of-the-art of the most common industrial methodologies to process PGMs. Additionally, some of them are specifically devoted to providing a thorough outline of PGM recovery from spent automotive catalysts (SACs) [7][8][9][22][24][27][29][33][7,8,9,22,24,27,29,33], thus, informing about the general composition of SACs and how they are primarily processed for pyro-and/or hydrometallurgical treatments. It is worthwhile to mention that the use of ILs [12][30][31][12,30,31] and biometallurgical [23][24][26][27][28][29][32][23,24,26,27,28,29,32] recent advances to process PGMs have also been comprehensively summarised.
The oxidation state +4 for Pd is rare, and the equilibrium redox potential for the transformation [PdCl4]2− ⇔ [PdCl6]2− is 1.29 V. For Pt, both oxidation states are common, with an equilibrium redox potential of 0.74 V for [PtCl4]2− ⇔ [PtCl6]2− [13]. [PdCl4]2− and [PtCl4]2− exhibit square planar configurations for four-coordinate d8 complexes, whereas [PtCl6]2− displays an octahedral configuration typical of six-coordinate d6 complexes.
Complex geometry can have a deep effect on their reactivity [36]. To assess the stability of Pd and Pt complexes in aqueous chloride solutions, the thermodynamic and kinetic contributions to the overall picture should be considered. Hence, under a given set of conditions, thermodynamic stability is generally described through the equilibrium constant for a particular reaction of the complex, whereas kinetic stability depends on the rate at which the complex is converted to an equilibrium concentration of products. In particular, the mentioned Pd(II), Pt(II), and Pt(IV) chlorocomplexes exhibit comparable thermodynamic equilibrium constants for the replacement of one chloride anion by a water molecule (4.6 × 10−2, 1.3 × 10−2, and 5.6 × 10−3, respectively); however, in the case of [PdCl4]2−, a chloride anion is rapidly replaced by water, with a half-life of the reaction of less than 0.1 s. Accordingly, this behaviour of the Pd(II) chlorocomplex is termed as labile to substitution by other ligands.
On the other hand, the aquation of [PtCl6]2− is too slow to be determined in the absence of Pt(II), which can catalyse the reaction; under these circumstances, the complex can be classified as being inert in ligand substitution reactions [36]. In the major part of the chloride concentrations used in the leaching solutions of spent catalysts, the oxidation potential is too high to allow the significant co-existence of Pt(II) species; hence, the main contribution of Pd(II) and Pt(IV) chlorocomplex species such as [PdCl4]2− and [PtCl6]2− should be expected. As pointed out previously, the [PdCl6]2− species is rare, as it only shows some stability in the presence of excess chlorine, and has a tendency to slowly decompose to the stable Pd(II) species [36]; in contrast, the Pt(IV) chlorocomplex anion is stable under similar conditions.
The exploitation of the kinetic different behaviours of the main Pd(II) and Pt(IV) chlorocomplex species is on the basis of flowsheet schemes developed to separate the two metal ions by SX, schemes that are currently adopted by the principal PGM industrial refiners [7][15][7,15].
Accordingly, the reactivity shown by Pd(II) and Pt(IV) towards a given extractant, the active ingredient of the organic solvent, is often rather different in nature, allowing an adequate separation of both metal ions. As an example, di-octylsulphide (DOS) is used in the INCO process to extract Pd(II), as depicted in Equation (1) [15].
In Equation (1), (aq) denotes species in the aqueous solution, and (org) denotes species in the organic solvent. This reaction belongs to the type of system usually known as compound formation through ligand substitution, profiting from the labile characteristics of the [PdCl4]2− chlorocomplex. On the other hand, Pt(IV) is often extracted through the formation of ion pairs in an organic phase, exemplified by the application of a protonated tertiary amine (R3NH+ Cl−), Equation (2), which is used in the Matthey–Rustenberg process [15].
Pt(IV) can nevertheless be extracted through solvation, and an example is its extraction as a solvate in the INCO process—Equation (3)—when tributylphosphate (TBP) is used [15].
Furthermore, there are also examples of Pd(II) extraction through ion pair formation, as is the case of its preliminary extraction by protonated tertiary amines prior to the interaction with hydroxyoxime derivatives in the Matthey–Rustenberg process [15]. This approach accelerates the SX process since the direct Pd(II) extraction by hydroxyoxime derivatives is slow. The involved reactions are depicted in Equations (4) and (5) (HL represents the hydroxyoxime derivative).
A summary of the most significant SX schemes to recover Pd(II) and Pt(IV) directly from pregnant liquors of spent catalysts, published in the last 13 years, is displayed in Table 2 in chronological order. If the works dealing with investigations involving model solutions of SACs were considered, the entries shown in Table 2 would, at least, double. Additionally, only the best results found in each publication of Table 2 are highlighted.
As can be observed in Table 2, the majority of extractants that have been investigated for recovering Pd(II) and Pt(IV) from leaching solutions of spent catalysts are not commercially available and have been specifically synthesized instead. Within the extractants commercially supplied, Pt(IV) recovery by tertiary amines Alamine 336 and 308 [37][38][42][37,38,42] and ammonium salt Aliquat 336 [38][43][38,43] deserve mention; as an example, Aliquat 336 in toluene was used to accomplish Pt(IV) and Fe(III) extraction from a spent petroleum catalyst leaching liquor, containing 45 mgL−1 Pt(IV), 62 mgL−1 Fe(III), 23 mgL−1 Si, and 2.7 gL−1 Al. Fe(III) was successfully removed from the loaded Aliquat 336 by scrubbing with dilute HCl solution. After Fe(III) removal, Pt(IV) remaining in the loaded Aliquat 336 was completely stripped by using an HClO4 solution [43]. More recently, Cyphos IL 101 has also shown good extraction results for Pt(IV) and Pd(II) recovery from highly concentrated HCl solutions [61][63][64][61,63,64]. Although Aliquat 336 has been used for a long time in SX, it, like Cyphos IL 101, is an ionic liquid (IL) and both extractants are ionic salts in the liquid state over a wide range of temperatures, mainly because of having cations and anions that are well differentiated in size [12]. More detailed information about the performance of commercially available ILs in the recovery of Pt(IV) and Pd(II) will be provided and discussed in a later section.
In sequence, some of the most promising SX systems depicted in Table 2 are further detailed and discussed below.
The extractant 1,2-bis (2-methoxyethylthio) benzene has been extensively investigated and tested to extract Pd(II) from a SAC leach liquor [40]. The extractant dissolved in 1,2-dichlorobenzene allowed efficient and selective Pd(II) recovery over Pt(IV) and Rh(III), and over several other metals co-existing in the real 2 M Cl− leaching solution. The stability of the extractant in successive extraction–stripping cycles has been cautiously demonstrated, showing a very good reproducibility of results when an acidified thiourea aqueous phase was employed to strip Pd(II) [40]. However, the use of 1,2-dichlorobenzene as the diluent and the unfavourable kinetics involved—24 h for extraction, and 3 h for stripping—are disadvantages of this extraction system for future practical applications. Pd(II) is extracted by 1,2-bis (2-methoxyethylthio) benzene (L) through compound formation, leading to the formation of PdCl2L and PdCl2L2 species [40].
Several thiacalix[4] and thiacalix[6]arene derivatives bearing different substituent groups of the O-thiocarbamoyl, phosphonomethyl, and dialkylamino types have been synthesized by the Yamada group to recover mainly Pd(II), but also Pt(IV), from leaching liquors of SACs [44][45][46][47][50][56][44,45,46,47,50,56]. There is a common feature in all those works, which is the testing of the organic solvent performance with SAC liquors that have been highly diluted, and therefore, not being representative of the leaching solutions often involved in the hydrometallurgical industry. As can be seen in Table 2, the tested calix[n]arenes are particularly adequate to extract Pt(IV) and Pd(II), but there are usually appreciable amounts of several contaminants that are also co-extracted. In addition to the general use of a diluent that is not appropriate to be employed in practical applications (chloroform), this drawback adds to the difficult syntheses that these compounds generally require. Pd(II) and Pt(IV) extraction reactions are mainly of compound formation type for Pd(II), e.g., [44][51][44,51], and ion pair formation for Pt(IV) and also for Pd(II) [45][50][56][45,50,56].
Probably inspired by the promising extraction results provided by calix[n]arene derivatives, the Yamada group pursued their research developing the synthesis methods for several other extractants with somewhat similar structures, all of them bearing one or more benzene rings, and always possessing sulphur atoms [48][49][53][55][60][65][48,49,53,55,60,65]. The structures of the best derivatives for Pd(II) and Pt(IV) recovery by SX reported in these works are illustrated in Figure 3.
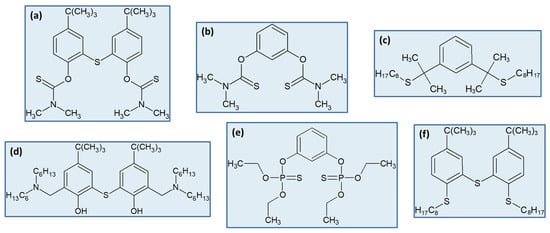 The Pd(II) extraction performances shown by all extractants displayed in Figure 3, and that of Pt(IV) extraction by the (d) derivative shown in Figure 3, have been thoroughly investigated from a fundamental point of view. Furthermore, the Pd(II) extraction, and sometimes stripping reactions involving thiourea, have been systematically studied by spectroscopic (FTIR, UV–visible, NMR) and single-crystal X-ray diffraction techniques [49][65][49,65], and DFT calculations [65], demonstrating the high quality of the research. All compounds extracted Pd(II) through compound formation [48][49][53][60][65][48,49,53,60,65], with the exception of the extractant shown in Figure 3d, which co-extracts Pd(II) and Pt(IV) through the formation of ion pairs [55].
There is a notable tendency to substitute chloroform, used as a diluent [48], with more suitable diluents for practical applications, e.g., kerosene [53], kerosene with 20% n-octanol [55], n-octanol only [60], and toluene [65]. Additionally, the determination of equilibrium extraction isotherms [48][60][48,60], and the reutilization performance of the solvents when subject to several extraction–stripping cycles [48][53][55][60][48,53,55,60], deserved the attention of the authors, as these are important features to be considered in industrial practice. Regrettably, only the work published in 2023 [65] studied the development of the SX system to recover Pd(II) from an undiluted SAC leachate, free from Pt(IV), since all previous works involved dilute SAC leaching solutions, as can be observed in Table 2.
The dithiophenol-based extractant with three sulphur atoms, depicted in Figure 3 as derivative (f), showed promising behaviour towards Pd(II) extraction from a real SAC solution with approximately 8 M HCl by carefully adjusting the extractant concentration. After extraction, the metal-loaded organic phase was scrubbed with water to remove the major part of the co-extracted Fe(III) from the liquor. Pd(II) was subsequently stripped from the organic phase by thiourea and the final aqueous solution contained Pd(II) only, but Fe(III), Al(III), and Ce(III) were still present in the organic phase after scrubbing [65]. Hence, the accumulation of these contaminating elements in the organic phase is predictable, inhibiting the complete recycling of the solvent when reutilized in sequential extraction–stripping cycles. This is generally a drawback that is difficult to surpass. Moreover, the kinetic profile of this SX system is surprising; after 24 h equilibration, the Pd(II) extraction efficiency is more or less elevated for HCl concentrations between 1 and 10 M (>85%), but after 6 h, it is almost 0% at 4 M HCl (but ≈ 95% at 8 M HCl). The authors opted to choose a 6 h contact time for the investigation of Pd(II) SX from the SAC liquor. Another disadvantage of this SX system is the rather complicated synthesis of extractant (f) in Figure 3, as four synthesis steps were necessary to produce the compound.
A thioamide and a thiodiglycolamide derivative were tested for their ability to selectively recover Pd(II) from the leachate of a spent hydrogenating catalyst from the petrochemical industry [54] (Figure 4). The leaching stage was optimized in order to maximize Pd(II) dissolution and minimize Al(III) co-extraction, but contents of Pd(II) and Al(III) up to 55 mg/L and 2750 to 4200 mg/L, respectively, were nevertheless achieved. Alternative leaching agents to partially replace HCl, namely magnesium and ammonium chlorides, were employed, with all leachates showing a total chloride ion concentration of 2 M. The assays revealed similar results for all leachates, a similar behaviour to that observed in the SX experiments involving the two organic extractants. Equilibrium Pd(II) extraction isotherms and SX reutilization data were achieved for all leaching solutions and showed very promising results. The identified drawback was the progressive accumulation of Al(III) in the organic solvents, which would certainly inhibit the reutilization of the solvent after a higher number of extraction–stripping cycles. Furthermore, Pd(II) stripping by the acidic thiourea solution became less efficient when the organic solvents were saturated with Pd(II) [54]. Both compounds extract Pd(II) through compound formation [67][68][67,68], although the extraction data achieved for the thiodiglycolamide derivative indicated the involvement of the protonated extractant in the Pd(II) extracted species [68].
The Pd(II) extraction performances shown by all extractants displayed in Figure 3, and that of Pt(IV) extraction by the (d) derivative shown in Figure 3, have been thoroughly investigated from a fundamental point of view. Furthermore, the Pd(II) extraction, and sometimes stripping reactions involving thiourea, have been systematically studied by spectroscopic (FTIR, UV–visible, NMR) and single-crystal X-ray diffraction techniques [49][65][49,65], and DFT calculations [65], demonstrating the high quality of the research. All compounds extracted Pd(II) through compound formation [48][49][53][60][65][48,49,53,60,65], with the exception of the extractant shown in Figure 3d, which co-extracts Pd(II) and Pt(IV) through the formation of ion pairs [55].
There is a notable tendency to substitute chloroform, used as a diluent [48], with more suitable diluents for practical applications, e.g., kerosene [53], kerosene with 20% n-octanol [55], n-octanol only [60], and toluene [65]. Additionally, the determination of equilibrium extraction isotherms [48][60][48,60], and the reutilization performance of the solvents when subject to several extraction–stripping cycles [48][53][55][60][48,53,55,60], deserved the attention of the authors, as these are important features to be considered in industrial practice. Regrettably, only the work published in 2023 [65] studied the development of the SX system to recover Pd(II) from an undiluted SAC leachate, free from Pt(IV), since all previous works involved dilute SAC leaching solutions, as can be observed in Table 2.
The dithiophenol-based extractant with three sulphur atoms, depicted in Figure 3 as derivative (f), showed promising behaviour towards Pd(II) extraction from a real SAC solution with approximately 8 M HCl by carefully adjusting the extractant concentration. After extraction, the metal-loaded organic phase was scrubbed with water to remove the major part of the co-extracted Fe(III) from the liquor. Pd(II) was subsequently stripped from the organic phase by thiourea and the final aqueous solution contained Pd(II) only, but Fe(III), Al(III), and Ce(III) were still present in the organic phase after scrubbing [65]. Hence, the accumulation of these contaminating elements in the organic phase is predictable, inhibiting the complete recycling of the solvent when reutilized in sequential extraction–stripping cycles. This is generally a drawback that is difficult to surpass. Moreover, the kinetic profile of this SX system is surprising; after 24 h equilibration, the Pd(II) extraction efficiency is more or less elevated for HCl concentrations between 1 and 10 M (>85%), but after 6 h, it is almost 0% at 4 M HCl (but ≈ 95% at 8 M HCl). The authors opted to choose a 6 h contact time for the investigation of Pd(II) SX from the SAC liquor. Another disadvantage of this SX system is the rather complicated synthesis of extractant (f) in Figure 3, as four synthesis steps were necessary to produce the compound.
A thioamide and a thiodiglycolamide derivative were tested for their ability to selectively recover Pd(II) from the leachate of a spent hydrogenating catalyst from the petrochemical industry [54] (Figure 4). The leaching stage was optimized in order to maximize Pd(II) dissolution and minimize Al(III) co-extraction, but contents of Pd(II) and Al(III) up to 55 mg/L and 2750 to 4200 mg/L, respectively, were nevertheless achieved. Alternative leaching agents to partially replace HCl, namely magnesium and ammonium chlorides, were employed, with all leachates showing a total chloride ion concentration of 2 M. The assays revealed similar results for all leachates, a similar behaviour to that observed in the SX experiments involving the two organic extractants. Equilibrium Pd(II) extraction isotherms and SX reutilization data were achieved for all leaching solutions and showed very promising results. The identified drawback was the progressive accumulation of Al(III) in the organic solvents, which would certainly inhibit the reutilization of the solvent after a higher number of extraction–stripping cycles. Furthermore, Pd(II) stripping by the acidic thiourea solution became less efficient when the organic solvents were saturated with Pd(II) [54]. Both compounds extract Pd(II) through compound formation [67][68][67,68], although the extraction data achieved for the thiodiglycolamide derivative indicated the involvement of the protonated extractant in the Pd(II) extracted species [68].
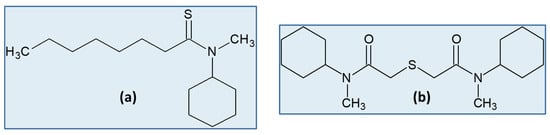 The thiodiglycolamide derivative—structure (b) in Figure 4—was also tested for its ability to recover Pt(IV) from a spent SAC leaching solution containing Pt as the only PGM and 8 M HCl [62]. Equilibrium extraction isotherms showed a very good loading profile of the thiodiglycolamide derivative towards Pt(IV), but the affinity to load Fe(III) was even higher. The net result was the inability of water to completely scrub Fe(III) from the organic phase in successive extraction–stripping cycles, and the same occurred with thiourea in HCl to strip Pt(IV), causing the saturation of the organic phase. In fact, both Fe(III) and Pt(IV) could not properly be removed from the organic solvent, although the results obtained with a model solution did not indicate that such behaviour would occur [62].
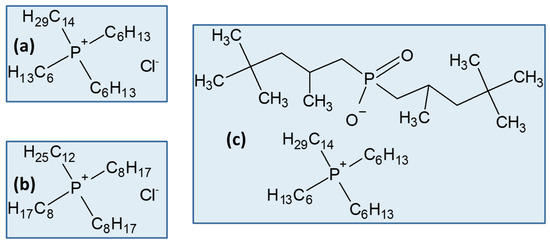
More recently, and after somewhat exhaustive research of the SX performance of ionic liquids (ILs) to recover PGMs from model solutions, e.g., [12], the first works involving real leachates of spent catalysts using commercial ILs as SX solvents began to appear in the literature [58][61][63][64][58,61,63,64]; however, to date, these studies only used phosphonium-based ILs. The structures of the tested ILs, all commercially available, are illustrated in Figure 5.
The thiodiglycolamide derivative—structure (b) in Figure 4—was also tested for its ability to recover Pt(IV) from a spent SAC leaching solution containing Pt as the only PGM and 8 M HCl [62]. Equilibrium extraction isotherms showed a very good loading profile of the thiodiglycolamide derivative towards Pt(IV), but the affinity to load Fe(III) was even higher. The net result was the inability of water to completely scrub Fe(III) from the organic phase in successive extraction–stripping cycles, and the same occurred with thiourea in HCl to strip Pt(IV), causing the saturation of the organic phase. In fact, both Fe(III) and Pt(IV) could not properly be removed from the organic solvent, although the results obtained with a model solution did not indicate that such behaviour would occur [62].
More recently, and after somewhat exhaustive research of the SX performance of ionic liquids (ILs) to recover PGMs from model solutions, e.g., [12], the first works involving real leachates of spent catalysts using commercial ILs as SX solvents began to appear in the literature [58][61][63][64][58,61,63,64]; however, to date, these studies only used phosphonium-based ILs. The structures of the tested ILs, all commercially available, are illustrated in Figure 5.
 Goto and co-workers [58] tested the SX performance of Pd(II) and Rh(III) from a spent SAC leachate using trioctyldodecylphosphonium chloride IL—(b) derivative in Figure 5. The authors used the undiluted IL to extract Pd(II) from the leaching SAC solution, containing approximately 5 M HCl, within a 10 min contact time. Approximately 99% Pd(II) was extracted, together with 50% Fe(III) and a small amount of other metals (<5%). A first Fe(III) scrubbing with sodium sulphite for 30 min and at 45 °C allowed 90% Fe(III) removal from the loaded organic phase. Subsequently, Pd(II) stripping was accomplished using a thiourea solution, with an efficiency of 90%. Rh(III) was extracted from the raffinate leftover from the first Pd(II) extraction stage by adding a fresh quantity of the IL, but adjustment of the HCl concentration to 1 M was necessary. For an efficient Rh(III) extraction, a 5 h contact time was needed. After Fe(III) scrubbing as previously described, Rh(III) was recovered using a 5 M HCl stripping solution. Both metal ions were extracted through ion pair formation [58]. The results presented in this publication are promising, but much work has still to be carried out, particularly to check the robustness of the solvent in successive extraction–stripping cycles and the effect of the accumulation of contaminants in the organic phase. Nothing was reported about any trouble arising from the viscosity of the IL in the SX essays; therefore, it is supposed there were not any constraints to point out.
The Rzelewska-Piekut group published a paper about the use of Cyphos IL 101 [61]—derivative (a) in Figure 5—to recover Pt(IV) and Rh(III) from leaching solutions of SACs. The authors investigated the best conditions to leach Pt(IV) and Rh(III), leading to the choice of a HCl, H2SO4, and H2O2 mixture as a feed solution to apply a SX scheme involving Cyphos IL 101 diluted in toluene. The results obtained were somehow encouraging: 100% Pt(IV) and 0% Rh(III) extractions, but 100% Fe(III) and Zn(II) removal were also verified. Contact with a nitric acid solution stripped 19% of Pt(IV), all Fe(III), and half of the Zn(II) from the organic medium. Further investigation of Pt(IV) stripping from the loaded organic phase needs to be carried out to improve the SX process.
Both Cyphos IL 101 and 104—derivatives (a) and (c) in Figure 5—were tested for their ability to recover Pt(IV), Pd(II), and Rh(III) from a SAC leaching solution by the Rzelewska-Piekut group again [63]. The suggested approach used several stages: (1) a preliminary leaching step by oxalic acid, to reduce the amount of non-precious metals (Al(III), Fe(III), Mg(II), and Zn(II)) leached in the second stage and leaving PGMs in the residue; (2) leaching of the residue with a mixture of HCl, H2SO4, and H2O2 to dissolve PGMs; (3) extraction of PGMs preferably by Cyphos IL 101 in toluene, for which 100% extraction of Pt(IV), Pd(II), and Fe(III) was achieved, together with 96%, 95%, and 84% extraction of Mg(II), Zn(II), and Cu(II), respectively; and (4) Pd(II) stripping by acidic thiourea, followed by Pt(IV) stripping by a nitric acid solution. The numbers do not lie: it was obviously possible to concentrate Pd(II) and Pt(IV) from the second leaching solution by SX, but a lot of work still has to be carried out to investigate how to remove the impurities that were not easily scrubbed from the loaded IL, whose accumulation will result in the inhibition of Pt(IV) and Pd(II) extraction in a short number of extraction–stripping cycles. Both Pt(IV) and Pd(II) were extracted by Cyphos IL 101 through ion pairs [61][63][61,63]. It should also be emphasized that, much of the data were presented without a logical placement; therefore, its organization is rather confusing.
Cyphos IL 101 in toluene was tested for its ability to recover Pt(IV) and Pd(II) from leachates of two SACs, one with Pt(IV) and Rh(III) and another with Pt(IV), Pd(II), and Rh(III) as PGMs [64]. An exhaustive investigation to find the best conditions to leach PGMs was accomplished, always with the goal of maximizing their dissolution and minimizing the presence of contaminants. Cyphos IL 101 showed very promising results regarding the uptake of Pd(II) and Pt(IV), with extraction percentages close to 100%, together with quantitative co-extraction of Fe(III) and Zn(II). Water scrubbing revealed poor washing percentages for Fe(III) and Zn(II) in both leachates, although the PGMs remained in the organic phase. There was inefficient Pd(II) stripping by acidic thiourea—only 34%—which was also without selectivity, as Pt(IV) and Fe(III) were co-stripped. Aqueous ammonia did not work for Pt(IV) stripping, showing better results for removing Zn(II), and especially Fe(III). In summary, the quantitative Pd(II) and Pt(IV) extractions are encouraging results, provided that the presence of Fe(III) and Zn(II) can be avoided. For that, the inclusion of a previous extraction step for base metal removal is a possibility, or the identification of appropriate scrubbing agents for both Fe(III) and Zn(II). For the latter situation, the scrubbing agents should not interfere with Pd(II) and/or Pt(IV) in the loaded Cyphos IL 101 organic phase [64].
Several alkoxyimine-1-propylpyridinium chloride derivatives were synthesized and tested for their ability to recover Pd(II) and Pt(IV) from real SACs solutions diluted in toluene [66]. These ILs were tested with leaching solutions of aqua regia and a mixture of HCl, H2SO4, and H2O2, with and without dilution. One pyridinium chloride derivative extracted 80% Pd(II) from two-fold diluted aqua regia, and > 65% Pt(IV) was extracted from the mixture of HCl, H2SO4, and H2O2 using two other IL derivatives. Separation of Pt(IV) from Pd(II) or Pd(II) from Pt(IV) was not possible by extraction with the pyridinium chloride salts used. In most cases, Pd(II) and Pt(IV) were co-extracted together into the organic phases, which were also contaminated with base metals such as Fe(III), Al(III), Mg(II), and Zn(II). Additionally, phase separation immediately after extraction was always slow, and only achieved after 24 h. Extraction of Pt(IV) and Pd(II) by alkoxyimine-1-propylpyridinium chloride derivatives occurs through ion pair formation [66].
The results obtained with Cyanex 471X (a commercial organic extractant developed for the selective extraction of Pd(II) over Pt(IV) in the 1980s) for Pd(II) extraction from a real SAC solution deserve a mention [64][69][64,69]. The active ingredient of Cyanex 471X is triisobutylphosphine sulphide (TIBPS). Adopting the recommended extraction procedure [69] (briefly, 15 min contact time for a kinetic separation of Pd(II) from Pt(IV) at 50 °C), it was possible to effectively separate Pd(II) from Pt(IV) from a real SAC leachate, and only Fe(III) was quantitatively co-extracted. Fe(III) was efficiently scrubbed by water (88%), and Pd(II) stripping was effectively accomplished by thiourea in HCl [64]. The Pd(II) percentage stripping reported—46%—is surely higher, as there was abundant precipitation of Pd species whose content was not considered in the overall Pd(II) recovery value. These results are encouraging, particularly for Pd(II) recovery, and deserve further investigation.
It is worthwhile to mention that there are other hydrometallurgical unit operations that have been actively investigated in recent years to recover platinum and palladium from real leachates of spent catalysts; these include ion exchange [70][71][72][73][74][75][70,71,72,73,74,75], applied on leachates coming from SACs [70][72][73][74][70,72,73,74] and industrial catalysts [71][75][71,75], and PGM precipitation [76][77][76,77] from SAC leachates. These works report advantages and constraints, but in comparison with SX, they are at an earlier stage of development, and therefore, still need improvement. The precipitation of PGM nanoparticles contaminated with base metals from SACs leachates, and the confirmation of the maintenance of their catalytic activity in chemical transformations [77] is an interesting finding; nevertheless, although being motivating from an in situ point of view (e.g., in-house recycling of catalysts) it does not seem promising for implementation by industry, as the selling of “no pure” catalysts should not be appealing for industries.
Goto and co-workers [58] tested the SX performance of Pd(II) and Rh(III) from a spent SAC leachate using trioctyldodecylphosphonium chloride IL—(b) derivative in Figure 5. The authors used the undiluted IL to extract Pd(II) from the leaching SAC solution, containing approximately 5 M HCl, within a 10 min contact time. Approximately 99% Pd(II) was extracted, together with 50% Fe(III) and a small amount of other metals (<5%). A first Fe(III) scrubbing with sodium sulphite for 30 min and at 45 °C allowed 90% Fe(III) removal from the loaded organic phase. Subsequently, Pd(II) stripping was accomplished using a thiourea solution, with an efficiency of 90%. Rh(III) was extracted from the raffinate leftover from the first Pd(II) extraction stage by adding a fresh quantity of the IL, but adjustment of the HCl concentration to 1 M was necessary. For an efficient Rh(III) extraction, a 5 h contact time was needed. After Fe(III) scrubbing as previously described, Rh(III) was recovered using a 5 M HCl stripping solution. Both metal ions were extracted through ion pair formation [58]. The results presented in this publication are promising, but much work has still to be carried out, particularly to check the robustness of the solvent in successive extraction–stripping cycles and the effect of the accumulation of contaminants in the organic phase. Nothing was reported about any trouble arising from the viscosity of the IL in the SX essays; therefore, it is supposed there were not any constraints to point out.
The Rzelewska-Piekut group published a paper about the use of Cyphos IL 101 [61]—derivative (a) in Figure 5—to recover Pt(IV) and Rh(III) from leaching solutions of SACs. The authors investigated the best conditions to leach Pt(IV) and Rh(III), leading to the choice of a HCl, H2SO4, and H2O2 mixture as a feed solution to apply a SX scheme involving Cyphos IL 101 diluted in toluene. The results obtained were somehow encouraging: 100% Pt(IV) and 0% Rh(III) extractions, but 100% Fe(III) and Zn(II) removal were also verified. Contact with a nitric acid solution stripped 19% of Pt(IV), all Fe(III), and half of the Zn(II) from the organic medium. Further investigation of Pt(IV) stripping from the loaded organic phase needs to be carried out to improve the SX process.
Both Cyphos IL 101 and 104—derivatives (a) and (c) in Figure 5—were tested for their ability to recover Pt(IV), Pd(II), and Rh(III) from a SAC leaching solution by the Rzelewska-Piekut group again [63]. The suggested approach used several stages: (1) a preliminary leaching step by oxalic acid, to reduce the amount of non-precious metals (Al(III), Fe(III), Mg(II), and Zn(II)) leached in the second stage and leaving PGMs in the residue; (2) leaching of the residue with a mixture of HCl, H2SO4, and H2O2 to dissolve PGMs; (3) extraction of PGMs preferably by Cyphos IL 101 in toluene, for which 100% extraction of Pt(IV), Pd(II), and Fe(III) was achieved, together with 96%, 95%, and 84% extraction of Mg(II), Zn(II), and Cu(II), respectively; and (4) Pd(II) stripping by acidic thiourea, followed by Pt(IV) stripping by a nitric acid solution. The numbers do not lie: it was obviously possible to concentrate Pd(II) and Pt(IV) from the second leaching solution by SX, but a lot of work still has to be carried out to investigate how to remove the impurities that were not easily scrubbed from the loaded IL, whose accumulation will result in the inhibition of Pt(IV) and Pd(II) extraction in a short number of extraction–stripping cycles. Both Pt(IV) and Pd(II) were extracted by Cyphos IL 101 through ion pairs [61][63][61,63]. It should also be emphasized that, much of the data were presented without a logical placement; therefore, its organization is rather confusing.
Cyphos IL 101 in toluene was tested for its ability to recover Pt(IV) and Pd(II) from leachates of two SACs, one with Pt(IV) and Rh(III) and another with Pt(IV), Pd(II), and Rh(III) as PGMs [64]. An exhaustive investigation to find the best conditions to leach PGMs was accomplished, always with the goal of maximizing their dissolution and minimizing the presence of contaminants. Cyphos IL 101 showed very promising results regarding the uptake of Pd(II) and Pt(IV), with extraction percentages close to 100%, together with quantitative co-extraction of Fe(III) and Zn(II). Water scrubbing revealed poor washing percentages for Fe(III) and Zn(II) in both leachates, although the PGMs remained in the organic phase. There was inefficient Pd(II) stripping by acidic thiourea—only 34%—which was also without selectivity, as Pt(IV) and Fe(III) were co-stripped. Aqueous ammonia did not work for Pt(IV) stripping, showing better results for removing Zn(II), and especially Fe(III). In summary, the quantitative Pd(II) and Pt(IV) extractions are encouraging results, provided that the presence of Fe(III) and Zn(II) can be avoided. For that, the inclusion of a previous extraction step for base metal removal is a possibility, or the identification of appropriate scrubbing agents for both Fe(III) and Zn(II). For the latter situation, the scrubbing agents should not interfere with Pd(II) and/or Pt(IV) in the loaded Cyphos IL 101 organic phase [64].
Several alkoxyimine-1-propylpyridinium chloride derivatives were synthesized and tested for their ability to recover Pd(II) and Pt(IV) from real SACs solutions diluted in toluene [66]. These ILs were tested with leaching solutions of aqua regia and a mixture of HCl, H2SO4, and H2O2, with and without dilution. One pyridinium chloride derivative extracted 80% Pd(II) from two-fold diluted aqua regia, and > 65% Pt(IV) was extracted from the mixture of HCl, H2SO4, and H2O2 using two other IL derivatives. Separation of Pt(IV) from Pd(II) or Pd(II) from Pt(IV) was not possible by extraction with the pyridinium chloride salts used. In most cases, Pd(II) and Pt(IV) were co-extracted together into the organic phases, which were also contaminated with base metals such as Fe(III), Al(III), Mg(II), and Zn(II). Additionally, phase separation immediately after extraction was always slow, and only achieved after 24 h. Extraction of Pt(IV) and Pd(II) by alkoxyimine-1-propylpyridinium chloride derivatives occurs through ion pair formation [66].
The results obtained with Cyanex 471X (a commercial organic extractant developed for the selective extraction of Pd(II) over Pt(IV) in the 1980s) for Pd(II) extraction from a real SAC solution deserve a mention [64][69][64,69]. The active ingredient of Cyanex 471X is triisobutylphosphine sulphide (TIBPS). Adopting the recommended extraction procedure [69] (briefly, 15 min contact time for a kinetic separation of Pd(II) from Pt(IV) at 50 °C), it was possible to effectively separate Pd(II) from Pt(IV) from a real SAC leachate, and only Fe(III) was quantitatively co-extracted. Fe(III) was efficiently scrubbed by water (88%), and Pd(II) stripping was effectively accomplished by thiourea in HCl [64]. The Pd(II) percentage stripping reported—46%—is surely higher, as there was abundant precipitation of Pd species whose content was not considered in the overall Pd(II) recovery value. These results are encouraging, particularly for Pd(II) recovery, and deserve further investigation.
It is worthwhile to mention that there are other hydrometallurgical unit operations that have been actively investigated in recent years to recover platinum and palladium from real leachates of spent catalysts; these include ion exchange [70][71][72][73][74][75][70,71,72,73,74,75], applied on leachates coming from SACs [70][72][73][74][70,72,73,74] and industrial catalysts [71][75][71,75], and PGM precipitation [76][77][76,77] from SAC leachates. These works report advantages and constraints, but in comparison with SX, they are at an earlier stage of development, and therefore, still need improvement. The precipitation of PGM nanoparticles contaminated with base metals from SACs leachates, and the confirmation of the maintenance of their catalytic activity in chemical transformations [77] is an interesting finding; nevertheless, although being motivating from an in situ point of view (e.g., in-house recycling of catalysts) it does not seem promising for implementation by industry, as the selling of “no pure” catalysts should not be appealing for industries.
 The authors claimed that, with the proposed solvometallurgical process, less harmful chemicals compared to those of the hydrometallurgical approaches were used, and the utilization of high temperatures and pressures and aggressive acidic leaching media (i.e., Cl2, HNO3, and HCl) were also avoided. Regarding the non-aqueous SX, the complete separation of individual PGMs and Fe(III) in a safe and simple way was accomplished as well. The assumption that acetonitrile is a harmless chemical solvent is, at least, dubious [82]. Even though revealing an apparent high potential for PGM recovery from SACs [79][81][79,81], the use of solvometallurgy as a viable alternative to the already mature hydrometallurgy needs to be much more developed and further evaluated.
The authors claimed that, with the proposed solvometallurgical process, less harmful chemicals compared to those of the hydrometallurgical approaches were used, and the utilization of high temperatures and pressures and aggressive acidic leaching media (i.e., Cl2, HNO3, and HCl) were also avoided. Regarding the non-aqueous SX, the complete separation of individual PGMs and Fe(III) in a safe and simple way was accomplished as well. The assumption that acetonitrile is a harmless chemical solvent is, at least, dubious [82]. Even though revealing an apparent high potential for PGM recovery from SACs [79][81][79,81], the use of solvometallurgy as a viable alternative to the already mature hydrometallurgy needs to be much more developed and further evaluated.

Figure 2.
Solvent extraction (SX) in hydrometallurgy.
2. Hydrometallurgy
As pointed out in the Introduction, the usual leaching media to dissolve PGMs are chloride aqueous solutions. The development of processes able to efficiently recover and separate platinum and palladium from complex chloride media must rely on knowledge about the nature and physico-chemical behaviour of their chlorocomplexes [13]. The data regarding the speciation of the most significant co-existing contaminants, as well as their main physico-chemical characteristics, are obviously valuable in accomplishing successful operations. The speciation and the kinetic/thermodynamic properties of all PGM chlorocomplexes are highly dependent on several factors, and the knowledge and exploitation of the most relevant ones have been the keys to the development of PGM separation schemes, which have been successfully applied by refiners to precious metal recovery (gold, silver, and PGMs) from primary sources since the 1970s. These factors are [13][35][13,35]:-
The oxidation and coordination numbers;
-
The size, charge, and structure of chlorocomplexes;
-
The kinetics of ligand exchange reactions of the type MXn + L → MXn−1L + X.
Table 1. Oxidation states, d-electron configurations, coordination numbers, and structure configurations of the most common Pd and Pt chlorocomplexes.
| Metals | Oxidation States | d-Electron Configurations | Coordination Numbers | Structure Configurations |
|---|---|---|---|---|
| Pd | +2 (+4) | d8 (d6) | 4 | 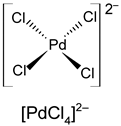 |
| Pt | +2, +4 | d8, d6 | 4, 6 | 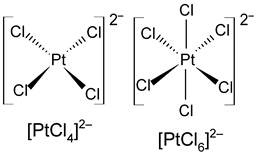 |
[PdCl
[PdCl
4
]
2−
(aq) + 2 DOS (org) ⇔ PdCl
2
(DOS)
2
(org) + 2 Cl
− (aq) (1)
(aq)
[PtCl
[PtCl
6
]
2−
(aq) + 2 R
3
NH
+
Cl
−
(org) ⇔ [(R
3
NH
+
)
2
PtCl
62−
] (org) + 2 Cl
− (aq) (2)
(aq)
H
H
2
PtCl
6
(aq) + 2 TBP (org) ⇔ H
2
PtCl
6 · 2 TBP (org) (3)
· 2 TBP (org)
[PdCl
[PdCl
4
]
2−
(aq) + 2 R
3
NH
+
Cl
−
(org) ⇔ [(R
3
NH
+
)
2
PdCl
42−
] (org) + 2 Cl
− (aq) (4)
(aq)
[(R
[(R
3
NH
+
)
2
PdCl
42−
] (org) + 2 HL (org) ⇔ PdL
2
(org) + 2 R
3
NH
+
Cl
− (org) + 2 HCl (aq) (5)
(org) + 2 HCl (aq)
Table 2. Most significant SX schemes to recover Pd(II) and Pt(IV) from pregnant liquors of spent catalysts (SAC: spent automobile catalyst; TU: thiourea).
| Target Metal(s) | Extractant(s) | Catalysts/Leaching Media for SX Application | SX Data | References |
|---|---|---|---|---|
| Pd, Pt | LIX84I (2-hydroxy-5-nonylacetophenone oxime) and Alamine 336 (trioctyl-decylamines) | First-generation SAC/ 3 M HCl |
LIX84I: 100% Pd extraction; 100% stripping with acidic TU; good selectivity Alamine 336: 100% Pt extraction; 100% stripping with acidic TU; Fe co-extraction, easily scrubbed |
[37] |
| Pt | Alamine 336; Aliquat 336 (trioctylmethyl ammonium chloride) |
Monometallic (Pt/Al2O3); multimetallic (PtSnIn/Al2O3)/1.4 and 0.5 M Cl−, respectively | >99.4% extraction; reductive stripping to Pt(II) by Na2S2O3; good selectivity | [38] |
| Pt | N,N’–dimethyl–N,N’–diphenyltetradecyl-malonamide (DMDPHTDMA) |
SAC/5M HCl | 99.5% extraction in presence of Sn; ≈79% stripping with (HCl + NaClO3) + NaOH; reasonable selectivity | [39] |
| Pd | 1,2-bis(2-methoxyethylthio) benzene |
SAC/2 M Cl− | 100% extraction; 100% stripping with acidic TU; excellent selectivity | [40] |
| Pd | Cyanex 923 (trialkylphosphine oxides, mainly with hexyl and octyl groups) | Alumina and coated ceramic honeycomb catalysts/5 M H2SO4 | 98.6% extraction; 100% stripping with HClO4; excellent selectivity over Al | [41] |
| Pd, Pt | Alamine 336 (Microfluidic SX) | SAC/pH 1.42 | Pd and Pt co-extraction | [42] |
| Pt | Aliquat 336 | Spent petroleum refining catalyst/3 M HCl | 100% extraction of Pt and Fe; 100% Fe scrubbing with dilute HCl; 100% Pt stripping by HClO4 | [43] |
| Pd | Thiacalix[6]arene and thiacalix[4]arene derivatives | SAC/pH 0.8 | 99.3% extraction; 100% stripping with TU; excellent selectivity, slight Zr co-extraction | [44] |
| Pt | Thiacalix[4]arene derivative | SAC/pH 0.8 | 88% extraction; 20% Pd co-extraction | [45] |
| Pd | Thiacalix[6]arene and thiacalix[4]arene derivatives | SAC/1 M HCl | 99% extraction; slight co-extraction of Zr, Pt, and Al | [46] |
| Pd | Thiacalix[6]arene derivative | SAC/pH 1.5 | 98% extraction; 98% stripping with acidic TU; 22% co-extraction of Zr | [47] |
| Pd | Acyclic thioamide derivative | SAC/0.1 M HCl | 96% extraction; 99.5% stripping with acidic TU; excellent selectivity | [48] |
| Pd | 1,3-bis (dimethylthio-carbamoyloxy)-benzene | SAC/1 M HCl | 99.9% extraction; > 99% stripping with acidic TU; excellent selectivity | [49] |
| Pd | Calix[4]arene-based n-dialkylamino extractants | SAC/0.06 M HCl | 88–92% extraction; 90–99% stripping with acidic TU; reasonable selectivity | [50] |
| Pd | Azothiacalix[4]arene derivative | SAC/1 M HCl | 99.7% extraction; >99% stripping with acidic TU; <10% Pt and La co-extraction | [51] |
| Pd | Heterocyclic dithioether ligands | SAC/0.5 M HCl | 99.9% extraction; >99% stripping with acidic TU; 11–17% Pt, 3–8% Rh, <4% other metals co-extraction | [52] |
| Pd | Sulphur–carbon–sulphur (SCS) pincer ligands | SAC/≈ 0.1 M HCl | 99.9% extraction; >99.9% stripping with acidic TU; excellent selectivity | [53] |
| Pd | Thioamide and thiodiglycolamide derivatives | Spent petrochemical catalyst/2M HCl (MgCl2 + NH4Cl) + H2O2 | 95–100% extraction; 98–99.5% stripping with acidic TU; Al slowly accumulates in the solvents | [54] |
| Pd, Pt | Thiodiphenol-based n-dialkylamino extractants | SAC/0.1 M HCl | 99.5% Pd, 99.3% Pt extraction; 99% Pd and Pt stripping with acidic TU; <3% other metals co-extraction | [55] |
| Pd, Pt | Calix[4]arene-based amino extractants containing n-alkyl moieties | SAC/pH 1.22 | 93–98% Pd, 92–94% Pt extraction; 95–99% Pd, 91–93% Pt stripping with acidic TU; <6% other metals co-extraction | [56] |
| Pd | Thioamide-modified calix[4]arene derivative |
SAC/0.06 M HCl | 99.9% extraction; 99.9% stripping with acidic TU; <2% other metals co-extraction | [57] |
| Pd | Phosphonium-based ionic liquid | SAC/5 M HCl | 90% extraction, 50% Fe co-extracted; Fe scrubbing by Na2SO3, Pd stripping by TU; small amounts of other metals co-extracted | [58] |
| Pd, Pt | Alamine 308 (trioctylamine); Cloud-point extraction |
SAC/unknown [HCl] | 81% Pd, 95% Pt | [59] |
| Pd | Thiophosphate-based extractant | SAC/0.06 M HCl | 99.9% extraction; 99% stripping with acidic TU; <1% other metals co-extraction | [60] |
| Pt, Rh | Cyphos IL 101 (trihexyl-tetradecyl- phosphonium chloride) |
SAC/10.7 M HCl + 1 M H2SO4 + H2O2 |
100% Pt, 0% Rh, 100% Fe, 100% Zn, 47% Pb extraction; 19% Pt and 100% Fe stripping with HNO3 | [61] |
| Pt | Thiodiglycolamide derivative | SAC/HCl + H2O2 (8 M HCl) |
100% extraction; 100% Fe co-extraction; Fe scrubbing by water and Pt stripping by acidic TU did not work | [62] |
| Pd, Pt | Cyphos IL 101 | SAC/10.7 M HCl + 1 M H2SO4 + H2O2 |
100% Pt, 100% Pd, 100% Fe, 96% Mg, 95% Zn, 84% Cu extraction; Pd stripping with acidic TU and Pt stripping with HNO3 | [63] |
| Pd, Pt | Cyanex 471X (triisobutylphosphine sulphide) and Cyphos IL 101 | SAC/HCl + H2O2 (6 M HCl) |
Cyanex 471X: 100% Pd extraction; 46% stripping with acidic TU; 99% Fe, scrubbed with water. Cyphos IL 101: 99% Pt, 100% Pd; 100% Fe, 100% Zn co-extraction; stripping inefficient |
[64] |
| Pd | Dithiophenol-based extractant bearing 3 S atoms |
SAC/HCl + H2O2 [HCl] ≈ 8 M |
100% Pd, 4% Al, 11% Cr, 24% Fe, 3% Ni, 9% Rh, 1% Ce extraction; Fe scrubbing with water, 100% Pd stripping with acidic TU | [65] |
| Pd, Pt | Pyridinium salt derivatives | SAC/aqua regia + H2O2 (half diluted); HCl + H2SO4+ H2O2 (half diluted) [H+] < 6 M |
One compound: 80% Pd, 0% Pt extraction; another compound: 70% Pt, 0% Pd; Fe and Zn co-extraction; Pd stripping by acidic TU | [66] |

Figure 3. Compounds recently developed by the Yamada group for the SX of Pd(II) and Pt(IV). Examples of structures: (a) acyclic thioamide derivative [48]; (b) SCS pincer ligand with 2 thioamide groups [49]; (c) SCS pincer ligand with 2 sulphide groups [53]; (d) thiodiphenol derivative with 2-substituted amino groups [55]; (e) thiophosphate-based extractant [60]; (f) dithiophenol-based extractant with 3 sulphur atoms [65].

Figure 4.
The structures of the thioamide (
a
) and thiodiglycolamide (

3. Solvometallurgy
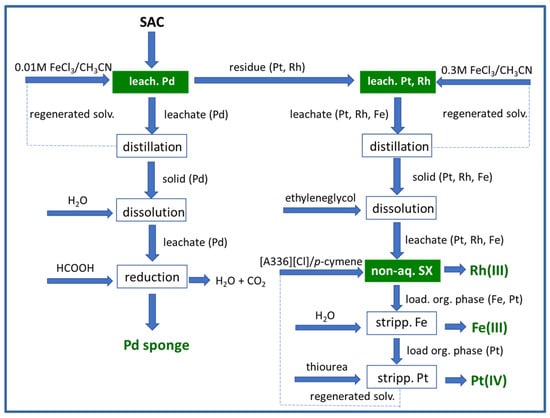
Solvometallurgy is the designation used to describe the integrated process of extracting metals from ores, industrial residues, scraps, and urban waste using non-aqueous solutions [10]. Non-aqueous does not mean that water is forbidden, but a low water content should be employed. The involved unit operations in solvometallurgy are solvent leaching; separation of the residue; purification of the leach solution by non-aqueous SX [11] or non-aqueous ion exchange; and metal recovery by precipitation or electrolysis in non-aqueous electrolytes. For solvometallurgical processes to be sustainable, they must be based on the use of green solvents, meaning that all toxic or environmentally harmful solvents must be avoided [10]. ILs are an example of alternative chemical compounds that are supposed to be “green”. It is, however, well known that some ILs are not as green as previously thought, and the investigation of their toxicity should be a priority [78]. As described previously, several ILs have been investigated for PGM recovery from model and real leaching solutions of spent catalysts, but not from the point of view of an integrated solvometallurgical process; therefore, the investigations which aimed to develop integrated non-aqueous processes, starting from the PGM-containing spent catalyst, and ending in the final metal recovery, will be mainly addressed under this heading, obviously focusing on the use of non-aqueous SX. Non-aqueous SX considers the use of two immiscible liquid phases, but neither is aqueous. Concerning PGM recycling from SACs, the main overall solvometallurgical operations developed under the scope of PLATIRUS, an EU Horizon 2020 project, have been published [79]. This article presents a PLATIRUS project overview, although without an in-depth technical analysis of each technology; it summarises the evaluation of the most promising technologies that were developed, their operation in cascade, the expected recycled PGM end-user validation, and the next steps required to prepare the industrial application and to further confirm the project’s potential. Several types of leaching procedures were investigated [79], including solvometallurgical and microwave-assisted options to separate PGMs from leachates, and “non-conventional liquid–liquid extraction” approaches were developed [79]. The microwave-assisted leaching option led to the concept of “split-anion extraction”. Basically, split-anion extraction is an SX process in which different anions are present in the aqueous and organic solutions. In the project, PGMs were extracted from the chloride leachates using the iodide form of the quaternary ammonium IL Aliquat 336, [A336][I], dissolved in p-cymene. The iodide anions, which have a stronger affinity for the organic phase, coordinate with PGMs to form stable iodo-complexes in the IL phase [79][80][79,80]. The investigators from the PLATIRUS EU project published another article describing the feasibility of an optimized integrated solvometallurgical approach to efficiently and selectively recover PGMs from SACs [81]. Two distinct solvoleaching steps were proposed: the first one was to selectively leach Pd(II) using a dilute solution of ferric chloride in acetonitrile, separating it from the other elements; the remaining residue undergoes a similar operation but using a more concentrated ferric chloride solution, again in acetonitrile, to extract Pt(IV), Rh(III), and Fe(III). After evaporation of the solvent, the residue was dissolved in ethylene glycol and, through non-aqueous SX, Pt(IV) and Fe(III) were extracted by the quaternary ammonium IL Aliquat 336 ([A336][Cl]) dissolved in p-cymene, leaving Rh(III) in the raffinate. Fe(III) and Pt(IV) were subsequently separated through selective stripping. The overall workflow described in [81] is detailed in Figure 6.
Figure 6. Simplified scheme showing the integrated solvometallurgical process to recover PGMs from SACs, developed under the PLATIRUS EU Horizon 2020 project (adapted from [81]).