Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Bojana Kožik.
LOXL2, a copper-dependent amine oxidase, has emerged as a promising therapeutic target in hepatocellular carcinoma (HCC). Increased LOXL2 expression in HCC has been linked with an aggressive phenotype and represents a poor prognostic factor.
- hepatocellular carcinoma (HCC)
- LOXL2
- tumor microenvironment (TME)
- extracellular matrix (ECM)
1. Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, accounting for more than 80% of cases [1]. With incidence and mortality rates increasing worldwide, it represents a global healthcare concern [1,2][1][2]. The underlying process in the development of HCC is chronic liver damage leading to cirrhosis, which is most often caused by chronic viral hepatitis (hepatitis B and C), alcohol abuse, and metabolic dysfunction-associated steatotic liver disease (MASLD) [3]. Around 80% of HCC arises from a cirrhotic liver, and among patients with cirrhosis of any etiology, one-third will develop HCC [1,4,5][1][4][5]. The pathogenesis of HCC is a complex multistep process that usually starts with liver injury and inflammation. Chronic liver inflammation triggers a fibrous process, which over time can progress to cirrhosis and is characterized by the extensive disruption of liver tissue architecture. Prolonged inflammation and cirrhosis create precancerous settings resulting in the generation of dysplastic foci and dysplastic nodules, which further accumulate genetic/epigenetic alterations, resulting in the development of hepatocellular carcinoma [6].
The most appropriate therapeutic option is determined based on the TNM tumor stage, degree of background liver damage, and the patient’s overall health [7]. The recommended therapy for an early-stage HCC is surgical resection, liver transplantation, or radiofrequency ablation. Transarterial chemoembolization (TACE) and radiation therapy, alone or in combination with systemic therapy, are used for intermediate-stage HCCs, while systemic therapy (chemotherapy, molecular targeted therapy, immunotherapy, and gene therapy) is the treatment of choice for advanced-stage HCCs [7,8][7][8]. Unfortunately, due to the asymptomatic nature of early-stage HCC, more than 60% of cases are detected in the intermediate or advanced stage, where curative therapeutic options are limited [9]. The backbone of systemic therapy for HCC is sorafenib and other multi-kinase inhibitors [7,10,11,12][7][10][11][12]. Moreover, ramucirumab, a vascular endothelial growth factor (VEGF) inhibitor and immune checkpoint inhibitors (nivolumab and pembrolizumab) are being increasingly used, either separately or in combination [7,13][7][13]. Although the choices for therapeutic strategies in advanced HCC are rapidly expanding, they provide a variable but still limited extension of survival, cause a wide range of side effects, and ultimately, lead to the development of tumor resistance, which is recognized as one of the biggest problems in the treatment of HCC [10].
The multitude of underlying mechanisms that are responsible for the development of resistance to therapy by HCC is not completely understood, although it has been shown that tumor microenvironment (TME) plays an important role. The tumor microenvironment has a crucial role in hepatocarcinogenesis and directly participates in the regulation of liver fibrosis and tumor-progressive processes, such as epithelial–mesenchymal transition (EMT), extracellular matrix (ECM) remodeling, migration, invasion, and metastasis [14]. Unraveling the complex interactions within the tumor microenvironment and targeting the components of the TME, such as ECM remodeling enzymes, might serve as a valuable strategy to improve the current therapeutic options and develop novel ones while attempting to re-sensitize resistant tumors to existing therapeutic agents [10,15,16][10][15][16].
In the last fifteen years, lysyl oxidase-like 2 (LOXL2) has emerged as one of the major mediators between tumor cells and TME. This research has implicated the involvement of LOXL2 in every step of tumor progression [17,18,19,20,21,22][17][18][19][20][21][22]. The involvement of LOXL2 has been reported in the regulation of cancer cell proliferation, epithelial–mesenchymal transition, migration, extravasation, and creating premetastatic niches at distant sites, as reviewed by Zhang et al. [23], Lin et al. [24], and Wen et al. [25]. The mechanisms through which LOXL2 affects tumor invasiveness can be used as a typical model of solid cancer progression and spreading [26]. In addition, LOXL2 regulates tumor-induced angiogenesis [27,28,29][27][28][29] and mediates the interaction between cancer cells and cancer-associated fibroblasts (CAF), and macrophages [22,30,31][22][30][31]. Taken together, LOXL2 represents a multifunctional protein, which is enrolled in almost every step of solid tumor propagation.
2. LOXL2 Introduction: LOX Family, Structure, and LOXL2 Function
LOXL2 is a secreted and copper-dependent amine oxidase that belongs to the lysyl oxidase (LOX) family, which consists of five homologous members: LOX and LOX-like l–4 (LOXL1–4) proteins [32,33,34,35,36,37][32][33][34][35][36][37]. The primary function of the LOX family enzymes is to catalyze the cross-linking of elastin and collagen by oxidation, which is essential for maintaining the rigidity, stability, and remodeling of the extracellular matrix (ECM) [38,39][38][39]. The human LOXL2 gene is positioned on chromosome 8p21-22 and encodes a 774 amino acid protein [26,40][26][40]. Structurally, the LOXL2 protein contains a variable N-terminal region and a highly conserved C-terminal region with catalytic activity (Figure 1). The catalytic domains at the C-terminus are conserved among LOX family members and consist of the copper-binding domain, lysyl tyrosine quinone (LTQ) element, which is required for cofactor formation, and a cytokine receptor-like (CRL) domain [41,42][41][42]. The LOXL2 N-terminal domain is more variable and includes a signal peptide and four scavenger receptor cysteine-rich (SRCR) elements, which are responsible for protein–protein interactions [41,43][41][43].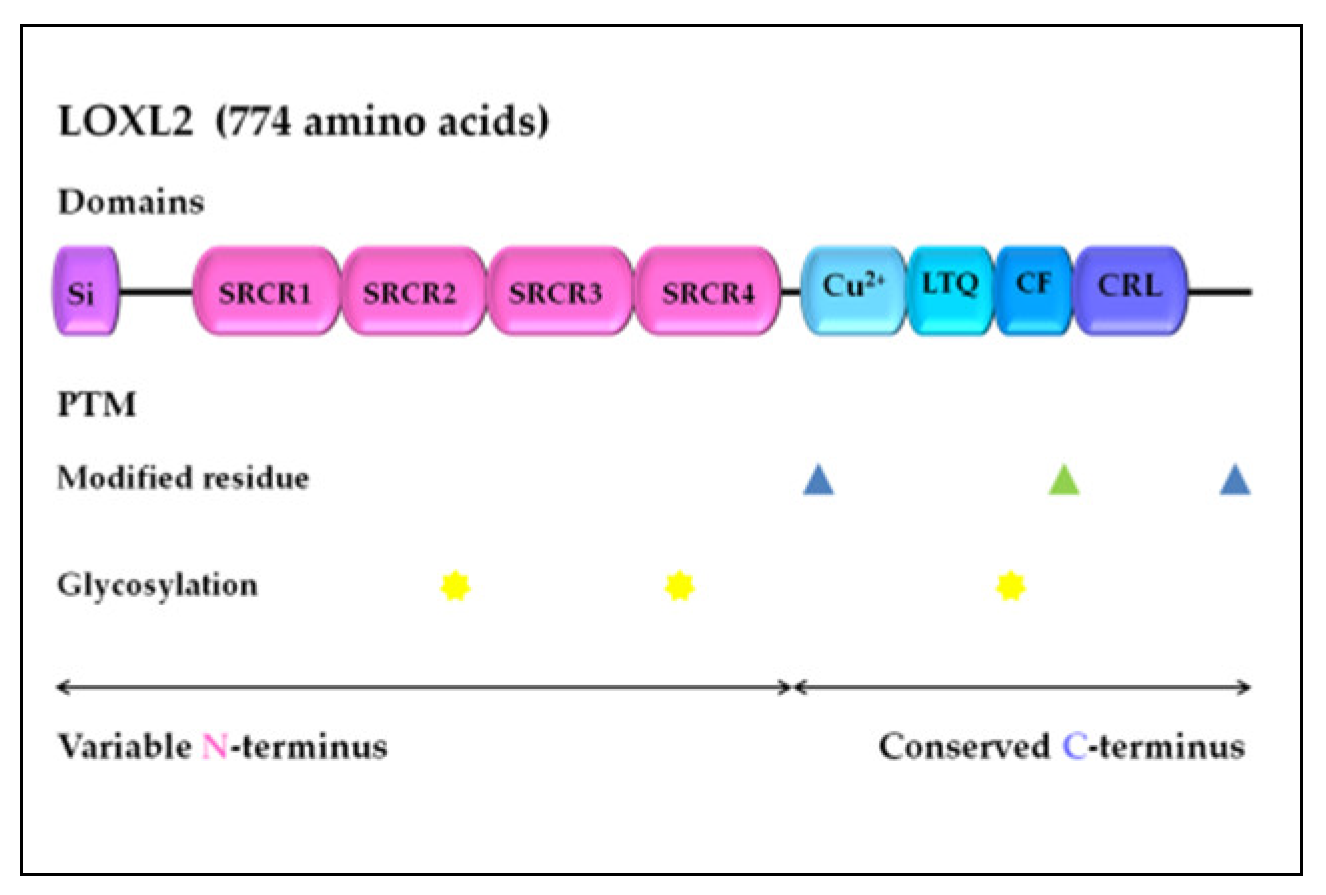
Figure 1. LOXL2 protein structure. Arrows indicate the variable N-terminal domain and conserved C-terminal domain consists of several different functional domains, represented by boxes. Post-translational modifications of LOXL2 were obtained using UniProt. Modified residues are represented by blue triangles (phosphoserine at 601 and 722) and green triangle (2′,4′,5′-topaquinone at 689), while N-linked glycans at N288, N455, and N644 are marked with yellow stars. Si, signal peptide; SRCR, scavenger receptor cysteine-rich domain; Cu2+, copper binding domain; LTQ, lysine tyrosylquinone cofactor residues; CF, cofactor formation; CRL, cytokine receptor-like domain; PTM, post-translational modifications.
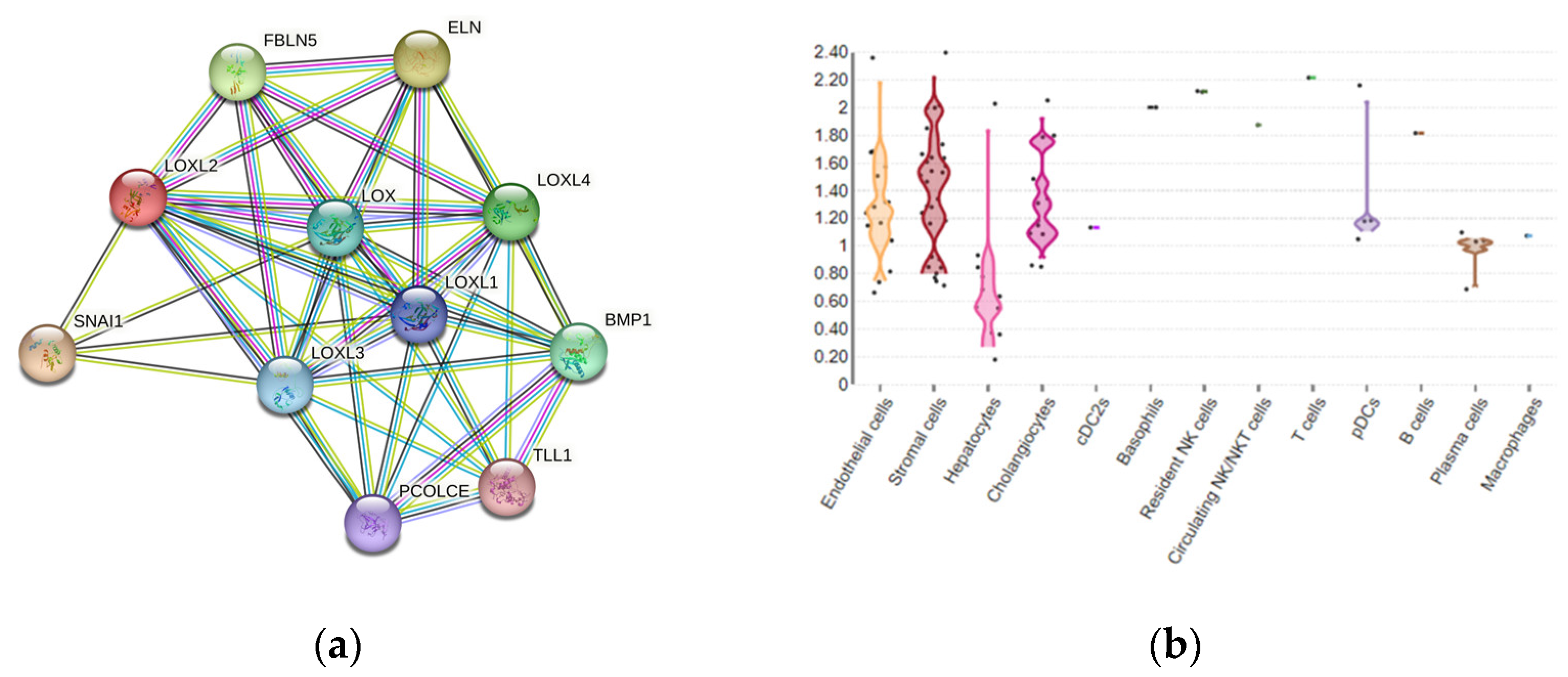
Figure 2. (a) LOXL2 protein interactome. Available on the String platform. SNAI1, zinc finger protein involved in the induction of the epithelial–mesenchymal transition (EMT); ELN, elastin; FBLN5, fibulin-5, which is essential for elastic fiber formation; PCOLCE, procollagen C-endopeptidase enhancer 1, which binds to the C-terminal propeptide of type I procollagen; TLL1, Tolloid-like protein 1, protease that processes procollagen C-propeptides; BMP1, bone morphogenetic protein 1, which cleaves the C-terminal propeptides of procollagen I, II, and III. (b) LOXL2 spatial expression in healthy liver cells.
3. LOXL2 Expression in HCC and Correlation with Clinical Parameters
LOXL2 is overexpressed in human HCC tissue compared to healthy liver tissue, at both the mRNA and protein levels [17,24,67,68,69,70,71,72][17][24][65][66][67][68][69][70]. LOXL2 protein expression has been shown to correlate to the amount of fibrosis in the tumor stroma and was more pronounced in the cytoplasm of cancer cells directly adjacent to a fibrous stroma, compared to centrally located cancer cells [71][69]. LOXL2 expression was positively correlated with the direct invasion of adjacent liver tissue [17], increased frequent portal vein invasion, poor tumor differentiation, and more advanced TNM stage [71][69]. Moreover, LOXL2 expression in HCC patients, especially high-risk HCCs (HCC tumor size > 5 cm, HCCs with portal vein invasion, poor differentiation, and TNM stage II or III), correlated with shorter overall survival, disease-free survival, disease-specific survival, and extrahepatic recurrence-free survival [24,69,70,71][24][67][68][69]. Hypoxia, chronic inflammation, and fibrosis have all been shown to induce LOXL2 expression in HCC [15,17][15][17]. Additionally, in human HCC tissue samples, the co-expression of LOXL2 and carbon anhydrase IX, which is a hypoxia-related biomarker, showed a better correlation with prognostic parameters compared to LOXL2 alone [71][69]. Interestingly, LOXL2 levels were significantly higher in the serum of HCC patients than in the serum of non-HCC patients [17], making LOXL2 a promising biomarker for HCC.4. LOXL2 and micro-RNAs in HCC
Recent studies have revealed that micro-RNAs (miRs) may be differentially expressed in HCC and that some of them function as negative epigenetic regulators of LOXL2, thereby affecting the LOXL2 TME-mediated processes. Wong et al. demonstrated that miR-26 and miR-29 suppress LOXL2 in HCC by directly binding to the 3′UTR of LOXL2 mRNA [17]. Specifically, miR29-a has been identified as a negative regulator of hypoxia-responsive genes, such as HIF-1α, VEGFa, LOX, and LOXL2 in HCC cell lines and HCC tissue functional studies [69,136][67][71]. Repression of miR-29a has been mediated by MYC; MYC amplification represents one of the earliest events in HCC development [137,138][72][73]. Recent bioinformatics analysis proposed hsa-miR-192-5p as a potential LOXL2 regulator in HCC; however, more in vivo studies are required to clarify these interactions between LOXL2 and specific micro-RNAs in the pathogenesis of HCC [139][74].5. LOXL2 as Potential Target for Treatment of HCC
The well-established treatment options for patients with HCC have limited clinical efficacy in the late stages of HCC due to severe fibrosis and cirrhosis [140][75]. Therefore, chemoresistant HCC cases require novel combined treatment strategies. Since LOXL2 is unquestionably involved in almost every step of HCC progression and dissemination, this protein fulfills conditions that are required for a potential target in developing molecular anticancer therapy. Considering the LOXL2 protein structure, two approaches for targeting this protein were considered in drug development studies. The first was based on the fact that LOXL2 demands copper ions for enzyme activity, and the second was to target the lysine tyrosylquinone region (LTQ), which possesses a cofactor binding function [141][76]. Designing highly selective LOXL2 inhibitors is promising and could be beneficial for HCC patients with LOXL2 overexpression since the downregulation of LOXL2 expression reduces tumor cell invasiveness and metastatic spread [142][77]. The first monoclonal LOXL2 antibody, AB0023, which specifically binds to the fourth SRCR domain was developed in 2010 and has been effective in clinical studies (Table 1) [49,127,143][48][78][79]. Its efficacy was demonstrated in both tumor xenografts as well as in liver fibrosis models [49,127][48][78]. On the other hand, simtuzumab, a humanized LOXL2 antibody (AB0024), has not achieved satisfactory results in clinical trials for fibrotic diseases and several solid tumors [144,145,146][80][81][82]. The lack of clinical effectiveness can be explained by the fact that specific antibody inhibitors probably only deactivate extracellular LOXL2, while the intracellular functions of LOXL2 remain preserved due to incomplete antibody internalization or the compensatory activity of other LOX family members [25,82][25][83]. In the search for more selective LOXL2 inhibitors, most efforts have been toward designing a small molecule inhibitor in order to increase specificity and efficacy, and to minimize its side effects [141][76]. According to the data available on the PHAROS web interface for exploring target/ligand interactions [147][84], a query for ”LOXL2” resulted in 227 active ligands (ChEMBL compounds with an activity cutoff of <30 nM), although clinical trials that focus on testing LOXL2 based-drugs for HCC are still lacking. One of the most explored LOX inhibitors is β-aminopropionitrile (BAPN), a small irreversible inhibitor that blocks the catalytic activity of all LOX members and displays specific affinity to LOXL2 [141,148][76][85]. Previous studies have shown significant tumor suppressive effects by BAPN in several solid tumors and tumor-cell lines [149,150,151,152,153][86][87][88][89][90]. Moreover, some studies demonstrated that BAPN affects the TME and impedes the interaction between cancer-associated fibroblasts and gastric cancer cells, resulting in a reduction in the frequency and size of the liver metastasis [154][91]. In HCC, BAPN inhibited tumor growth and angiogenesis in vivo and hampered the migration and invasion of HCC cell lines [70,118][68][92]. According to the study by Liu et al. [155][93], BAPN also showed an ameliorative effect in the CCl4-induced model of liver fibrosis. The first selective LOXL2 inhibitor, LOXL2-IN-1 hydrochloride, demonstrated potential for use in HCC treatment since it suppressed Snail1, HIF-1α, and VEGF, the main drivers of HCC progression, as mentioned above [24]. In recent years, the second generation of small-molecular-weight haloallylamine-based LOXL2 inhibitors was explored, including PXS-5338, PXS-5382, and PXS-5878, which showed promising results in inhibiting the catalytic activity of LOXL2 [156][94]. A dual LOXL2/LOXL3 inhibitor, PXS-5153A, was designed in 2019 by Schilter et al. [157][95] and also demonstrated ameliorating effects and a significant improvement in liver fibrosis.Table 1. LOXL2 inhibitors for HCC and liver fibrosis.
| Type | Agent | Target | References | |
|---|---|---|---|---|
| monoclonal antibody |
AB0023 | LOXL2 | [46,121,122] | [45][96][97] |
| AB0024 | LOXL2 | [123,124,125] | [98][99][100] | |
| small-molecule inhibitor |
BAPN | LOX/LOXL1-4 | [100,133,134] | [101][102][103] |
| LOXL2-IN-1 | LOXL2 | [122] | [97] | |
| PXS-5338 | LOXL2 | [156] | [94] | |
| PXS-5382 | LOXL2 | [156] | [94] | |
| PXS-5878 | LOXL2 | [156] | [94] | |
| PXS-5153A | LOXL2/LOXL3 | [157] | [95] | |
| (2-chloropyridin-4-yl) methenamine | LOXL2 | [158] | [104] |
References
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CACancer J. Clin. 2021, 71, 209–249.
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604.
- Affo, S.; Yu, L.-X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu. Rev. Pathol. 2017, 12, 153–186.
- Sangiovanni, A.; Prati, G.M.; Fasani, P.; Ronchi, G.; Romeo, R.; Manini, M.; Del Ninno, E.; Morabito, A.; Colombo, M. The Natural History of Compensated Cirrhosis Due to Hepatitis C Virus: A 17-Year Cohort Study of 214 Patients. Hepatology 2006, 43, 1303–1310.
- Dhanasekaran, R.; Bandoh, S.; Roberts, L.R. Molecular Pathogenesis of Hepatocellular Carcinoma and Impact of Therapeutic Advances. F1000Research 2016, 5, 879.
- Wege, H.; Li, J.; Ittrich, H. Treatment Lines in Hepatocellular Carcinoma. Visc. Med. 2019, 35, 266–272.
- Suresh, D.; Srinivas, A.N.; Prashant, A.; Harikumar, K.B.; Kumar, D.P. Therapeutic Options in Hepatocellular Carcinoma: A Comprehensive Review. Clin. Exp. Med. 2023.
- Altekruse, S.F.; McGlynn, K.A.; Reichman, M.E. Hepatocellular Carcinoma Incidence, Mortality, and Survival Trends in the United States from 1975 to 2005. J. Clin. Oncol. 2009, 27, 1485–1491.
- Bao, M.H.-R.; Wong, C.C.-L. Hypoxia, Metabolic Reprogramming, and Drug Resistance in Liver Cancer. Cells 2021, 10, 1715.
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390.
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2009, 10, 25–34.
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primer 2021, 7, 6.
- Novikova, M.V.; Khromova, N.V.; Kopnin, P.B. Components of the Hepatocellular Carcinoma Microenvironment and Their Role in Tumor Progression. Biochem. Biokhimiia 2017, 82, 861–873.
- Gong, L.; Zhang, Y.; Yang, Y.; Yan, Q.; Ren, J.; Luo, J.; Tiu, Y.C.; Fang, X.; Liu, B.; Lam, R.H.W.; et al. Inhibition of Lysyl Oxidase-like 2 Overcomes Adhesion-dependent Drug Resistance in the Collagen-enriched Liver Cancer Microenvironment. Hepatol. Commun. 2022, 6, 3194–3211.
- Hernandez–Gea, V.; Toffanin, S.; Friedman, S.L.; Llovet, J.M. Role of the Microenvironment in the Pathogenesis and Treatment of Hepatocellular Carcinoma. Gastroenterology 2013, 144, 512–527.
- Wong, C.C.-L.; Tse, A.P.-W.; Huang, Y.-P.; Zhu, Y.-T.; Chiu, D.K.-C.; Lai, R.K.-H.; Au, S.L.-K.; Kai, A.K.-L.; Lee, J.M.-F.; Wei, L.L.; et al. Lysyl Oxidase-like 2 Is Critical to Tumor Microenvironment and Metastatic Niche Formation in Hepatocellular Carcinoma. Hepatology 2014, 60, 1645–1658.
- Payne, S.L.; Hendrix, M.J.C.; Kirschmann, D.A. Paradoxical Roles for Lysyl Oxidases in Cancer—A Prospect. J. Cell. Biochem. 2007, 101, 1338–1354.
- Zhan, X.; Jiao, J.; Zhang, H.; Li, C.; Zhao, J.; Liao, L.; Wu, J.; Wu, B.; Wu, Z.; Wang, S.; et al. A Three-Gene Signature from Protein-Protein Interaction Network of LOXL2- and Actin-Related Proteins for Esophageal Squamous Cell Carcinoma Prognosis. Cancer Med. 2017, 6, 1707–1719.
- Moreno-Bueno, G.; Salvador, F.; Martín, A.; Floristán, A.; Cuevas, E.P.; Santos, V.; Montes, A.; Morales, S.; Castilla, M.A.; Rojo-Sebastián, A.; et al. Lysyl Oxidase-like 2 (LOXL2), a New Regulator of Cell Polarity Required for Metastatic Dissemination of Basal-like Breast Carcinomas. EMBO Mol. Med. 2011, 3, 528–544.
- Park, J.S.; Lee, J.; Lee, Y.S.; Kim, J.K.; Dong, S.M.; Yoon, D.S. Emerging Role of LOXL2 in the Promotion of Pancreas Cancer Metastasis. Oncotarget 2016, 7, 42539–42552.
- Torres, S.; Garcia-Palmero, I.; Herrera, M.; Bartolomé, R.A.; Peña, C.; Fernandez-Aceñero, M.J.; Padilla, G.; Peláez-García, A.; Lopez-Lucendo, M.; Rodriguez-Merlo, R.; et al. LOXL2 Is Highly Expressed in Cancer-Associated Fibroblasts and Associates to Poor Colon Cancer Survival. Clin. Cancer Res. 2015, 21, 4892–4902.
- Zhang, Y.; Liu, W.; Xu, J. Prognostic Utility and Clinical Significance of Lysyl Oxidase-like 2 Protein Expression in Digestive System Cancers. J. Cell. Physiol. 2019, 234, 20713–20720.
- Lin, H.-Y.; Li, C.-J.; Yang, Y.-L.; Huang, Y.-H.; Hsiau, Y.-T.; Chu, P.-Y. Roles of Lysyl Oxidase Family Members in the Tumor Microenvironment and Progression of Liver Cancer. Int. J. Mol. Sci. 2020, 21, 9751.
- Wen, B.; Xu, L.-Y.; Li, E.-M. LOXL2 in Cancer: Regulation, Downstream Effectors and Novel Roles. Biochim. Biophys. Acta BBA-Rev. Cancer 2020, 1874, 188435.
- Wu, L.; Zhu, Y. The Function and Mechanisms of Action of LOXL2 in Cancer (Review). Int. J. Mol. Med. 2015, 36, 1200–1204.
- Peng, T.; Deng, X.; Tian, F.; Li, Z.; Jiang, P.; Zhao, X.; Chen, G.; Chen, Y.; Zheng, P.; Li, D.; et al. The Interaction of LOXL2 with GATA6 Induces VEGFA Expression and Angiogenesis in Cholangiocarcinoma. Int. J. Oncol. 2019, 55, 657–670.
- Wang, C.; Xu, S.; Tian, Y.; Ju, A.; Hou, Q.; Liu, J.; Fu, Y.; Luo, Y. Lysyl Oxidase-Like Protein 2 Promotes Tumor Lymphangiogenesis and Lymph Node Metastasis in Breast Cancer. Neoplasia 2019, 21, 413–427.
- Shao, B.; Zhao, X.; Liu, T.; Zhang, Y.; Sun, R.; Dong, X.; Liu, F.; Zhao, N.; Zhang, D.; Wu, L.; et al. LOXL2 Promotes Vasculogenic Mimicry and Tumour Aggressiveness in Hepatocellular Carcinoma. J. Cell. Mol. Med. 2019, 23, 1363–1374.
- Barker, H.E.; Bird, D.; Lang, G.; Erler, J.T. Tumor-Secreted LOXL2 Activates Fibroblasts through FAK Signaling. Mol. Cancer Res. 2013, 11, 1425–1436.
- Xing, X.; Wang, Y.; Zhang, X.; Gao, X.; Li, M.; Wu, S.; Zhao, Y.; Chen, J.; Gao, D.; Chen, R.; et al. Matrix Stiffness-mediated Effects on Macrophages Polarization and Their LOXL2 Expression. FEBS J. 2021, 288, 3465–3477.
- Mäki, J.M.; Kivirikko, K.I. Cloning and Characterization of a Fourth Human Lysyl Oxidase Isoenzyme. Biochem. J. 2001, 355, 381–387.
- Molnar, J.; Fong, K.S.K.; He, Q.P.; Hayashi, K.; Kim, Y.; Fong, S.F.T.; Fogelgren, B.; MolnarneSzauter, K.; Mink, M.; Csiszar, K. Structural and Functional Diversity of Lysyl Oxidase and the LOX-like Proteins. Biochim. Biophys. Acta BBA-Proteins Proteom. 2003, 1647, 220–224.
- Kenyon, K.; Modi, W.S.; Contente, S.; Friedman, R.M. A Novel Human CDNA with a Predicted Protein Similar to Lysyl Oxidase Maps to Chromosome 15q24-Q25. J. Biol. Chem. 1993, 268, 18435–18437.
- Kim, Y.; Boyd, C.D.; Csiszar, K. A New Gene with Sequence and Structural Similarity to the Gene Encoding Human Lysyl Oxidase. J. Biol. Chem. 1995, 270, 7176–7182.
- Saito, H.; Papaconstantinou, J.; Sato, H.; Goldstein, S. Regulation of a Novel Gene Encoding a Lysyl Oxidase-Related Protein in Cellular Adhesion and Senescence. J. Biol. Chem. 1997, 272, 8157–8160.
- Huang, Y. Cloning and Characterization of a Human Lysyl Oxidase-like 3 Gene (HLOXL3). Matrix Biol. 2001, 20, 153–157.
- Siegel, R.C.; Pinnell, S.R.; Martin, G.R. Cross-Linking of Collagen and Elastin. Properties of Lysyl Oxidase. Biochemistry 1970, 9, 4486–4492.
- Pinnell, S.R.; Martin, G.R. The Cross-Linking of Collagen and Elastin: Enzymatic Conversion of Lysine in Peptide Linkage to Alpha-Aminoadipic-Delta-Semialdehyde (Allysine) by an Extract from Bone. Proc. Natl. Acad. Sci. USA 1968, 61, 708–716.
- Jourdan-Le Saux, C.; Le Saux, O.; Donlon, T.; Boyd, C.D.; Csiszar, K. The Human Lysyl Oxidase-Related Gene (LOXL2) Maps between Markers D8S280 and D8S278 on Chromosome 8p21.2–P21.3. Genomics 1998, 51, 305–307.
- Csiszar, K. Lysyl Oxidases: A Novel Multifunctional Amine Oxidase Family. In Progress in Nucleic Acid Research and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 70, pp. 1–32. ISBN 978-0-12-540070-1.
- Lucero, H.A.; Kagan, H.M. Lysyl Oxidase: An Oxidative Enzyme and Effector of Cell Function. Cell. Mol. Life Sci. 2006, 63, 2304–2316.
- Xiao, Q.; Ge, G. Lysyl Oxidase, Extracellular Matrix Remodeling and Cancer Metastasis. Cancer Microenviron. 2012, 5, 261–273.
- Boufraqech, M.; Zhang, L.; Nilubol, N.; Sadowski, S.M.; Kotian, S.; Quezado, M.; Kebebew, E. Lysyl Oxidase (LOX) Transcriptionally Regulates SNAI2 Expression and TIMP4 Secretion in Human Cancers. Clin. Cancer Res. 2016, 22, 4491–4504.
- Hornstra, I.K.; Birge, S.; Starcher, B.; Bailey, A.J.; Mecham, R.P.; Shapiro, S.D. Lysyl Oxidase Is Required for Vascular and Diaphragmatic Development in Mice. J. Biol. Chem. 2003, 278, 14387–14393.
- Dongiovanni, P.; Meroni, M.; Baselli, G.A.; Bassani, G.A.; Rametta, R.; Pietrelli, A.; Maggioni, M.; Facciotti, F.; Trunzo, V.; Badiali, S.; et al. Insulin Resistance Promotes Lysyl Oxidase Like 2 Induction and Fibrosis Accumulation in Non-Alcoholic Fatty Liver Disease. Clin. Sci. 2017, 131, 1301–1315.
- Zhao, W.; Yang, A.; Chen, W.; Wang, P.; Liu, T.; Cong, M.; Xu, A.; Yan, X.; Jia, J.; You, H. Inhibition of Lysyl Oxidase-like 1 (LOXL1) Expression Arrests Liver Fibrosis Progression in Cirrhosis by Reducing Elastin Crosslinking. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2018, 1864, 1129–1137.
- Ikenaga, N.; Peng, Z.-W.; Vaid, K.A.; Liu, S.B.; Yoshida, S.; Sverdlov, D.Y.; Mikels-Vigdal, A.; Smith, V.; Schuppan, D.; Popov, Y.V. Selective Targeting of Lysyl Oxidase-like 2 (LOXL2) Suppresses Hepatic Fibrosis Progression and Accelerates Its Reversal. Gut 2017, 66, 1697–1708.
- Guilliams, M.; Bonnardel, J.; Haest, B.; Vanderborght, B.; Wagner, C.; Remmerie, A.; Bujko, A.; Martens, L.; Thoné, T.; Browaeys, R.; et al. Spatial Proteogenomics Reveals Distinct and Evolutionarily Conserved Hepatic Macrophage Niches. Cell 2022, 185, 379–396.e38.
- Human All Liver Cells. Available online: https://www.livercellatlas.org/umap-humanAll.php (accessed on 10 June 2023).
- Wang, T.-H.; Hsia, S.-M.; Shieh, T.-M. Lysyl Oxidase and the Tumor Microenvironment. Int. J. Mol. Sci. 2016, 18, 62.
- Xu, L.; Go, E.P.; Finney, J.; Moon, H.; Lantz, M.; Rebecchi, K.; Desaire, H.; Mure, M. Post-Translational Modifications of Recombinant Human Lysyl Oxidase-like 2 (RhLOXL2) Secreted from Drosophila S2 Cells. J. Biol. Chem. 2013, 288, 5357–5363.
- Luo, W.; Chang, R.; Zhong, J.; Pandey, A.; Semenza, G.L. Histone Demethylase JMJD2C Is a Coactivator for Hypoxia-Inducible Factor 1 That Is Required for Breast Cancer Progression. Proc. Natl. Acad. Sci. USA 2012, 109, E3367–E3376.
- Zhu, Y.; Zhu, M.-X.; Zhang, X.-D.; Xu, X.-E.; Wu, Z.-Y.; Liao, L.-D.; Li, L.-Y.; Xie, Y.-M.; Wu, J.-Y.; Zou, H.-Y.; et al. SMYD3 Stimulates EZR and LOXL2 Transcription to Enhance Proliferation, Migration, and Invasion in Esophageal Squamous Cell Carcinoma. Hum. Pathol. 2016, 52, 153–163.
- Liu, C.; Guo, T.; Sakai, A.; Ren, S.; Fukusumi, T.; Ando, M.; Sadat, S.; Saito, Y.; Califano, J.A. A Novel Splice Variant of LOXL2 Promotes Progression of Human Papillomavirus–Negative Head and Neck Squamous Cell Carcinoma. Cancer 2020, 126, 737–748.
- Lv, G.-Q.; Zou, H.-Y.; Liao, L.-D.; Cao, H.-H.; Zeng, F.-M.; Wu, B.-L.; Xie, J.-J.; Fang, W.-K.; Xu, L.-Y.; Li, E.-M. Identification of a Novel Lysyl Oxidase-like 2 Alternative Splicing Isoform, LOXL2 Δe13, in Esophageal Squamous Cell Carcinoma. Biochem. Cell Biol. 2014, 92, 379–389.
- Qu, Y.; Xiao, H.; Xiao, W.; Xiong, Z.; Hu, W.; Gao, Y.; Ru, Z.; Wang, C.; Bao, L.; Wang, K.; et al. Upregulation of MIAT Regulates LOXL2 Expression by Competitively Binding MiR-29c in Clear Cell Renal Cell Carcinoma. Cell. Physiol. Biochem. 2018, 48, 1075–1087.
- Fukumoto, I.; Kikkawa, N.; Matsushita, R.; Kato, M.; Kurozumi, A.; Nishikawa, R.; Goto, Y.; Koshizuka, K.; Hanazawa, T.; Enokida, H.; et al. Tumor-Suppressive MicroRNAs (MiR-26a/b, MiR-29a/b/c and MiR-218) Concertedly Suppressed Metastasis-Promoting LOXL2 in Head and Neck Squamous Cell Carcinoma. J. Hum. Genet. 2016, 61, 109–118.
- Ye, M.; Zhang, J.; Guo, T.; Pan, X. MiR-504 Inhibits Cell Proliferation and Invasion by Targeting LOXL2 in Non Small Cell Lung Cancer. Biomed. Pharmacother. 2018, 97, 1289–1295.
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in Health and Disease. Nat. Rev. Nephrol. 2019, 15, 346–366.
- Go, E.P.; Moon, H.-J.; Mure, M.; Desaire, H. Recombinant Human Lysyl Oxidase-like 2 Secreted from Human Embryonic Kidney Cells Displays Complex and Acidic Glycans at All Three N-Linked Glycosylation Sites. J. Proteome Res. 2018, 17, 1826–1832.
- Wang, W.; Wang, X.; Yao, F.; Huang, C. Lysyl Oxidase Family Proteins: Prospective Therapeutic Targets in Cancer. Int. J. Mol. Sci. 2022, 23, 12270.
- Ferreira, S.; Saraiva, N.; Rijo, P.; Fernandes, A.S. LOXL2 Inhibitors and Breast Cancer Progression. Antioxidants 2021, 10, 312.
- Moon, H.-J.; Finney, J.; Xu, L.; Moore, D.; Welch, D.R.; Mure, M. MCF-7 Cells Expressing Nuclear Associated Lysyl Oxidase-like 2 (LOXL2) Exhibit an Epithelial-to-Mesenchymal Transition (EMT) Phenotype and Are Highly Invasive in Vitro. J. Biol. Chem. 2013, 288, 30000–30008.
- Wang, M.; Zhao, X.; Zhu, D.; Liu, T.; Liang, X.; Liu, F.; Zhang, Y.; Dong, X.; Sun, B. HIF-1α Promoted Vasculogenic Mimicry Formation in Hepatocellular Carcinoma through LOXL2 up-Regulation in Hypoxic Tumor Microenvironment. J. Exp. Clin. Cancer Res. 2017, 36, 60.
- Zhao, N.; Chen, C.; Guo, Y.; Liu, T.; Che, N.; Zhang, D.; Liang, X.; Zhang, Y.; Zhao, X. LOXL2 Serves as a Prognostic Biomarker for Hepatocellular Carcinoma by Mediating Immune Infiltration and Vasculogenic Mimicry. Dig. Liver Dis. 2023, 55, 661–672.
- Yang, Y.-L.; Tsai, M.-C.; Chang, Y.-H.; Wang, C.-C.; Chu, P.-Y.; Lin, H.-Y.; Huang, Y.-H. MIR29A Impedes Metastatic Behaviors in Hepatocellular Carcinoma via Targeting LOX, LOXL2, and VEGFA. Int. J. Mol. Sci. 2021, 22, 6001.
- Umezaki, N.; Nakagawa, S.; Yamashita, Y.; Kitano, Y.; Arima, K.; Miyata, T.; Hiyoshi, Y.; Okabe, H.; Nitta, H.; Hayashi, H.; et al. Lysyl Oxidase Induces Epithelial-mesenchymal Transition and Predicts Intrahepatic Metastasis of Hepatocellular Carcinoma. Cancer Sci. 2019, 110, 2033–2043.
- Choi, J.; Chung, T.; Rhee, H.; Kim, Y.-J.; Jeon, Y.; Yoo, J.E.; Noh, S.; Han, D.H.; Park, Y.N. Increased Expression of the Matrix-Modifying Enzyme Lysyl Oxidase-Like 2 in Aggressive Hepatocellular Carcinoma with Poor Prognosis. Gut Liver 2019, 13, 83–92.
- Wu, L.; Zhang, Y.; Zhu, Y.; Cong, Q.; Xiang, Y.; Fu, L. The Effect of LOXL2 in Hepatocellular Carcinoma. Mol. Med. Rep. 2016, 14, 1923–1932.
- Huang, Y.-H.; Lian, W.-S.; Wang, F.-S.; Wang, P.-W.; Lin, H.-Y.; Tsai, M.-C.; Yang, Y.-L. MiR-29a Curbs Hepatocellular Carcinoma Incidence via Targeting of HIF-1α and ANGPT2. Int. J. Mol. Sci. 2022, 23, 1636.
- Sequera, C.; Grattarola, M.; Holczbauer, A.; Dono, R.; Pizzimenti, S.; Barrera, G.; Wangensteen, K.J.; Maina, F. MYC and MET Cooperatively Drive Hepatocellular Carcinoma with Distinct Molecular Traits and Vulnerabilities. Cell Death Dis. 2022, 13, 994.
- Dey, S.; Kwon, J.J.; Liu, S.; Hodge, G.A.; Taleb, S.; Zimmers, T.A.; Wan, J.; Kota, J. MiR-29a Is Repressed by MYC in Pancreatic Cancer and Its Restoration Drives Tumor-Suppressive Effects via Downregulation of LOXL2. Mol. Cancer Res. MCR 2020, 18, 311–323.
- Wang, X.; Wu, S.; Yang, Y.; Zhao, J. LncRNA CARMN Affects Hepatocellular Carcinoma Prognosis by Regulating the MiR-192-5p/LOXL2 Axis. Oxidative Med. Cell. Longev. 2022, 2022, 9277360.
- Zhu, Y.; Zheng, B.; Wang, H.; Chen, L. New Knowledge of the Mechanisms of Sorafenib Resistance in Liver Cancer. Acta Pharmacol. Sin. 2017, 38, 614–622.
- Hajdú, I.; Kardos, J.; Major, B.; Fabó, G.; Lőrincz, Z.; Cseh, S.; Dormán, G. Inhibition of the LOX Enzyme Family Members with Old and New Ligands. Selectivity Analysis Revisited. Bioorg. Med. Chem. Lett. 2018, 28, 3113–3118.
- Rodríguez, C.; Rodríguez-Sinovas, A.; Martínez-González, J. Lysyl Oxidase as a Potential Therapeutic Target. Drug News Perspect. 2008, 21, 218–224.
- Barry-Hamilton, V.; Spangler, R.; Marshall, D.; McCauley, S.; Rodriguez, H.M.; Oyasu, M.; Mikels, A.; Vaysberg, M.; Ghermazien, H.; Wai, C.; et al. Allosteric Inhibition of Lysyl Oxidase–like-2 Impedes the Development of a Pathologic Microenvironment. Nat. Med. 2010, 16, 1009–1017.
- Rodriguez, H.M.; Vaysberg, M.; Mikels, A.; McCauley, S.; Velayo, A.C.; Garcia, C.; Smith, V. Modulation of Lysyl Oxidase-like 2 Enzymatic Activity by an Allosteric Antibody Inhibitor. J. Biol. Chem. 2010, 285, 20964–20974.
- Verstovsek, S.; Savona, M.R.; Mesa, R.A.; Dong, H.; Maltzman, J.D.; Sharma, S.; Silverman, J.; Oh, S.T.; Gotlib, J. A Phase 2 Study of Simtuzumab in Patients with Primary, Post-Polycythaemia Vera or Post-Essential Thrombocythaemia Myelofibrosis. Br. J. Haematol. 2017, 176, 939–949.
- Harrison, S.A.; Abdelmalek, M.F.; Caldwell, S.; Shiffman, M.L.; Diehl, A.M.; Ghalib, R.; Lawitz, E.J.; Rockey, D.C.; Schall, R.A.; Jia, C.; et al. Simtuzumab Is Ineffective for Patients with Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology 2018, 155, 1140–1153.
- Hecht, J.R.; Benson, A.B.; Vyushkov, D.; Yang, Y.; Bendell, J.; Verma, U. A Phase II, Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab in Combination with FOLFIRI for the Second-Line Treatment of Metastatic KRAS Mutant Colorectal Adenocarcinoma. Oncologist 2017, 22, 243-e23.
- Liburkin-Dan, T.; Toledano, S.; Neufeld, G. Lysyl Oxidase Family Enzymes and Their Role in Tumor Progression. Int. J. Mol. Sci. 2022, 23, 6249.
- Pharos. Available online: https://pharos.nih.gov/targets/LOXL2 (accessed on 10 June 2023).
- Sampath Narayanan, A.; Siegel, R.C.; Martin, G.R. On the Inhibition of Lysyl Oxidase by β-Aminopropionitrile. Biochem. Biophys. Res. Commun. 1972, 46, 745–751.
- Shi, L.; Zhang, N.; Liu, H.; Zhao, L.; Liu, J.; Wan, J.; Wu, W.; Lei, H.; Liu, R.; Han, M. Lysyl Oxidase Inhibition via β-Aminoproprionitrile Hampers Human Umbilical Vein Endothelial Cell Angiogenesis and Migration Invitro. Mol. Med. Rep. 2018, 17, 5029–5036.
- Kirschmann, D.A.; Seftor, E.A.; Fong, S.F.T.; Nieva, D.R.C.; Sullivan, C.M.; Edwards, E.M.; Sommer, P.; Csiszar, K.; Hendrix, M.J.C. A Molecular Role for Lysyl Oxidase in Breast Cancer Invasion. Cancer Res. 2002, 62, 4478–4483.
- Abourbih, D.A.; Di Cesare, S.; Orellana, M.E.; Antecka, E.; Martins, C.; Petruccelli, L.A.; Burnier, M.N. Lysyl Oxidase Expression and Inhibition in Uveal Melanoma. Melanoma Res. 2010, 20, 97–106.
- Bondareva, A.; Downey, C.M.; Ayres, F.; Liu, W.; Boyd, S.K.; Hallgrimsson, B.; Jirik, F.R. The Lysyl Oxidase Inhibitor, β-Aminopropionitrile, Diminishes the Metastatic Colonization Potential of Circulating Breast Cancer Cells. PLoS ONE 2009, 4, e5620.
- Yang, X.; Li, S.; Li, W.; Chen, J.; Xiao, X.; Wang, Y.; Yan, G.; Chen, L. Inactivation of Lysyl Oxidase by β-Aminopropionitrile Inhibits Hypoxia-Induced Invasion and Migration of Cervical Cancer Cells. Oncol. Rep. 2013, 29, 541–548.
- Li, Q.; Zhu, C.-C.; Ni, B.; Zhang, Z.-Z.; Jiang, S.-H.; Hu, L.-P.; Wang, X.; Zhang, X.-X.; Huang, P.-Q.; Yang, Q.; et al. Lysyl Oxidase Promotes Liver Metastasis of Gastric Cancer via Facilitating the Reciprocal Interactions between Tumor Cells and Cancer Associated Fibroblasts. eBioMedicine 2019, 49, 157–171.
- Ninomiya, G.; Yamada, S.; Hayashi, M.; Takeda, S.; Suenaga, M.; Takami, H.; Kanda, M.; Iwata, N.; Niwa, Y.; Tanaka, C.; et al. Significance of Lysyl Oxidase-like2 Gene Expression on the Epithelial-mesenchymal Status of Hepatocellular Carcinoma. Oncol. Rep. 2018, 39, 2664–2672.
- Liu, S.B.; Ikenaga, N.; Peng, Z.; Sverdlov, D.Y.; Greenstein, A.; Smith, V.; Schuppan, D.; Popov, Y. Lysyl Oxidase Activity Contributes to Collagen Stabilization during Liver Fibrosis Progression and Limits Spontaneous Fibrosis Reversal in Mice. FASEB J. 2016, 30, 1599–1609.
- Findlay, A.; Turner, C.; Schilter, H.; Deodhar, M.; Zhou, W.; Perryman, L.; Foot, J.; Zahoor, A.; Yao, Y.; Hamilton, R.; et al. An Activity-Based Bioprobe Differentiates a Novel Small Molecule Inhibitor from a LOXL2 Antibody and Provides Renewed Promise for Anti-Fibrotic Therapeutic Strategies. Clin. Transl. Med. 2021, 11, e572.
- Schilter, H.; Findlay, A.D.; Perryman, L.; Yow, T.T.; Moses, J.; Zahoor, A.; Turner, C.I.; Deodhar, M.; Foot, J.S.; Zhou, W.; et al. The Lysyl Oxidase like 2/3 Enzymatic Inhibitor, PXS-5153A, Reduces Crosslinks and Ameliorates Fibrosis. J. Cell. Mol. Med. 2019, 23, 1759–1770.
- Tse, A.P.-W.; Sze, K.M.-F.; Shea, Q.T.-K.; Chiu, E.Y.-T.; Tsang, F.H.-C.; Chiu, D.K.-C.; Zhang, M.S.; Lee, D.; Xu, I.M.-J.; Chan, C.Y.-K.; et al. Hepatitis Transactivator Protein X Promotes Extracellular Matrix Modification through HIF/LOX Pathway in Liver Cancer. Oncogenesis 2018, 7, 44.
- Fan, Z.; Zheng, W.; Li, H.; Wu, W.; Liu, X.; Sun, Z.; Hu, H.; Du, L.; Jia, Q.; Liu, Q. LOXL2 Upregulates Hypoxia-inducible Factor-1α Signaling through Snail-FBP1 Axis in Hepatocellular Carcinoma Cells. Oncol. Rep. 2020, 43, 1641–1649.
- Pang, R.W.C.; Joh, J.W.; Johnson, P.J.; Monden, M.; Pawlik, T.M.; Poon, R.T.P. Biology of Hepatocellular Carcinoma. Ann. Surg. Oncol. 2008, 15, 962–971.
- Fang, J.; Zhou, H.; Zhang, C.; Shang, L.; Zhang, L.; Xu, J.; Zheng, L.; Yuan, Y.; Guo, R.; Jia, W.; et al. A Novel Vascular Pattern Promotes Metastasis of Hepatocellular Carcinoma in an Epithelial–Mesenchymal Transition–Independent Manner. Hepatology 2015, 62, 452–465.
- Kerbel, R.S. Tumor Angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049.
- Tacke, F.; Luedde, T.; Trautwein, C. Inflammatory Pathways in Liver Homeostasis and Liver Injury. Clin. Rev. Allergy Immunol. 2009, 36, 4–12.
- Sun, B.; Zhang, D.; Zhao, N.; Zhao, X. Epithelial-to-Endothelial Transition and Cancer Stem Cells: Two Cornerstones of Vasculogenic Mimicry in Malignant Tumors. Oncotarget 2017, 8, 30502–30510.
- Sun, T.; Sun, B.; Zhao, X.; Zhao, N.; Dong, X.; Che, N.; Yao, Z.; Ma, Y.; Gu, Q.; Zong, W.; et al. Promotion of Tumor Cell Metastasis and Vasculogenic Mimicry by Way of Transcription Coactivation by Bcl-2 and Twist1: A Study of Hepatocellular Carcinoma. Hepatology 2011, 54, 1690–1706.
- Hutchinson, J.H.; Rowbottom, M.W.; Lonergan, D.; Darlington, J.; Prodanovich, P.; King, C.D.; Evans, J.F.; Bain, G. Small Molecule Lysyl Oxidase-like 2 (LOXL2) Inhibitors: The Identification of an Inhibitor Selective for LOXL2 over LOX. ACS Med. Chem. Lett. 2017, 8, 423–427.
More
