Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mark F. Wiser and Version 2 by Fanny Huang.
Plasmodium falciparum can cause a severe disease with high mortality. A major factor contributing to the increased virulence of P. falciparum, as compared to other human malarial parasites, is the sequestration of infected erythrocytes in the capillary beds of organs and tissues. This sequestration is due to the cytoadherence of infected erythrocytes to endothelial cells.
- malaria
- Plasmodium falciparum
- cytoadherence
- PfEMP1
1. Introduction
Clinical manifestations associated with malaria can range from asymptomatic carriage to a febrile illness to severe organ dysfunction resulting in death [1][148]. Febrile illness without organ dysfunction is often called uncomplicated malaria and exhibits non-specific symptoms such as fever, nausea, and headache. Complicated malaria refers to severe malaria associated with organ dysfunction. Approximately one percent of falciparum malaria cases develop into severe disease [1][2][148,149]. Case fatality rates of severe falciparum malaria range from 5% to 50%, depending on the extent of organ involvement and availability of therapy. Multiple organs can be affected during complicated malaria and the manifestations are determined by the affected organ(s) (Table 1). Patients can exhibit several of these manifestations either simultaneously or sequentially. In addition, children and adults often exhibit different severe manifestations of malaria [3][150]. The prognosis of individuals exhibiting any of the indicators of complicated malaria is poor, and without treatment the risk of death increases.
Table 1. Manifestations of severe malaria and indicators of poor prognosis a.
| Manifestation | Features |
|---|---|
| Severe anemia | Primarily in young children and defined as hematocrit < 15% or hemoglobin < 50 g/L in the presence of parasitemia. |
| Cerebral malaria | An unrousable coma in the presence of parasitemia and not attributable to another cause. |
| Respiratory distress | Defined by labored breathing and pulmonary edema that can progress to an acute respiratory distress syndrome requiring mechanical ventilation. |
| Impaired consciousness | An impaired consciousness that is less pronounced than the unrousable coma associated with cerebral malaria. |
| Prostration or weakness | Patients are unable to sit or walk, which is not attributable to neurological or other explanations. |
| Convulsions | Three or more repeated generalized convulsions observed within 24 h. |
| Acidosis | An important cause of death due to the accumulation of organic acids, including lactic acid, and compounded by ketoacidosis and acute kidney injury. |
| Hypoglycemia | Results from increased glucose consumption in the tissues and impaired hepatic glucogenesis. Often concomitant with lactic acidosis. |
| Jaundice | Results from a combination of hemolysis and hepatocyte damage and defined by elevated serum bilirubin in the presence of parasitemia. |
| Renal impairment | Defined by low urine output and high serum creatinine or urea levels despite adequate hydration. |
| Abnormal bleeding | Recurrent or prolonged bleeding from nose, gums, or venipuncture sites. |
| Coagulopathy | Activation of blood coagulation including disseminated intravascular coagulation or depletion of platelets. |
| Circulatory collapse (shock) | Defined as systolic blood pressure < 70 mm Hg in malaria patients and accompanied by cold clammy skin. |
| Hyperpyrexia | Core body temperature > 40 °C and may be associated with rapid heart rate and occasionally delirium. |
| Hyperparasitemia | Poor prognosis associated with >10% parasitized erythrocytes. |
Several factors are involved in the development of severe malaria [4][151]. These include host genetics, patient age, and prior exposures to P. falciparum, which all can affect parasitemia. It is widely accepted that hyperparasitemia is associated with a poor prognosis and the total parasite biomass correlates with severe disease [5][152]. However, some children can tolerate extremely high parasitemia without exhibiting severe disease [6][153]. Generally, exposure-dependent acquisition of immunity decreases the development of severe malaria, but there are exceptions [7][154].
2. Cerebral Malaria, Respiratory Distress, and Severe Anemia Are Common Manifestations of Complicated Malaria
The three most common manifestations of severe falciparum malaria are cerebral malaria, respiratory distress, and severe anemia in children [8][155]. Cerebral malaria is characterized by an impaired consciousness and other neurological symptoms [9][156]. Patients typically present with fever and severe headache for several days followed by drowsiness, confusion, repeated seizures, convulsions, and ultimately an unrousable coma. Swelling of the brain and hemorrhage into the brain tissue are also present. Common co-morbidities include respiratory distress, hypoglycemia, and acidosis. Metabolic acidosis, as manifested by respiratory distress, has emerged as a central feature of severe falciparum malaria and is a better predictor of death than cerebral malaria or severe anemia [1][10][148,157]. The first signs of lung injury are rapid and difficult breathing accompanied by pulmonary edema. This pulmonary edema can progress to acute respiratory distress syndrome and even respiratory failure.
Severe anemia is due to both the increased destruction of erythrocytes and decreased production of new erythrocytes [11][158]. In addition to the erythrocytes that are destroyed by the parasite during blood-stage schizogony as part of the parasite’s life cycle, non-infected erythrocytes are destroyed at higher rates due to complement-mediated lysis and phagocytosis mediated by immune complex deposition or complement activation. Furthermore, there is a decreased production of erythrocytes during infection, resulting in less replacement of the lost erythrocytes. Acute kidney injury is also associated with high mortality during severe falciparum malaria [12][159].
3. Sequestration of the Infected Erythrocytes in Microvasculature Is a Major Factor in Disease Pathogenesis
The cytoadherence of parasitized erythrocytes to the capillary endothelium of vital organs, such as brain, bone marrow, lungs, kidneys, or intestines, certainly plays a major role in complicated falciparum malaria. The sequestration of parasitized erythrocytes results in microvascular obstruction, localized inflammation, increased vascular permeability, coagulation disorders, and ultimately organ dysfunction (Figure 1).
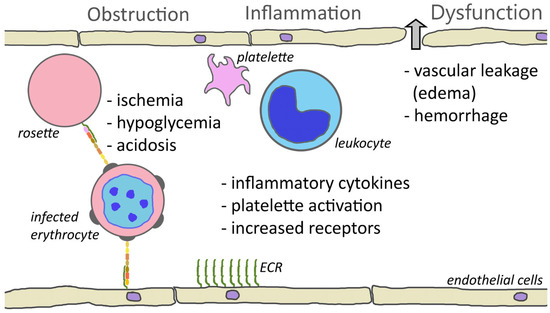
Figure 1. Cytoadherence-mediated pathogenesis. One consequence of cytoadherence is the obstruction of capillaries. Rosetting and platelet-mediated clumping may augment this obstruction. Obstruction of blood vessels results in reduced blood flow and ischemia. This decreased perfusion combined with parasite metabolism may contribute to hypoglycemia and acidosis. Cytoadherence also inflames the endothelium and attracts leukocytes and activates platelets. This inflammation and increased levels of inflammatory cytokines cause a loosening of the tight junctions between endothelial cells and results in leakage of fluid into the tissues or hemorrhaging (block arrow). Inflammation also increases the expression of endothelial cell receptors (ECRs) and may augment cytoadherence.
The adherence of infected erythrocytes in the capillaries obstructs blood flow and deprives tissues of nutrients and oxygen. This ischemia may be enhanced by rosettes [13][160] or platelet-induced clumps [14][145]. In addition, the metabolism of the parasite further complicates ischemia. The parasite has a high demand for glucose and can lead to localized hypoglycemia. Furthermore, the parasite exhibits an anaerobic metabolism and converts glucose into lactic acid via glycolysis, thus promoting acidosis. Infection also increases the anaerobic metabolism of the host tissues, which further increases the hypoglycemia and acidosis [15][161]. Metabolic acidosis is a key feature of severe falciparum malaria and is a major contributing factor to respiratory distress.
In addition to the obstruction of capillaries and ischemia, cytoadherence causes increased production of inflammatory cytokines—especially TNF-α—and endothelium inflammation is a major feature of severe malaria [16][17][20,162]. Activated endothelial cells also express higher levels of potential PfEMP1 receptors such as ICAM1, P-selectin, and E-selectin. This increased expression of cytoadherence receptors can enhance sequestration. The inflammatory cytokines also lead to endothelium dysfunction and increased vascular permeability in the affected tissues and organs. For example, pulmonary edema is a major feature of respiratory distress. Likewise, edema and the swelling of the brain due to the disruption of the blood–brain barrier is a common feature of cerebral malaria [18][163]. Inflammation also results in the activation of platelets and platelet accumulation is significantly higher in cerebral malaria patients than in uncomplicated malaria [19][164].
4. Expression of Specific PfEMP1 Alleles Is Associated with Severe Disease and Organ Specific Clinical Manifestations
A logical inference from the wide range of endothelial cell receptors recognized by PfEMP1 is that different PfEMP1 alleles may be responsible for the organ specificity of the various complications associated with severe falciparum malaria [4][20][93,151]. Indeed, it has been demonstrated that parasites isolated from different organs express different variants of PfEMP1 [21][165]. For example, there are correlations between the expression of specific PfEMP1 alleles and the development of cerebral malaria or placental malaria. However, specific PfEMP1 alleles that target infected erythrocytes to the lungs, bone marrow, kidneys, or other organs associated with complicated malaria have not yet been identified. Nonetheless, it is likely that specific PfEMP1 alleles may have a tropism for a particular tissue or organ, and this organ specific tropism could account for the wide range of clinical manifestations that are associated with complicated malaria (see Table 1).
Several studies have shown that the expression of specific PfEMP1 alleles is correlated with the risk of developing severe disease [9][11][22][19,156,158]. For example, PfEMP1 alleles with EPCR binding capabilities tend to be associated with severe disease and alleles with CD36 binding capabilities tend to be associated with milder disease [23][24][25][122,166,167]. In particular, the expression of PfEMP1 of domain cassettes 8 or 13, which bind to both EPCR and ICAM1, are associated with cerebral malaria [26][27][168,169]. Consistent with a possible role in cerebral malaria, members of DC8 and DC13 exhibit preferential adherence to brain endothelial cells [28][29][170,171]. However, questions have been raised about the role of ICAM1-binding PfEMP1 alleles in cerebral malaria [30][172]. Heparan sulfate-binding alleles [31][173] and gC1qR-binding alleles [32][33][130,174] have also been implicated in severe disease.
A good example of a specific PfEMP1 allele that is correlated with a specific disease manifestation is the association of var2csa expression with placental malaria. The expression of var2csa is significantly upregulated following the selection for adhesion to CSA in vitro [34][175]. Furthermore, var2csa is the predominant var gene that is expressed in parasites isolated from the placenta [35][36][37][133,176,177]. Placental sequestration impacts both mother and fetus, contributing to premature delivery, intrauterine growth retardation, stillbirth, maternal anemia, and increased neonatal and maternal mortality [38][178]. In addition, it is known that the severity of placental malaria decreases with subsequent pregnancies. This decrease in disease severity in multigravida women is likely due to antibodies directed against the var2csa variant of PfEMP1, which would develop as a result of acquiring malaria during a previous pregnancy [39][179]. Thus, multigravida women have better immunity directed at var2csa than primigravida women. In this regard, clinical trials with var2csa as a potential vaccine are underway [40][180].
