You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Waqas Latif.
White Rot Fungi (WRF) are a class of microorganisms widely understood for their ability to break down an extensive range of pollutants generally found in industrial wastewater. WRF usually carry out the degradation process with ligninolytic enzyme by targeting complex industrial pollutants, such as aromatic hydrocarbons, dyes, pharmaceuticals, and products of personal care. The unique enzymatic system of WRF converts the complex and harmful industrial pollutants into harmless end and byproducts, thus minimizing the impact on the environment and ecosystem.
- White Rot Fungi
- wastewater treatment
- biodegradation
- bioremediation
- lignin-degrading enzymes
- industrial wastewater
1. Introduction
Although the rapid development of the industrial sector has contributed substantially to the world economy and human welfare, it has consequences in terms of waste generation, resource consumption, and energy use, which required the development of ad hoc strategies and countermeasures to contain their impacts [1,2,3,4][1][2][3][4]. Among the most impactful consequences related to human activities is the generation of huge volumes of wastewater, which is rich in a wide variety of pollutants and is liable for serious environmental pollution and public health risks [5,6][5][6]. The exact nature of pollutants being produced and released into the environment depends on the type of industry. The pollutants may be organic compounds, heavy metals, textile dyes, and any other persistent chemicals of environmental concern [7]. When these diverse pollutants are discharged into the environment, like in natural water bodies, they cause water pollution, which ultimately poses serious risks to aquatic life and public health [8,9,10][8][9][10].
The currently in-use traditional wastewater treatment methods, such as activated sludge process, coagulation, flocculation, and adsorption, have been used commonly to address issues associated with industrial wastewater pollution [11,12,13][11][12][13]. Although these conventional methods have been proven over time and again to be very effective in treating commonly occurring environmental pollutants, these methods are not effective in degrading industrial pollutants of a complex and recalcitrant nature, such as aromatic dyes and heavy metals. Apart from this bottleneck, these conventional methods produce a wide range of secondary pollutants during the process of degradation that are harmful to the environment [9].
The limitations of traditional wastewater treatment systems, such as the inability to degrade pollutants of a complex nature and the production of environmentally toxic byproducts, have led to the exploration of novel and innovative treatment methods. Most of the newly developed techniques employ biological methods, involving microorganisms because of their natural ability to biouptake and degrade pollutants of a complex nature [10,14,15,16,17,18][10][14][15][16][17][18]. Among a wide variety of microorganisms, White Rot Fungi (WRF) have recently gained substantial attention from scientists due to their exceptional ability to produce different extracellular lignin-degrading enzymes, such as lignin peroxidase, manganese peroxidase, and laccase. These extracellular enzymes enable WRF to degrade and mineralize industrial/environmental pollutants of a complex nature into less toxic or non-toxic compounds [19]. The extracellular enzyme-producing capability of WRF makes them an appealing candidate in the field of bioremediation to degrade complex/resistant pollutants emanating from different industries, such as the textile, paper and pulp, pharmaceutical, and/or agrochemical industries, etc. [20].
In the recent past, researchers have been focusing specifically on WRF’s biodegradation potential and its performance optimization, as well as WRF’s effective integration in already existing wastewater treatment facilities [21]. Mycoremediation involving White Rot Fungi (WRF) is a growing area of interest for researchers to address challenges associated with industrial wastewater treatment specifically involving complex organic pollutants and heavy metals. WRF fungi can offer effective wastewater treatment solutions at an industrial scale.
2. Merits of WRF-Based Alternative Treatment Techniques
Considering the limitations associated with conventional wastewater treatment methods, such as the inability of these techniques to degrade pollutants of a complex nature, high operating cost, and generation of secondary pollutants, there is an increasing demand for alternative wastewater treatment methods that can address all these limitations. This has led to the increasing interest of researchers in bioremediation, especially mycoremediation, such as the employment of White Rot Fungi, which provide a promising solution to all these issues.2.1. Broad-Spectrum Biodegradation Capabilities
White Rot Fungi (WRF) are known for their distinctive capability to produce extracellular lignin-degrading enzymes, for example, manganese peroxidase, lignin peroxidase, and laccase. These extracellular enzymes help WRF oxidize and mineralize/degrade a vast variety of industrial pollutants, such as recalcitrant, resistant, and toxic compounds, which are not easily degradable by traditional wastewater treatment methods. Additionally, WRF can absorb and adsorb various heavy metals from industrial wastewater. These multidimensional and broad-spectrum capabilities of WRF to degrade and adsorb/absorb various industrial pollutants make them an ideal option for treatment [35][22].2.2. Environmentally Sustainable Technology
Industrial pollutants degradation by White Rot Fungi (WRF)-based system is considered as an environmentally safe and sustainable approach, as WRFs rely on the natural process of degradation and adsorption/absorption, rather than intensive use of chemicals (flocculants and coagulants) and energy, which further adds to environmental problems in the form of secondary pollutants and greenhouse gases emissions [36][23].2.3. Adaptability to Different Bioremediation Treatment Technologies
White Rot Fungi (WRF)-based wastewater treatment is very flexible, and it can be used in combination with other state-of-the-art bioremediation techniques, such as solid-state fermentation, submerged fermentation, immobilized fungal systems, and enzymatic treatment using isolated WRF enzymes. This flexibility grants the development of customized treatment systems to address the specialized needs of different industrial sectors and wastewater compositions [37,38][24][25].2.4. Potential for Combined Conventional Treatment Approaches
WRF-based wastewater treatment systems can be combined with conventional treatment systems, such as the activated sludge process, Advanced Oxidation Process (AOP), or adsorption, to enhance the already present/designed wastewater treatment system. Combining this comparatively new and innovative technology with conventional methods will help overcome each technique’s limitations, resulting in a more thorough and effective treatment of industrial and otherwise resistant chemical pollutants [39,40][26][27]. Although WRF-based wastewater treatment systems have many advantages over conventional treatment methods, there are certain challenges that need to be addressed, such as enhancement of the technology for commercial and industrial applications [41][28], operational parameters optimization according to the need and type of industry, and assurance of cost-effectiveness. However, rigorous, and widely expanding research is currently being carried out on the degradation capabilities of White Rot Fungi (WRF), which will pave the way for this technology in industrial and commercial wastewater treatment in the near future [42][29].3. White Rot Fungi: An Overview
White Rot Fungi (WRF) belong to the basidiomycetes group. These species are important in the degradation and natural decomposition of lignocellulosic material, like decayed wood and plant residues [43][30]. White Rot Fungi have the unique ability to naturally decompose the lignin, which is a complex and recalcitrant biopolymer. This unique ability of WRF has attracted the attention of researchers looking for alternatives to conventional wastewater treatment plants that are incapable of treating complex chemical pollutants [44][31].3.1. Taxonomy and General Characteristics
Agaricomycetes is a class of Fungi within the Phylum Basidomycota, to which White Rot Fungi (WRF) belongs [45][32]. The most commonly and vastly studied genera of WRF are Phanerochaete, Trametes, Pleurotus, and Ganoderma. The Fungi from these genera are typically known and characterized by their capability to naturally decompose lignocellulosic material, including lignin, hemicellulose, and cellulose [43][30]. White Rot Fungi are filamentous fungi, typically characterized by thread-like structures known as hyphae. Hyphae, when combined, form a structure known as Mycelium. WRF usually exhibit sexual reproduction by producing basidiospores, produced by specialized fungal structures known as basidia [46][33]. As far as the habitats of WRF are concerned, these can be commonly found in environments like decaying wood, compost piles, and forest soils. In such environments, WRFs play an important role in nutrient cycling and carbon sequestration [47][34].3.2. Lignin-Degrading Enzyme System
White Rot Fungi (WRF) are capable of producing extracellular lignin-degrading enzymes, like lignin peroxidase (LiP), manganese peroxidase (MnP), and laccase. The excellent natural decomposition (of recalcitrant industrial chemicals) properties of WRF are mainly due to these extracellular lignin-degrading enzymes. These enzymes speed up the process of lignin oxidation and depolymerisation, which further assist WRF to approach the hemicellulose and cellulose components of lignocellulosic materials for the sake of energy and nutrient incorporation [48,49][35][36]. Lignin Peroxidase (LiP): LiP, a heme-containing enzyme, acts as a catalyst for the oxidation of various aromatic and non-aromatic compounds. LiP has the capability to directly attack the lignin by breaking the C-C and C-O bonds present within the complex structure of lignin. High redox potential is a usual characteristic of the LiP enzyme. This characteristic permits LiP to naturally decompose and oxidize several recalcitrant pollutants, like PAHs, textile dyes, and many phenolic compounds [50][37]. Manganese Peroxidase (MnP): Another extracellular enzyme WRF produces is MnP. MnP is also a heme-containing enzyme, which assists in the oxidation of Mn(II) to Mn(III). In return, Mn(III) can degrade a variety of phenolic and non-phenolic compounds. Due to Mn(III) production, MnP can indirectly attack and degrade lignin through oxidation [51][38]. Laccase: Another extracellular multicopper oxidase enzyme produced by WRF is laccase. Laccase helps in speeding up the oxidation of phenolic and non-phenolic compounds, with the associated reduction of molecular oxygen to water. Although the redox potential of laccase is lower than the other two enzymes, i.e., MnP and LiP, the enzyme has the capability to degrade and oxidize a range of pollutants, such as phenols, textile dyes, and endocrine-disrupting compounds [51][38].3.3. Non-Lignin-Degrading Enzyme System
Apart from the lignin-degrading enzymes in WRF, other non-lignin-degrading enzymes, such as cellobiose dehydrogenases, versatile peroxidases, and hydrolases, have been found to contribute to the transformation of diverse polluting substances. Cellobiose dehydrogenases play a role in the breakdown of cellulose and lignocellulosic biomass by generating reactive oxygen species. CDHs are capable of oxidizing cellobiose and other cello-oligosaccharides, freeing electrons that can be transported to various electron acceptors, including pollutants, accelerating their degradation [52][39]. Moreover, hydrolases are responsible for the hydrolysis of numerous pollutant compounds, such as Esters, Amides, Glycosides, Nitriles, Phosphates, and Sulphates [53][40]. Likewise, peroxidases, including versatile peroxidases (VPs) and manganese peroxidases (MnPs), have been identified to influence the degradation of diverse pollutants. VPs are able to oxidize a wide range of aromatic compounds, while MnPs demonstrate degrading ability towards lignin and recalcitrant pollutants [54][41]. VP shows a visible substrate specificity and can oxidize both high-redox-potential compounds, such as dyes, and low-redox-potential compounds, such as phenolic compounds and lignin [55][42].3.4. Laccase Isozymes System
During laccase production, White Rot Fungi (WRF) have the notable capability to induce the production of laccase isozymes in the presence of xenobiotic substances/pollutants. These isozymes show similar enzymatic activities on mandatory laccases, but are specifically produced in response to the presence of hazardous substances [56][43]. When WRF encounter hazardous pollutants, like xenobiotics, they trigger a signaling cascade that leads to the activation of specific genes responsible for the synthesis of laccase isozymes [57][44]. These isozymes support the activity of laccases by increasing the range of substrates that can be treated and enhancing the overall degradation efficiency. The isozymes are involved in the degradation of many recalcitrant pollutants, such as polycyclic aromatic hydrocarbons (PAHs), pharmaceuticals, and other xenobiotics [58][45].3.5. Mechanism of Pollution Degradation by WRF
As already discussed, White Rot Fungi are gaining attention to treat industrial wastewater containing various complex pollutants due to their ability to produce extracellular lignin-degrading enzymes [51][38]. Figure 1 shows WRF’s generic pollutants degradation mechanism.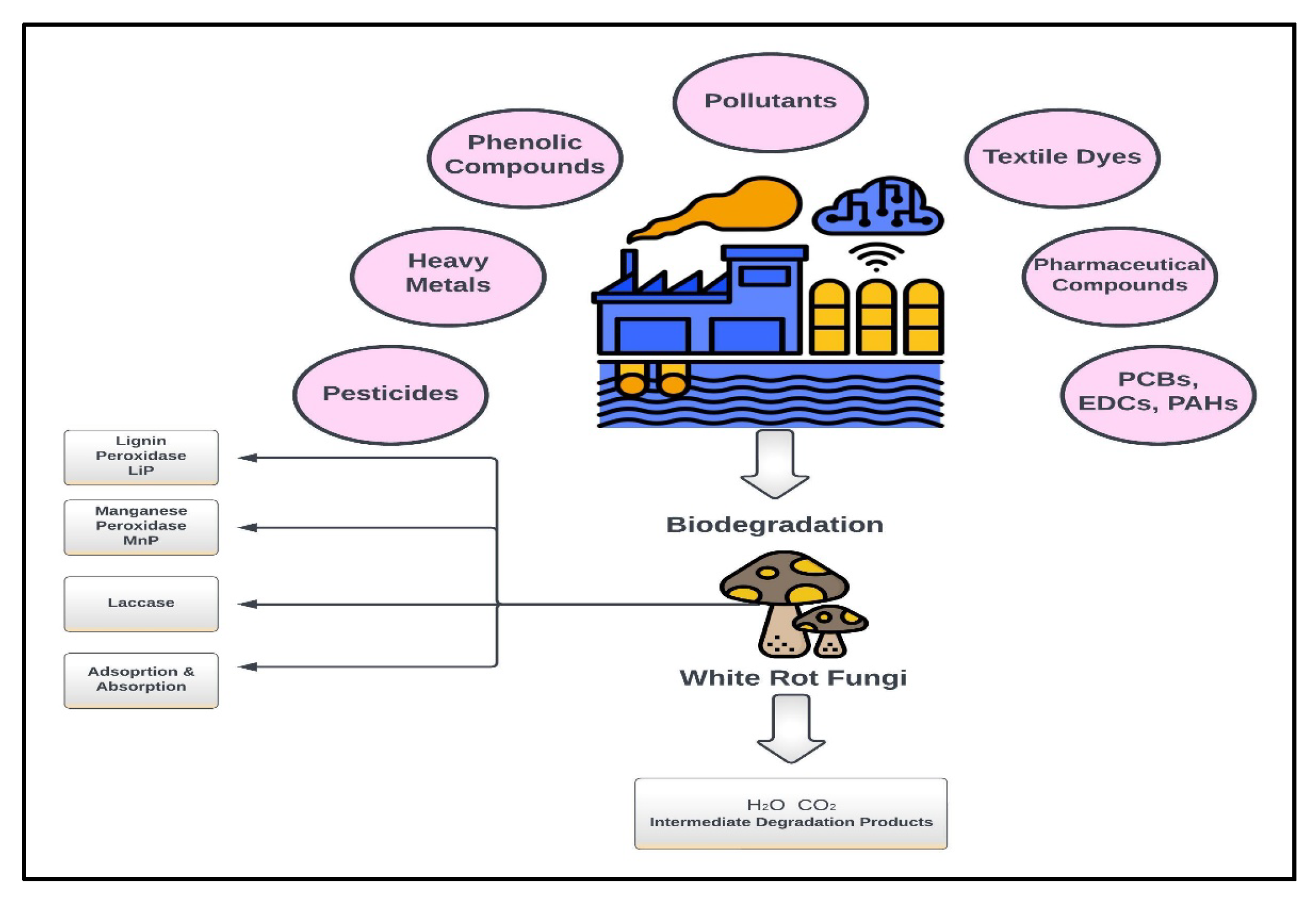
Figure 1.
Generic pollutants degradation mechanism of WRF.
3.5.1. Generation of Reactive Radicals
The extracellular lignin-degrading enzyme production by White Rot Fungi consists mainly of LiP and MnP. These two enzymes are responsible for producing reactive radicals with the assistance of redox reactions. These reactive radicals are non-specific oxidizing agents, which can degrade a range of complex and recalcitrant pollutants present in industrial wastewater. The reactive radicals carry out the oxidation of recalcitrant pollutants through several breakdown processes, including the removal of hydrogen atoms, chemical bond breakdown, and several other oxidative transformations [59,60][46][47].3.5.2. Co-Metabolism and Cometabolites
During the process of co-metabolism, extracellular enzymes of WRF, along with the degradation and oxidation of lignin, can oxidize other pollutants without assimilating any energy or nutrients from the process. On the other hand, co-metabolites refer to the auxiliary or secondary products produced during the degradation of primary pollutants by WRF. These co-metabolites can enhance the lignin-degrading capability of WRF, which further facilitates the treatment of industrial wastewater pollutants [61][48].3.5.3. Enzyme-Mediated Oxidation
Laccase is a multicopper oxidase enzyme, which can degrade a range of phenolic and non-phenolic compounds. Laccase has less redox potential than MnP and LiP, but its activity can be enhanced with the help of small molecules mediators, which can expand the range of substrates/pollutants that can be oxidized by the enzyme [62][49]. In fact, these mediators can transfer electrons between the enzyme and pollutants, thus facilitating the breakdown and oxidation of the pollutant [63][50]. The mediators generally applied in WRF-based bioremediation are the low-molecular-weight redox mediators, such as synthetic dyes and phenolic compounds [64][51]. These mediators act as electron shuttles, assisting the transfer of electrons between the enzymes and recalcitrant pollutants. They can accept electrons from the ligninolytic enzymes generated by WRF and transport them to the target pollutants, efficiently starting the degradation process. The availability of mediators in the reaction medium has numerous valuable effects on enzymatic reactions. Firstly, mediators can directly work together with the enzymes to augment their stability and activity. By acting as electron carriers, mediators can reproduce the active form of enzymes and inhibit their inactivation due to oxidative damage [65][52]. This process permits the enzymes to effectively take part in the degradation of pollutants over prolonged periods. Secondly, mediators can broaden the range of pollutants that can be treated by WRF. A few recalcitrant pollutants, like polycyclic aromatic hydrocarbons (PAHs) and chlorinated compounds, are intrinsically resistant to direct enzymatic attack by WRF’s ligninolytic enzymes. However, the addition of mediators can overcome this limitation by allowing indirect degradation pathways. The mediators support the production of reactive radicals, like hydroxyl radicals, which can react with and treat recalcitrant pollutants [66][53]. The selection of appropriate mediators is essential for optimizing the bioremediation process. The selection of a mediator relies on the specific pollutants targeted for treatment and the enzymatic systems of the WRF species being employed. Various mediators may exhibit varying efficiencies and selectivity in increasing the degradation of specific pollutants.3.5.4. Biosorption and Bioprecipitation
Apart from enzyme-based degradation and oxidation of recalcitrant pollutants, White Rot Fungi can employ the processes of biosorption and bioprecipitation to remove certain pollutants from industrial wastewater [67][54]. The fungal cell wall is usually filled with chitin and certain other polymers, which help the fungal cell wall to bind with and store metal ions and some other pollutants through several interactions, like electrostatic interactions, complexation, and ion exchange [68][55]. Additionally, WRF can alter the pH or redox conditions of the wastewater treatment system, which causes metal ions to precipitate as insoluble salts or hydroxides [69][56].3.5.5. Acid-Based Bioremediation Mechanism
In addition to enzymes, acids, including oxalic acid, play a substantial role in the bioremediation of pollutants and the breakdown of xenobiotic compounds by White Rot Fungi (WRF). An important characteristic of oxalic acid is its contribution in the chelation of metal ions. WRF, for example, Phanerochaete chrysosporium, are able to produce oxalic acid as a metabolic byproduct during the degradation of lignocellulosic materials. The produced oxalic acid can chelate with metal ions available in the environment, like iron, manganese, and copper ions [70][57]. This chelation process assists in mustering these metal ions and making them available for supplementary enzymatic reactions involved in the degradation of pollutants. Likewise, the acidic circumstances generated by oxalic acid can accelerate the generation and activity of ligninolytic enzymes in WRF. The increased enzymatic activity under acidic pH conditions can induce the breakdown of complex pollutants, including aromatic hydrocarbons, dyes, and pharmaceuticals [71,72][58][59]. The mixed action of oxalic acid and ligninolytic enzymes permits the efficient breakdown and detoxification of these hazardous compounds.3.6. Degradation of Industrial Pollutants by WRF
White Rot Fungi (WRF), due to their exceptional extracellular lignin-degrading enzymes producing ability, coupled with biosorption and bioprecipitation capabilities, have shown promising results in treating industrial wastewater pollutants of a complex nature [73][60]. In this section, the role of WRF in degrading various pollutants emanating from different industrial setups will be discussed.3.6.1. Phenolic Compounds
Phenolic compounds are common pollutants in industrial wastewater originating from various industries, including textile, pulp and paper, and petrochemical [74][61]. Typical phenolic compounds are cresols, phenols, and chlorophenols, all of which can be easily degraded by WRF’s extracellular enzymes. These lignin enzymes produce reactive radicals that, in turn, oxidize and degrade phenolic compounds of various types into less toxic and simpler substances, or these enzymes can even mineralize these compounds into CO2 and water [75][62].3.6.2. Dyes and Textile Wastewater
The textile industry is rapidly growing worldwide. The industry generates large amounts of wastewater containing many synthetic dyes, mostly recalcitrant and toxic [78][63]. Common dyes in textile industry wastewater are Azo dyes, anthraquinone, and triphenylmethane dyes, which can be easily degraded by WRF’s extracellular enzymes, especially MnP and laccase [59][46]. In some cases, redox mediators can augment the enzymatic oxidation of dyes, resulting in quicker and more effective decolorization [79][64].3.6.3. Pesticides and Agrochemicals
The foremost and major usage point of pesticides is the agricultural sector. These pesticides can contaminate waterbodies through surface water runoff or through percolation and leaching to underground water bodies [82][65]. The pesticides used in agricultural sectors are organochlorines, organophosphates, and carbamates. Most of the pesticides can easily be degraded by the WRF’s mechanism of extracellular enzyme production. These enzymes release reactive radicals that can cleave bonds present in pesticide molecules, thus transforming them into non-toxic or less toxic byproducts [83][66].3.6.4. Pharmaceuticals and Personal Care Products
Due to their persistence in the environment and association with ecotoxicological impacts, Pharmaceuticals and Personal Care Products (PPCPs) have recently emerged as notable pollutants in industrial wastewater requiring immediate attention [85][67]. The most common PPCPs include, but are not limited to, antibiotics, hormones, and anti-inflammatory drugs. All these PPCPs can be degraded by WRF-produced extracellular lignin-degrading enzymes, coupled with the enhancement of the degradation efficiency by the induction of redox mediators [42][29].3.6.5. Heavy Metals
In addition to degradation of organic pollutants, White Rot Fungi (WRF) play a considerable role in the remediation of heavy metal-contaminated environments. WRF carry out heavy metals remediation by the process of biosorption. The fungal cell walls contain many functional groups, such as carboxyl, amino, and hydroxyl groups, which exhibit a high affinity for metal ions. These functional groups can bind to heavy metal ions available in the surrounding environment, leading to their sequestration and immobilization on the fungal biomass [92][68]. In addition to biosorption, WRF can employ enzymatic processes for the remediation of heavy metals. Specific enzymes released by WRF, such as laccases and peroxidases, are involved in the oxidation and reduction reactions of metal ions. Laccases participate in the oxidation of metal ions, leading to their precipitation or conversion into less toxic forms [56][43].3.6.6. Other Pollutants
Apart from the industrial pollutants discussed in the previous section, White Rot Fungi can degrade and oxidize many other pollutants of environmental concern that cannot be treated by conventional wastewater treatment plants, including Poly Aromatic Hydrocarbons (PAHs), Poly Chlorinated Biphenyls (PCBs), and Endocrine-Disrupting Compounds (EDCs) [98][69]. The unique ability of WRF to produce lignin-degrading extracellular enzymes helps in degrading and oxidizing these compounds [59,60][46][47]. White Rot Fungi can degrade several industrial pollutants of a complex nature and can be an excellent option for wastewater treatment in the near future. However, in order to make practical and commercially viable WRF-based wastewater treatment systems, it is important to determine the optimized conditions for WRF and the factors that can affect the performance of these fungal species.References
- Osman, A.I.; Chen, L.; Yang, M.; Msigwa, G.; Farghali, M.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Cost, Environmental Impact, and Resilience of Renewable Energy under a Changing Climate: A Review. Environ. Chem. Lett. 2023, 21, 741–764.
- Spagnuolo, A.; De Santo, G.; Vetromile, C.; Masiello, A.; Di Costanzo, P.; Esposito, S.; Buono, U.; Di Cicco, M.R.; Lubritto, C. Characterizing Passenger-Ship Emissions: Towards Improved Sustainability for MedMar Fleet (Gulf of Naples). Energy Effic. 2022, 15, 55.
- Vetromile, C.; Spagnuolo, A.; Petraglia, A.; Masiello, A.; Di Cicco, M.R.; Lubritto, C. Pre- and Post-Operam Comparison of the Energy Consumption of a Radio Base Station under Energy Efficiency Actions. Energy Build. 2021, 236, 110772.
- Udugama, I.A.; Petersen, L.A.H.; Falco, F.C.; Junicke, H.; Mitic, A.; Alsina, X.F.; Mansouri, S.S.; Gernaey, K.V. Resource Recovery from Waste Streams in a Water-Energy-Food Nexus Perspective: Toward More Sustainable Food Processing. Food Bioprod. Process. 2020, 119, 133–147.
- Di Cicco, M.R.; Spagnuolo, A.; Masiello, A.; Vetromile, C.; Nappa, M.; Lubritto, C. Energetic and Environmental Analysis of a Wastewater Treatment Plant through Static and Dynamic Monitoring Activities. Int. J. Environ. Sci. Technol. 2020, 17, 4299–4312.
- Roy, S.; Garg, A.; Garg, S.; Tran, T.A. (Eds.) Advanced Industrial Wastewater Treatment and Reclamation of Water: Comparative Study of Water Pollution Index during Pre-Industrial, Industrial Period and Prospect of Wastewater Treatment for Water Resource Conservation; Springer: Cham, Switzerland, 2022.
- Bugajski, P.M.; Nowobilska-Majewska, E.; Kurek, K. The Variability of Pollution Load of Organic, Biogenic and Chromium Ions in Wastewater Inflow to the Treatment Plant in Nowy Targ. J. Water Land Dev. 2017, 35, 11–17.
- Lam, S.-M.; Choong, M.-K.; Sin, J.-C.; Zeng, H. Synchronous Organics Removal and Copper Reduction in Semiconductor Wastewater with Energy Recuperation via Photocatalytic Fuel Cell. E3S Web Conf. 2020, 167, 01002.
- Kow, S.-H.; Fahmi, M.R.; Azner Abidin, C.Z.; Ong, S.-A.; Ibrahim, A.H.; Sabri, S.N.; Razali, N.A. Oxidation of P-Cresol by Ozonation. Sains Malays. 2018, 47, 1085–1091.
- Maallah, R.; Chtaini, A. Phenol Removal from Wastewaters by Electrochemical Biosensor. Am. Int. J. Biol. Life Sci. 2019, 1, 24–32.
- Di Cicco, M.; Masiello, A.; Spagnuolo, A.; Vetromile, C.; Borea, L.; Giannella, G.; Iovinella, M.; Lubritto, C. Real-Time Monitoring and Static Data Analysis to Assess Energetic and Environmental Performances in the Wastewater Sector: A Case Study. Energies 2021, 14, 6948.
- Di Cicco, M.R.; Iovinella, M.; Palmieri, M.; Lubritto, C.; Ciniglia, C. Extremophilic Microalgae Galdieria Gen. for Urban Wastewater Treatment: Current State, the Case of “POWER” System, and Future Prospects. Plants 2021, 10, 2343.
- Di Cicco, M.R.; Spagnuolo, A.; Masiello, A.; Vetromile, C.; Nappa, M.; Corbo, G.; Lubritto, C. Energy monitoring of a wastewater treatment plant in Salerno, Campania Region (southern Italy). In Proceedings of the Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability: Proceedings of the 2nd WaterEnergyNEXUS Conference, November 2018, Salerno, Italy, 19 September 2019; Springer: Berlin/Heidelberg, Germany, 2020.
- Lovinella, M.; Lombardo, F.; Ciniglia, C.; Palmieri, M.; Di Cicco, M.R.; Trifuoggi, M.; Race, M.; Manfredi, C.; Lubritto, C.; Fabbricino, M.; et al. Bioremoval of Yttrium (III), Cerium (III), Europium (III), and Terbium (III) from Single and Quaternary Aqueous Solutions Using the Extremophile Galdieria Sulphuraria (Galdieriaceae, Rhodophyta). Plants 2022, 11, 1376.
- Sirakov, M.; Palmieri, M.; Iovinella, M.; Davis, S.J.; Petriccione, M.; Di Cicco, M.R.; De Stefano, M.; Ciniglia, C. Cyanidiophyceae (Rhodophyta) Tolerance to Precious Metals: Metabolic Response to Palladium and Gold. Plants 2021, 10, 2367.
- Palmieri, M.; Iovinella, M.; Davis, S.J.; Di Cicco, M.R.; Lubritto, C.; Race, M.; Papa, S.; Fabbricino, M.; Ciniglia, C. Galdieria Sulphuraria ACUF427 Freeze-Dried Biomass as Novel Biosorbent for Rare Earth Elements. Microorganisms 2022, 10, 2138.
- Iovinella, M.; Carbone, D.A.; Cioppa, D.; Davis, S.J.; Innangi, M.; Esposito, S.; Ciniglia, C. Prevalent PH Controls the Capacity of Galdieria Maxima to Use Ammonia and Nitrate as a Nitrogen Source. Plants 2020, 9, 232.
- Manfredi, C.; Amoruso, A.J.; Ciniglia, C.; Iovinella, M.; Palmieri, M.; Lubritto, C.; El Hassanin, A.; Davis, S.J.; Trifuoggi, M. Selective Biosorption of Lanthanides onto Galdieria Sulphuraria. Chemosphere 2023, 317, 137818.
- Li, Z.; Wang, X.; Ni, Z.; Bao, J.; Zhang, H. In-Situ Remediation of Carbofuran-ContaminatedSoil by Immobilized White-Rot Fungi. Pol. J. Environ. Stud. 2020, 29, 1237–1243.
- Honda, Y.; Tanigawa, E.; Tsukihara, T.; Nguyen, D.X.; Kawabe, H.; Sakatoku, N.; Watari, J.; Sato, H.; Yano, S.; Tachiki, T.; et al. Stable and Transient Transformation, and a Promoter Assay in the Selective Lignin-Degrading Fungus, Ceriporiopsis Subvermispora. AMB Express 2019, 9, 92.
- Zhang, J.; Chi, Y.; Feng, L. The Mechanism of Degradation of Alizarin Red by a White-Rot Fungus Trametes Gibbosa. BMC Biotechnol. 2021, 21, 64.
- Duran-Rivera, B.; Moreno-Suarez, J.R.; García-Ramírez, S.I. Decolorization of a Textile Effluent and Methylene Blue by Three White Rot Fungi (WRF), at Pilot and Laboratory Scale. Bionatura 2018, 3, 709–714.
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of Fungi in Bioremediation of Emerging Pollutants. Front. Mar. Sci. 2023, 10, 1070905.
- Guillaume, A.; Thorigné, A.; Carré, Y.; Vinh, J.; Levavasseur, L. Contribution of Proteases and Cellulases Produced by Solid-State Fermentation to the Improvement of Corn Ethanol Production. Bioresour. Bioprocess. 2019, 6, 7.
- Mérillon, J.-M.; Ramawat, K.G. (Eds.) Fungal Metabolites; Springer International Publishing: Cham, Switzerland, 2017.
- Stoller, M.; Sacco, O.; Sannino, D.; Chianese, A. Successful Integration of Membrane Technologies in a Conventional Purification Process of Tannery Wastewater Streams. Membranes 2013, 3, 126–135.
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the Conventional Biological Wastewater Treatment to Hybrid Processes, the Evaluation of Organic Micropollutant Removal: A Review. Water Res. 2017, 111, 297–317.
- Sá, H.; Michelin, M.; Tavares, T.; Silva, B. Current Challenges for Biological Treatment of Pharmaceutical-Based Contaminants with Oxidoreductase Enzymes: Immobilization Processes, Real Aqueous Matrices and Hybrid Techniques. Biomolecules 2022, 12, 1489.
- Zhuo, R.; Fan, F. A Comprehensive Insight into the Application of White Rot Fungi and Their Lignocellulolytic Enzymes in the Removal of Organic Pollutants. Sci. Total Environ. 2021, 778, 146132.
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of Lignocellulose Using Ligninolytic Enzymes from White-Rot Fungi. Heliyon 2022, 8, e08865.
- Kulshreshtha, S.; Onyedinma, U.; Siddhant, P. (Eds.) Recent Advances in Mushroom Cultivation Technology and Its Application (Volume 2), 1st ed.; Bright Sky Publications: Delhi, India, 2022.
- Lundell, T.K.; Mäkelä, M.R.; De Vries, R.P.; Hildén, K.S. Genomics, lifestyles and future prospects of wood-decay and litter-decomposing Basidiomycota. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 70, pp. 329–370.
- Taherzadah, M.J.; Ferreira, J.; Pandey, A. (Eds.) Current Developments in Biotechnology and Bioengineering Filamentous Fungi Biorefinery, 1st ed.; Elsevier Publications: Amsterdam, The Netherlands, 2022.
- Purchase, D. (Ed.) Fungal Applications in Sustainable Environmental Biotechnology; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2016.
- Ntwampe, S.; Chowdhury, F.; Sheldon, M.; Volschenk, H. Overview of Parameters Influencing Biomass and Bioreactor Performance Used for Extracellular Ligninase Production from Phanerochaete Chrysosporium. Braz. Arch. Biol. Technol. 2010, 53, 1057–1066.
- Souza, D.F.D.; Costa, S.C.D.; Dacome, A.S.; Souza, C.G.M.D.; Bracht, A.; Peralta, R.M. Pentachlorophenol Removal by Pleurotus Pulmonarius in Submerged Cultures. Braz. Arch. Biol. Technol. 2011, 54, 357–362.
- Ullrich, R.; Hofrichter, M. Enzymatic Hydroxylation of Aromatic Compounds. Cell. Mol. Life Sci. 2007, 64, 271–293.
- Kumar, A.; Chandra, R. Ligninolytic Enzymes and Its Mechanisms for Degradation of Lignocellulosic Waste in Environment. Heliyon 2020, 6, e03170.
- Chen, H. Biotechnology of Lignocellulose: Theory and Practice; Springer: Berlin/Heidelberg, Germany; Chemical Industry Press: Dordrecht, The Netherlands; New York, NY, USA, 2014.
- Saini, J.K.; Himanshu; Hemansi; Kaur, A.; Mathur, A. Strategies to Enhance Enzymatic Hydrolysis of Lignocellulosic Biomass for Biorefinery Applications: A Review. Bioresour. Technol. 2022, 360, 127517.
- Tuomela, M. Biodegradation of Lignin in a Compost Environment: A Review. Bioresour. Technol. 2000, 72, 169–183.
- Ruiz-Dueñas, F.J.; Lundell, T.; Floudas, D.; Nagy, L.G.; Barrasa, J.M.; Hibbett, D.S.; Martínez, A.T. Lignin-Degrading Peroxidases in Polyporales: An Evolutionary Survey Based on 10 Sequenced Genomes. Mycologia 2013, 105, 1428–1444.
- Baldrian, P. Fungal Laccases—Occurrence and Properties. FEMS Microbiol. Rev. 2006, 30, 215–242.
- Eggert, C.; Temp, U.; Eriksson, K.E. The Ligninolytic System of the White Rot Fungus Pycnoporus Cinnabarinus: Purification and Characterization of the Laccase. Appl. Environ. Microbiol. 1996, 62, 1151–1158.
- Abdel-Azeem, A.M.; Gherbawy, Y.A.; Sabry, A.M. Enzyme Profiles and Genotyping of Chaetomium Globosum Isolates from Various Substrates. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2016, 150, 420–428.
- Sun, S.; Liu, P.; Ullah, M. Efficient Azo Dye Biodecolorization System Using Lignin-Co-Cultured White-Rot Fungus. J. Fungi 2023, 9, 91.
- Morsi, R.; Bilal, M.; Iqbal, H.M.N.; Ashraf, S.S. Laccases and Peroxidases: The Smart, Greener and Futuristic Biocatalytic Tools to Mitigate Recalcitrant Emerging Pollutants. Sci. Total Environ. 2020, 714, 136572.
- Hadibarata, T.; Kristanti, R.A. Fate and Cometabolic Degradation of BenzoPyrene by White-Rot Fungus Armillaria Sp. F022. Bioresour. Technol. 2012, 107, 314–318.
- Upadhyay, P.; Shrivastava, R.; Agrawal, P.K. Bioprospecting and Biotechnological Applications of Fungal Laccase. 3 Biotech 2016, 6, 15.
- Mester, T.; Tien, M. Oxidation Mechanism of Ligninolytic Enzymes Involved in the Degradation of Environmental Pollutants. Int. Biodeterior. Biodegrad. 2000, 46, 51–59.
- Gianfreda, L.; Xu, F.; Bollag, J.-M. Laccases: A Useful Group of Oxidoreductive Enzymes. Bioremediat. J. 1999, 3, 1–26.
- Singh, G.; Dwivedi, S.K.; Mishra, J. Role of fungal enzymes in the removal of Azo Dyes. In Microbial Enzymes: Roles and Applications in Industries; Arora, N.K., Mishra, J., Mishra, V., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2020; Volume 11, pp. 231–257.
- Gunawardana, B.; Singhal, N.; Swedlund, P. Degradation of Chlorinated Phenols by Zero Valent Iron and Bimetals of Iron: A Review. Environ. Eng. Res. 2011, 16, 187–203.
- Silva, A.; Delerue-Matos, C.; Figueiredo, S.; Freitas, O. The Use of Algae and Fungi for Removal of Pharmaceuticals by Bioremediation and Biosorption Processes: A Review. Water 2019, 11, 1555.
- Javanbakht, V.; Alavi, S.A.; Zilouei, H. Mechanisms of Heavy Metal Removal Using Microorganisms as Biosorbent. Water Sci. Technol. 2014, 69, 1775–1787.
- Chandra, P.; Enespa. Mycoremediation of environmental pollutants from contaminated soil. In Mycorrhizosphere and Pedogenesis; Varma, A., Choudhary, D.K., Eds.; Springer: Singapore, 2019; pp. 239–274.
- Pointing, S. Feasibility of Bioremediation by White-Rot Fungi. Appl. Microbiol. Biotechnol. 2001, 57, 20–33.
- Cullen, D.; Kersten, P.J. Enzymology and molecular biology of lignin degradation. In Biochemistry and Molecular Biology; Brambl, R., Marzluf, G.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 249–273.
- Kellner, H.; Luis, P.; Zimdars, B.; Kiesel, B.; Buscot, F. Diversity of Bacterial Laccase-like Multicopper Oxidase Genes in Forest and Grassland Cambisol Soil Samples. Soil Biol. Biochem. 2008, 40, 638–648.
- Asgher, M.; Bhatti, H.N.; Ashraf, M.; Legge, R.L. Recent Developments in Biodegradation of Industrial Pollutants by White Rot Fungi and Their Enzyme System. Biodegradation 2008, 19, 771–783.
- Farhan Hanafi, M.; Sapawe, N. A Review on the Water Problem Associate with Organic Pollutants Derived from Phenol, Methyl Orange, and Remazol Brilliant Blue Dyes. Mater. Today Proc. 2020, 31, A141–A150.
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging Contaminants of High Concern and Their Enzyme-Assisted Biodegradation—A Review. Environ. Int. 2019, 124, 336–353.
- Islam, T.; Islam, T.; Repon, R. Synthetic Dyes for Textile Colouration: Process, Factors and Environmental Impact. Text. Leather Rev. 2022, 5, 327–373.
- Dafale, N.; Rao, N.N.; Meshram, S.U.; Wate, S.R. Decolorization of Azo Dyes and Simulated Dye Bath Wastewater Using Acclimatized Microbial Consortium—Biostimulation and Halo Tolerance. Bioresour. Technol. 2008, 99, 2552–2558.
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112.
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R. A Review on Catalytic-Enzyme Degradation of Toxic Environmental Pollutants: Microbial Enzymes. J. Hazard. Mater. 2021, 419, 126451.
- Hanay, Ö.; Türk, H. An Overview on Usage of Nanoscale Zero Valent Iron for Pharmaceuticals Elimination. Eskişehir Tek. Üniversitesi Bilim Teknol. Derg. B Teor. Bilim. 2019, 7, 222–239.
- Baldrian, P. Interactions of Heavy Metals with White-Rot Fungi. Enzym. Microb. Technol. 2003, 32, 78–91.
- Bharati, S.L.; Chaurasia, P.K.; Das, P.S. (Eds.) Research Advancements in Pharmaceutical, Nutritional, and Industrial Enzymology; Advances in Medical Technologies and Clinical Practice; IGI Global: Hershey, PA, USA, 2018.
More
