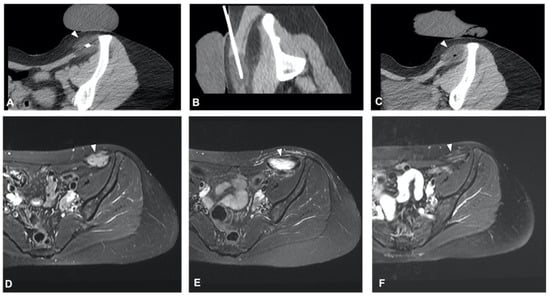
In the rapidly evolving field of interventional oncology, minimally invasive methods, including CT-guided cryoablation, play an increasingly important role in tumor treatment, notably in bone and soft tissue cancers. Cryoablation works using compressed gas-filled probes to freeze tumor cells to temperatures below −20 °C, exploiting the Joule–Thompson effect. This cooling causes cell destruction by forming intracellular ice crystals and disrupting blood flow through endothelial cell damage, leading to local ischemia and devascularization. Coupling this with CT technology enables precise tumor targeting, preserving healthy surrounding tissues and decreasing postoperative complications.
- interventional radiology
- metastatic neoplasms
- orthopedic surgery
- palliative medicine
- cryoablation
1. Introduction
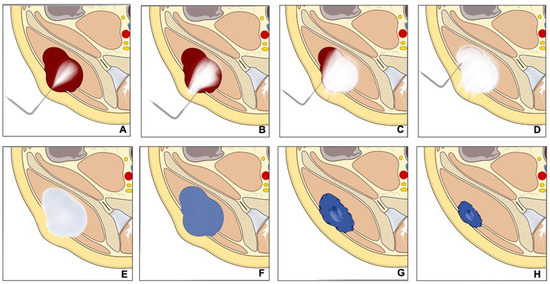
2. Malignant Bone and Soft Tissue Tumors
Percutaneous cryoablation is becoming an increasingly accepted option within the multidisciplinary sarcoma board for the treatment of primary bone and soft tissue tumors, applicable for selected cases. Despite surgical intervention being the mainstay for treating primary, non-metastatic bone, and soft tissue tumors, the local control of recurring bone and soft tissue sarcoma (STS) continues to be a challenging task. It mainly hinges on the disease prognosis as per the guidelines of the European Society for Medical Oncology (ESMO) [10][11][10,11]. Surgical resection is the common protocol for localized conditions, while chemotherapy or radiation therapy may be employed for more extensive diseases or recurrences [10][11][12][10,11,12]. Lately, minimally invasive techniques such as radiofrequency ablation, microwave ablation, or cryoablation have been proposed as potential surgical alternatives for some selected recurrent bone and soft tissue tumors [13][14][15][16][17][13,14,15,16,17].
Some initial studies evaluated the therapeutic effect of Cryoablation for the treatment of a variety of primary bone and soft tissue malignancies with promising results; however, the scientific evidence is still limited. Moreover, there is a recognized need for the standardization of selection criteria for percutaneous cryoablation. Lippa et al. [12] aimed to identify these criteria, finding high agreement for all proposed criteria between two readers. Eligibility for cryoablation was significantly associated with tumors located deeply, with great axes ≤ 5 cm, high local tumor aggressiveness, and a diagnosis of differentiated myxoid liposarcoma or myxofibrosarcoma.
2.1. Recurrent Retroperitoneal Soft Tissue Tumors
2.2. Sacrococcygeal Tumors and Chordoma
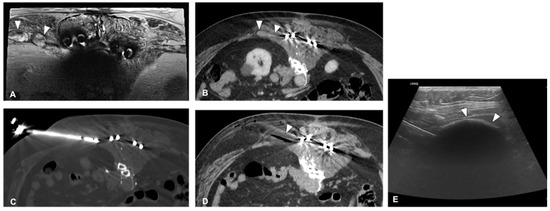
3. Bone Metastases
3.1. Pain Palliation and Disease Control
The clinical effect in pain reduction and safety of cryoablation for metastatic bone disease has been investigated by several studies in recent years [34][35][36][37][38][40,43,44,46,48] (Figure 3). A recent multicenter prospective study by Jennings et al. [39][38] assessed the clinical efficacy of cryoablation as a pain palliating method for patients with metastatic bone disease. The main goal was the pain score change from pre-treatment to the eighth-week follow-up, with participants monitored for 24 weeks post-treatment. A cohort of 66 participants (average age 60.8 years, 53% male) was recruited and underwent percutaneous cryoablation; 65 completed the follow-up. The average change in pain score from baseline to the eighth week decreased by 2.61 points. Average pain scores improved by 2 points at the first week and attained clinically significant levels (a decrease of more than 2 points) post the eighth week, with scores continuing to improve throughout the follow-up period. Quality of life was enhanced, opioid doses were steady, and functional status remained unchanged over six months. Severe adverse events were reported in three participants.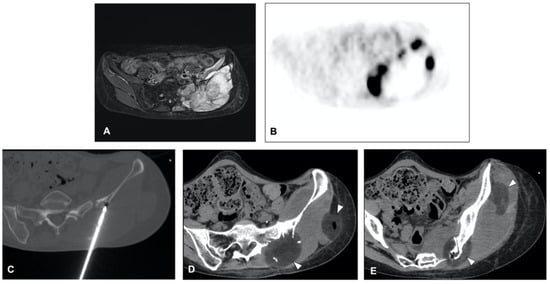
3.2. Application to Spinal Metastases
The treatment of spinal metastases is notoriously difficult due to the sensitive nature of the spine, with both watchful waiting and active treatment carrying significant risks of local complications [42][43][56,57]. Cryoablation for spinal metastases is often conducted alone or frequently in combination with vertebral augmentation techniques such as cementoplasty [44][51]. In a study by Autrusseau et al. [44][51], 31 patients (including 36 spinal metastases in 32 sessions) received cryoablation for pain relief, and 10 patients (10 metastases in 10 sessions) for local control. The procedure successfully alleviated pain in 93.8% of palliative sessions, with the average pain scores notably decreasing at 24 h, 1 month, and at the final follow-up (approximately 16.5 months). For those patients needing local tumor control, primary clinical success was achieved in 60% of cases with about 25 months of median follow-up. The overall complication rate was 8%, with no reported secondary fractures or thermal nerve injuries.3.3. Application to Sternal Metastases
Due to its high success rate and the safety provided by real-time visualization of the ice formation, cryoablation can be employed to treat highly sensitive body areas. A study by Hegg et al. [45][42] sought to evaluate the safety and efficacy of cryoablation for sternal metastases. The retrospective review included 12 patients with 12 sternal metastases. The results indicated that cryoablation provided pain relief, as shown by a drop in average pain score from 7.0 to 1.8. Local tumor control was achieved in 80% of the patients treated for this purpose. The study concluded that cryoablation is a safe and potentially effective treatment for painful sternal metastases.3.4. Evaluation of Post-Ablation Area
Evaluating the treated area after cryoablation is crucial in determining the success of the procedure and detecting any local tumor recurrence. In this regard, Gravel et al. [46][53] conducted a study to assess the effectiveness of post-ablation MRI in identifying cases of incomplete treatment of spinal osseous metastases following cryoablation. The study involved 54 spinal bone metastases in 39 patients. The classification of MRI images into four categories resulted in a sensitivity of 77.3% and specificity of 85.9% in identifying residual tumors.3.5. Technical Consideration for Neuroprotection
Preserving neural structures is paramount when treating lesions close to the spine or major peripheral nerves. Kurup et al. [47][59] explored the use of motor evoked potential (MEP) monitoring during the cryoablation procedure of musculoskeletal tumors to reduce the likelihood of nerve damage. This study included 59 procedures on 64 tumors in 52 patients, with tumors located in various sites such as the spine, sacrum, retroperitoneum, pelvis, and extremities. During these procedures, MEP monitoring identified significant decreases in MEPs in 32% of the cases, with transient decreases in 25% and persistent decreases in 7%. Out of the four patients with persistent decreases in MEPs, two experienced motor deficits post-ablation3.6. Technical Consideration for Bone Reinforcement
When conducting ablation on large bone sections or bones that bear weight [48][47], bone reinforcement might be required to avoid post-procedural pathologic fractures. Combining cryoablation with cement stabilization has been reported as highly effective by several studies. Masala et al. [49][60] studied the efficacy of combining cryoablation and vertebroplasty (CVT) vs. vertebroplasty alone in 46 patients with a single vertebral metastasis. They used the Visual Analog Scale (VAS) and the Oswestry Disability Index (ODI) to measure pain levels and quality of life. Although both treatment groups showed a significant reduction in VAS and ODI scores, more notable improvements were observed in the CVT group at various follow-up stages, suggesting CVT as a safe, effective option for pain relief and disability improvement.3.7. Combination Treatment
Sundararajan et al. [50][63] proposed a sequential interventional therapy involving embolization, cryoablation, and osteoplasty for patients with osseous neoplasms, who were unresponsive to conventional treatment3.8. Complications
Despite cryoablation being a minimally invasive procedure guided by CT, it carries a small risk of complications. Auloge et al. [51][64] evaluated the complications and related risk factors in bone tumor cryoablation. The study involved 239 patients who underwent cryoablation for 320 primary or metastatic bone tumors from 2008 to 2017. The overall complication rate was 9.1%, with serious complications making up 2.5% of this total. The most common major complication was secondary fractures, which represented 1.2% of the cases.4. Benign Bone Tumors
4.1. Osteoid Osteoma
Osteoid osteoma (OO) is a small, benign tumor primarily found in the bones of young people and children. Even though it only accounts for approximately 10% to 12% of all benign bone tumors, it can significantly affect the quality of life, causing pain and bone deformity, especially in children [52][53][54][65,66,67]. The treatment for OO has seen a considerable evolution over the years. Traditional surgical removal was once the main treatment approach, but technological advancements have facilitated a shift toward less invasive methods like radiofrequency ablation (RFA) [55][56][57][58][68,69,70,71]. Meng et al. [59][72] conducted a study comparing the safety and effectiveness of percutaneous CT-guided cryoablation of OO to surgical curettage. Both treatment approaches reported a 100% technical success rate. However, patients treated with cryoablation spent significantly less time in the hospital than those undergoing surgery, and both groups showed notable improvement in postoperative Visual Analog Scale (VAS) pain scores.4.2. Osteoblastoma
Cryoablation was also found to be a viable treatment for osteoblastoma in the study by Cazzato et al. [60][78] Technical success was achieved in all cases, and primary clinical success was 100% and 78% at 1 and 12 months of follow-up, respectively. Notably, this study emphasized the need for comprehensive protective measures due to the frequent close proximity of critical structures.4.3. Bone Cyst and Aneurysmal Bone Cyst
Bone cysts are fluid-filled holes that develop within bones. They are commonly found in children and adolescents, and most often occur in the long bones of the body such as the femur or the humerus. Most bone cysts do not cause symptoms and are often discovered incidentally during an X-ray performed for other reasons. However, in some cases, they can cause pain or lead to fractures [61][79]. Aneurysmal bone cysts (ABCs), on the other hand, are an uncommon type of bone cyst that is blood-filled rather than fluid-filled. They can occur at any age but are most commonly diagnosed in individuals under the age of 20. ABCs are expansile and can cause pain, swelling, and deformities in the affected bone. They can also lead to fractures due to the weakening of the bone structure.5. Desmoid Tumors
Desmoid tumors are rare benign tumors originating from musculoaponeurotic structures [62][85]. Despite their benign nature, they can display local aggressiveness, causing disability and sometimes pain. The ESMO advises initial observation and subsequent medical treatment for progressing tumors. Cryoablation, an interventional radiology technique, is recommended for desmoid tumor patients due to its ability to induce cell death through multiple cycles of freezing [63][64][65][86,87,88] (Figure 45).