Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yulan Hu and Version 2 by Rita Xu.
Mass spectrometry, chromatography, spectroscopy, nuclear magnetic, proteomics, and immunoassay are used to analyse protein materials. Proteomics techniques and enzyme-linked immunosorbent assay (ELISA) technology are two of the most common methods for detecting ancient proteins.
- cultural heritage conservation
- protein
- enzyme-linked immunosorbent assay (ELISA)
1. Introduction
In the conservation of cultural heritage and archaeological excavations, the materials, and especially the key components, such as the binder of murals and paintings and the organic components of building mortars, are not only closely related to human production life, material life, spiritual life, and all aspects of social life, but they also serve as significant carriers of historical information. In addition, they are the focus of research on traditional building techniques, the prevention and control of the deterioration of cultural relic materials, the elucidation of the physical and chemical causes of material deterioration, and the development of future protective measures based on analysis and testing [1].
Proteins are one of the most significant components of cultural heritage artefacts and are frequently employed as essential additives [2][3][4][2,3,4]. Additionally, proteins can be used to restore and reinforce cultural heritage artefacts [5][6][7][5,6,7]. It is difficult to analyze protein-containing cultural heritage artefacts due to the limited number of available samples or the low protein content of the samples. Moreover, after prolonged exposure to multiple environmental factors, proteins encounter a variety of issues, including ageing, degradation, and contamination [8][9][8,9]. The earliest research on protein detection in artefacts of cultural heritage dates back to the 1950s [10]. Archaeologists used mass spectrometry (MS) to detect amino acids, the building blocks of proteins, in archaeological and paleontological artefacts [11]. Since the 1980s, physical chemistry techniques such as mass spectrometry, chromatography, and spectroscopy have been utilised extensively in the field of cultural heritage research and conservation. However, these methods have the disadvantage of requiring a large number of samples, making it challenging to distinguish the complex protein components in cultural heritage objects and lacking species specificity [12]. In recent years, with the development of biotechnology, bioinformatics technology, and MS, proteomics techniques and enzyme-linked immunosorbent assay (ELISA) techniques have been increasingly applied to the study of proteins in cultural relics materials, and these two techniques offer high sensitivity and low detection limits [13].
2. ELISA Technology
2.1. The Concept of Enzyme-Linked Immunosorbent Assay
ELISA is a labelled immunoassay technique that combines the specific binding reaction of antigen–antibody with the color-rendering reaction of enzymes and catalytic substrate to enhance sensitivity [3] by amplifying the signal. In a published article promoting the establishment and development of ELISA [14][67], Swedish researchers Engvall and Perlmann first proposed the use of ELISA for the quantitative detection of antibodies during the 1970s. The substrate is added after the enzyme is labelled on the antibody, the antigen is immunologically bound to the enzyme-labeled antibody, and then the antigen is specifically bound to the enzyme-labeled antibody. The product of the colour reaction between the substrate and the enzyme is coloured. The colour intensity of the product correlates positively with the quantity of antigen or antibody in the test substance. Using an enzyme marker, the absorbance of the product at a particular wavelength can be measured, and the antigen or antibody can be quantitatively analysed. An antigen is a molecule that can stimulate an organism’s immune system, and an antibody is a glycoprotein that can bind to an antigen in a specific manner. Proteins can be used as an antigen to generate antibodies, and for immune experiments to generate specific antibodies, only the protein must be extracted and purified. The direct method, double antibody sandwich method, indirect method, and competition method are typical ELISA detection techniques used for protein analysis of cultural relic materials [15][68] (Figure 1). The direct method involves adsorbing the antigen onto a solid-phase carrier, incubating it at an appropriate temperature and relative humidity, and then washing away any unbound antigens and impurities. To bind other unbound sites on the solid-phase carrier, a high concentration of nonspecific proteins and enzyme-labeled specific antibodies are added. At an optimal temperature and relative humidity, the antigen and antibody react completely, and the sample is then rinsed to remove unreacted antibodies. Finally, the chromogenic substrate is added, and within a certain amount of time, the enzyme-catalyzed colour develops. The experimental operation requirements for the labelling reaction of antibodies are high; each antigen detected by the direct method must be labelled with a specific antibody that interacts with it, resulting in a high cost of detection. Using the sandwich method, a known antibody is affixed to the surface of a solid-phase carrier, followed by the test sample. If the sample contains an antigen, specific binding will occur; then, an enzyme-labeled antibody is added to produce an antibody–antigen–enzyme-labeled antibody “sandwich” structure. After the addition of the substrate, the antigen in the sample is detected and analysed using a color-generating reaction. Double antibody sandwiching is a common technique for detecting macromolecular antigens [16][69]. However, when using the double antibody sandwich method to detect each antigen, the specific antibody that reflects that antigen must be labelled; when combined with indirect methods, each antigen requires two antibodies, making the double antibody sandwich method more expensive. The indirect technique can be used to detect antigens. The principle is to adsorb the antigen to be tested onto the solid phase, followed by the addition of specific antibodies and enzyme-labeled antibodies. Alternately, after the antigen has been adsorbed in the solid phase, the antibody to be examined and the enzyme-labeled antibody are successively added. In the indirect method, the colour intensity is directly proportional to the concentrations of the coated antigen and the primary antibody. The binding between the first and second antibodies is based on the principle that cross-reactions can occur between distinct subclasses of the same antibody. Therefore, specific antibodies can be applied directly to ELISA without being labelled, preserving the high activity of the specific antibody, reducing the number of experimental steps, and saving time and money. Consequently, indirect methods are the most popular. It is also the most popular method for detecting proteins in cultural relics [17][70]. The competition method is distinguished by the interaction between the antigen being tested and the enzyme-labeled antigen and the solid-phase antibody. The more the enzyme-labelled antigen binds to the solid-phase antibody, and the darker the colour, the lower the antigen concentration in the test sample. The intensity of the colour of the competitive solution is directly proportional to the antigen concentration in the mixed solution and inversely proportional to the antigen concentration on the solid carrier. This method is primarily used to determine small-molecule hapten [18][19][20][71,72,73].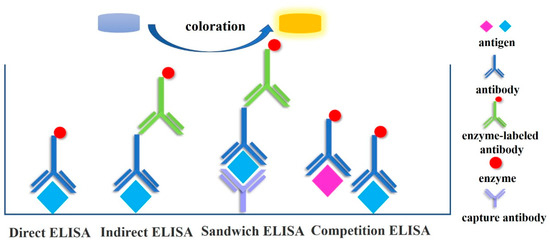
Figure 1. Common detection methods of ELISA.
