Liver disease are associated with a wide spectrum of neurological changes, of which the best known is hepatic encephalopathy (HE). Historically, hyperammonemia, was considered the main etiological factor; however, recent studies demonstrated a key role of neuroinflammation in the development of neurological complications in this setting. Neuroinflammation is characterized by activation of microglial cells and brain secretion of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, which alter neurotransmission, leading to cognitive and motor dysfunction. Changes in the gut microbiota resulting from liver disease play a crucial role in the pathogenesis of neuroinflammation. Dysbiosis and altered intestinal permeability, resulting in bacterial translocation and endotoxemia, are responsible for systemic inflammation, which can spread to brain tissue and trigger neuroinflammation. In addition, metabolites derived from the gut microbiota can act on the central nervous system and facilitate the development of neurological complications, exacerbating clinical manifestations.
- neuroinflammation
- microglia
- gut microbiota
- gut–liver–brain axis
- hepatic encephalopathy
- chronic liver disease
- ALF
1. Introduction
Although HE pathogenic mechanisms are still not fully elucidated, ammonia has always been considered the main causative factor [4]. However, it has been shown that ammonia levels do not correlate with the severity of HE and that HE can also manifest in patients with normal ammonia levels, hinting at the presence of other contributing factors, such as systemic inflammation and oxidative stress [5][6]. Recently several studies have suggested a key role of neuroinflammation in this setting [7]. Indeed, systemic inflammation and hyperammonemia stimulate in concert neuroinflammation [8].
2. Pathophysiology of Neuroinflammation
Neuroinflammation refers to the inflammatory response that develops within the central nervous system following several insults, such as infections, traumatic injury, or exposure to toxic metabolites [9]. Microglia and astrocytes, the main brain innate immune cells, drive this process by producing several pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, chemokines, including C-C motif chemokine ligand 2 (CCL2), CCL5, and C-X-C motif chemokine ligand 1 CXCL1, secondary messengers, such as nitric oxide (NO) and prostaglandins, and reactive oxygen species [10]. Additionally, endothelial cells and peripheral immune cells act in promoting this inflammatory status [11]. Under normal conditions, the central nervous system (CNS) is protected from the entrance of potentially pathological agents into the cerebral circulation thanks to the blood–brain barrier (BBB), a highly selective structure made of endothelial cells and astrocytes [12]. The integrity of the BBB is guaranteed by tight junction (TJs) proteins such as occludin and claudin-5 [13]. Following injury and systemic inflammation, TJs undergo a dysregulation process that affects the integrity of the BBB, increasing the permeability of dangerous molecules that promote brain inflammation. Activated microglia and astrocytes in turn favor BBB dysfunction, exacerbating this process [14]. Microglia is made of CNS resident innate immune cells derived from myeloid lineage. They constitute about 10% of the total CNS cells [15]. Microglia play an active role in fundamental brain processes, such as neurogenesis, synaptic pruning and plasticity, and immune surveillance [16]. In physiological conditions, microglia are quiescent, but they actively monitor the surrounding parenchymal environment with their branching processes [17]. In response to stimulations such as pro-inflammatory cytokines or other pathological molecules, microglial cells become activated and produce inflammatory cytokines and chemokines to prevent CNS damage [18]. However, when chronically activated, microglia plays a key role in the paradoxical propagation of neuroinflammation, leading to neurodegeneration [19]. Astrocytes are the most abundant glial cells in the CNS, representing, with their processes, a critical component of the BBB together with endothelial cells [20]. Astrocytes also provide metabolic support to neurons, regulate cerebral blood flow, and modulate synapses formation and synaptic transmission, through the uptake and release of neurotransmitters [21][22]. Activated microglia releases IL-1α, TNFα, and complement 1q (C1q), and is responsible, together with peripheral inflammatory cytokines and signals, for astrocytes activation. In this way, a gliosis response occurs, which is characterized by the upregulation of glial fibrillary acid protein (GFAP) expression and gliotic scar formation [23][24]. Neuroinflammation is normally part of a protective physiological process. However, its chronic and excessive activation triggers the development of brain damage with synaptic consequences, cell loss, and impaired neurogenesis [15] that, altogether, lead to manifestations related to nervous system dysfunctions, such as anxiety, depression, memory loss and cognitive impairment [25].3. Gut–Liver Axis Contribution to Systemic Inflammation
The term gut microbiota refers to all the microorganisms, including bacteria, viruses, fungi, archaea, and protozoa that inhabit the human gut and live in a mutualistic and symbiotic relationship with the host [26]. More than 100 trillions of microorganisms form the gut microbiota, the composition of which varies along the gastrointestinal tract and is influenced by genetic and environmental factors, such as early life events (i.e., mode of delivery, breastfeeding), diet, lifestyle, and exposure to drugs[27]. A growing body of evidence highlighted the importance of a balanced gut microbiota in maintaining host’s health given its role in several important functions for the organism [26][28][29][30]. Indeed, the gut microbiota is involved in the metabolism of undigested carbohydrates, producing short chain fatty acids (SCFAs) such as butyrate, propionate, and acetate, which not only are a source of energy for the organism and enterocytes [30][31], but also guarantee the integrity of the intestinal barrier and maintain intestinal motility . Butyrate intervenes in the maintenance of the gut barrier integrity by regulating tight junction proteins, such as claudin-1 and zonula occludens-1 [32]. SCFAs, and especially butyrate, were shown to modulate the immune response, and consequently, liver inflammation [33]. Gut microbiota, and in particular, Lactobacillus, Bifidobacterium, and Enterococcus, convert primary bile acids derived from the liver into secondary bile acids, which exert antimicrobial effects and contribute to the homeostasis of the intestinal epithelial barrier and vascular barrier, through the interaction with the farnesoid X receptor (FXR) [34][35]. In recent decades, increasing attention was paid to the close relationship between the gut microbiota and the liver. This strong bidirectional connection, known as the gut–liver–axis, is realized by the portal vein and the biliary tract; thus, gut-derived metabolites can reach the liver, which, in turn, releases bile acids as well as other mediators back into the intestine [36]. The intestinal barrier, composed of structural elements such as mucus layer, epithelial cells, vascular barrier, immune cells, and soluble mediators, plays a critical role in this interaction, limiting the systemic spread of toxins and pathogenic molecules [37]. Indeed, through the portal vein the liver receives about 70% of its blood supply from the intestine, it is constantly exposed only to small amounts of bacteria and bacterial products [34]. These products, in normal conditions, are eliminated by resident immune cells like Kupffer cells, dendritic cells, natural killer (NK) cells and lymphocytes [35] preventing their systemic spread, thus preserving a condition of immune tolerance [38][39].4. Role of the Gut Microbiota in Hepatic Encephalopathy and Neuroinflammation
A strong interplay was demonstrated between the gut microbiota and the central nervous system. This bidirectional network realizes the gut–brain–axis [25][44]. Different systems act together in this key channel, especially the enteric nervous system, the endocrine, and the immune system [45][46]. Indeed, the gut microbiota influences the function and development of the CNS by modulating signals via the vagus nerve, through the production of hormones and neurotransmitters, and the stimulation of the neuroimmune pathway by cytokines secretion [47]. On the other hand, the CNS uses these pathways to modulate intestinal secretory and immune functions, motility, and barrier permeability [48]. In the setting of liver disease, HE is typically related to gut–liver–brain axis dysfunction. The pathogenesis of HE is still not fully clarified, although high brain ammonia levels were always considered a major etiological factor [49]. Ammonia is a by-product of nitrogen metabolism, principally derived from the metabolic activity of urease-producing bacteria in the gut and the deamination of glutamine by the enzyme glutaminase present in the enterocytes of the small intestine and the colon[4]. Other organs, such as muscles, brain, and kidney, participate to a lesser extent in ammonia metabolism [40]. In normal conditions, ammonia is transported to the liver through the portal vein, where it enters in the urea cycle and is converted into urea, which is subsequently excreted through the kidneys [50]. In case of liver dysfunction, ammonia metabolism is impaired, resulting in a significant increase in serum ammonia [51]. However, it was shown that ammonia levels do not correlate exactly with the severity of HE[52]. This indicates that other factors ranging from intestinal dysbiosis, systemic inflammation, and neuroinflammation intervene in the pathogenesis of HE [53]. Many studies confirmed the role of gut microbiota dysregulation in HE; therefore, most of the therapies used in its treatment act on microbiota modulation [51,53]. The over-abundance of ammonia in HE can be in part explained by an overgrowth of urease-producing bacteria, as demonstrated by the presence of a greater population of urease-producing Proteobacteria in patients with HE and poor cognition [54]. Neuroinflammation was recently suggested to represent another crucial factor in the pathogenesis of neurological impairment in liver disease [55]. Following liver dysfunction, hyperammonemia, circulating bile acids, and systemic inflammation are able to activate microglia, promoting neuroinflammation [7]. Gut microbiota, being one of the main actors in the development of systemic inflammation and in ammonia metabolism, plays a critical role in the pathogenesis of neuroinflammation (Figure 1) [25]. This relation establishes a connection between hepatic inflammation and neuroinflammation. Indeed, gut dysbiosis, SIBO, and intestinal barrier dysfunction lead to increased bacterial translocation and release in circulation of bacterial products, such as LPS, peptidoglycan, flagellin, and bacterial DNA [53][56]. These PAMPs interact with TLR-4 on the membrane of reticuloendothelial cells of the liver, such as Kupffer cells. This interaction in turn favors the activation of NF-kB and MyD88, triggering the release of inflammatory cytokines such as TNF-α, IL-6, and IL-1β by immune cells, leading to systemic inflammation [57][58][59]. This inflammatory process is responsible for blood–brain barrier dysfunction and neuroinflammation.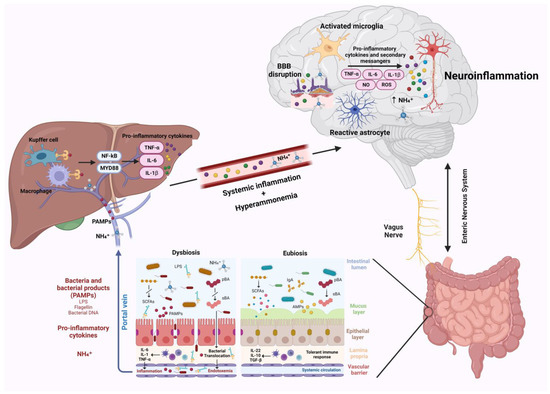
5. Neuroinflammation in Acute Liver Failure and Chronic Liver Disease
6. Intestinal Microbiota Modulation as Treatment Strategy and Emerging Therapies
6.1. Rifaximin
Rifaximin is an eubiotic compound currently approved for the treatment of overt HE [2][70]. Several studies looked at how rifaximin can help the nervous system recover from neuroinflammation. Mangas-Losada et al. administered rifaximin 1200 mg/day for six months to 22 cirrhotic patients with MHE. No significant changes in liver function, hemoglobin, or ammonia serum level were found, while immunological alterations showed a remarkable improvement in responder patients. In particular, pro-inflammatory CD14++CD16+ monocytes decreased in favor of anti-inflammatory CD14++CD16− monocytes; auto-reactive CD4+CD28− T-lymphocytes also decreased, while non-reactive CD4+CD28+ T-lymphocytes increased with the disappearance of CD69, a marker of early activation. Th22 CD4+ subsets and follicular Th diminished, as well as many pro-inflammatory cytokines, and levels of immunoglobulins normalized. Conversely, non-responders showed only a reduction in IL-6, CCL20, and T lymphocytes differentiation to Th22, and did not present increased expression of CD69 before treatment [71]. Other evidence in mice showed that rifaximin reduces neuroinflammation and cognitive impairment through microbiota modulation and promotion of the gut barrier integrity [72]. Furthermore, rifaximin favors the growth of gut bacteria associated with production of SCFAs [73], which are able to cross the BBB and exert anti-inflammatory properties [74].6.2. Lactulose
Lactulose is a nonabsorbable disaccharide approved for the treatment, prevention, and secondary prophylaxis of overt HE [75]. Some evidence showed effectiveness also in MHE and covert HE [2]. Both systemic inflammation and hyperammonemia, which lead to lactate accumulation in the brain, are responsible for microglial activation and neuroinflammation and contribute to HE [76][77]. Studies in both rat models and cirrhotic patients with MHE demonstrate that lactulose administration lowers serum endotoxins and pro-inflammatory cytokines such as TNFα, IL-2, IL-6, IL-13, and IL-18 [78][79][80]. Through its cathartic action and the acidification of the intestinal environment, lactulose also reduces ammonia levels in the blood. Indeed, gut bacteria metabolize lactulose producing SCFAs, such as lactic and acetic acid, which lower colonic pH. An acidic environment decreases the content of urease-producing bacteria and favors the production of non-absorbable ammonium (NH4+), which cannot pass the gut barrier [81].6.3. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
A recent study using rat models of HE with hyperammonemia reported a beneficial effect of the NSAID ibuprofen. A significant improvement in spatial memory and anxiety was registered after treatment with ibuprofen; the combination of ibuprofen and the antioxidant 1,8-cineol also increased the superoxide dismutase activity and significantly reduced oxidative stress [82]. Another pre-clinical study in rat models of HE reported a complete reversal of hypokinesia due to increased extracellular glutamate in substantia nigra pars reticulata (SNr) in rats with portacaval shunts (PCS) treated with ibuprofen 30 mg/kg. At the molecular level, this therapy normalized the amount of glutamate transporters GLT-1 and of excitatory amino acid carrier 1 (EAAC-1) and decreased by 53% extracellular glutamate in SNr of PCS rats [83]. Despite these positive results, NSAID therapy is burdened by unacceptable toxicities, such as renal damage and gastropathy in cirrhotic patients [84][85].6.4. Fecal Microbiota Transplantation
FMT, through its ability to modulate gut microbiota, can potentially reverse all the consequences of gut dysbiosis, such as increased gut barrier permeability, bacterial translocation, and systemic inflammation. Several animal models suggested a beneficial effect of FMT on neuroinflammation. In a rat model of HE induced by the administration of CCl4, FMT was able to improve cognitive functions and HE, improved gut barrier permeability and significantly decreased ammonia serum levels and the expression of TLR4 and TLR9, two important receptors involved in the inflammatory response. Overall, these effects led to a strong reduction in pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, pointing out how FMT could be useful in modulating systemic inflammation and, consequently, neuroinflammation [86].6.5. Probiotics, Prebiotics and Postbiotics
Several studies report the gut microbiota modulatory properties of probiotics, thus they are supposed to help in counteracting the mechanisms of neuroinflammation and HE in patients with liver disease. Probiotics reduce the overgrowth of pathogenic bacteria, maintain the integrity of tight junction proteins strengthening the gut barrier, and decrease intestinal bacteria translocation, with consequent reduction in endotoxemia and systemic inflammation [87][88][89][90][91][92]. In addition, Lactobacillus has the ability to inhibit gut urease-producing bacteria and to acidify intestinal environment with the consequent reduction in serum ammonia levels [93]. A randomized controlled trial involving 120 cirrhotic patients who recovered from an episode of HE proved the superiority of VSL#3, a group of eight probiotics, over placebo in improving Child Turcotte Pugh (CTP) and MELD scores and lowering the rate of HE recurrence and hospitalization[90]. However, the effectiveness of probiotics compared to lactulose is uncertain [94].6.6. Challenges of Proposed Treatments
Rifaximin and lactulose are effective, low-cost drugs characterized by a good tolerability and safety profile [95][96][97]; however, some adverse effects, such as bloating, may reduce quality of life and decrease the adherence to therapy [96]. In addition, in cirrhotic patients, circulating levels of rifaximin, which has usually a negligible absorption, may rise due to increased intestinal permeability, with the risk of altering the safety profile of the drug [98]. Several studies demonstrate the efficacy of FMT as a new treatment of HE. However, there is still no standardization about the route of administration, dosage, or the ideal bacterial consortium to be adopted for the transplant. This is made more difficult by the fact that each donor has a peculiar microbiome, which is complex to analyze [99][100]. Up to date, FMT appears to be a safe treatment, although risks for the potential bacterial dissemination in the bloodstream were reported [101].In conclusion, neuroinflammation appears to be a promising and blooming area of study for the treatment and prevention of HE. The currently available therapeutic strategies appear to be partially effective in modulating neuroinflammation, so it is desirable to identify new effective weapons that are also easily applicable in clinical practice.
References
- 1. Bajaj, J.S. Hepatic Encephalopathy: Classification and Treatment. . J. Hepatol. 2018,, 68, 838–839.
- Montagnese, S.; Rautou, P.-E.; Romero-Gómez, M.; Larsen, F.S.; Shawcross, D.L.; Thabut, D.; Vilstrup, H.; Weissenborn, K. EASL Clinical Practice Guidelines on the Management of Hepatic Encephalopathy. J. Hepatol. 2022, 77, 807–824.
- Amodio, P. Hepatic Encephalopathy: Diagnosis and Management. Liver Int. 2018, 38, 966–975.
- Aldridge, D.R.; Tranah, E.J.; Shawcross, D.L. Pathogenesis of Hepatic Encephalopathy: Role of Ammonia and Systemic Inflammation. J. Clin. Exp. Hepatol. 2015, 5, S7–S20.
- Butterworth, R.F. Hepatic Encephalopathy in Cirrhosis: Pathology and Pathophysiology. . Drugs 2019, 79, 17–21.
- Haj, M.; Rockey, D.C. Ammonia Levels Do Not Guide Clinical Management of Patients with Hepatic Encephalopathy Caused by Cirrhosis. Am. J. Gastroenterol. 2020, 115, 723–728.
- McMillin, M.; DeMorrow, S. Neuroinflammatory Signals during Acute and Chronic Liver Diseases. In Mechanisms of Neuroinflam-mation; Abreu, G.E.A., Ed.; InTech: Houston, TX, USA, 2017.
- Aitbaev, K.A.; Murkamilov, I.T.; Fomin, V.V. Liver Diseases: The Pathogenetic Role of the Gut Microbiome and the Potential of Treatment for Its Modulation. Ter. Arkhiv 2017, 89, 120–128.
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172.
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil Is in the Details. J. Neurochem. 2016, 139, 136–153.
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate Immune Activation in Neurodegenerative Disease. Nat. Rev. Immunol. 2014, 14, 463–477.
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015, 36, 569–577.
- Stamatovic, S.; Keep, R.; Andjelkovic, A. Brain Endothelial Cell-Cell Junctions: How to “Open” the Blood Brain Barrier. Curr. Neuropharmacol. 2008, 6, 179–192.
- Sun, Y.; Koyama, Y.; Shimada, S. Inflammation from Peripheral Organs to the Brain: How Does Systemic Inflammation Cause Neuroinflammation? Front. Aging Neurosci. 2022, 14, 903455.
- Salter, M.W.; Stevens, B. Microglia Emerge as Central Players in Brain Disease. Nat. Med. 2017, 23, 1018–1027.
- Tran, V.T.A.; Lee, L.P.; Cho, H. Neuroinflammation in Neurodegeneration via Microbial Infections. Front. Immunol. 2022, 13, 907804.
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma In Vivo. Science 2005, 308, 1314–1318.
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The Role and Consequences. Neurosci. Res. 2014, 79, 1–12.
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell. Neurosci. 2018, 12, 488.
- Eroglu, C.; Barres, B.A. Regulation of Synaptic Connectivity by Glia. Nature 2010, 468, 223–231.
- Pekny, M.; Pekna, M.; Messing, A.; Steinhäuser, C.; Lee, J.-M.; Parpura, V.; Hol, E.M.; Sofroniew, M.V.; Verkhratsky, A. Astrocytes: A Central Element in Neurological Diseases. Acta Neuropathol. 2016, 131, 323–345.
- Chiareli, R.A.; Carvalho, G.A.; Marques, B.L.; Mota, L.S.; Oliveira-Lima, O.C.; Gomes, R.M.; Birbrair, A.; Gomez, R.S.; Simão, F.; Klempin, F.; et al. The Role of Astrocytes in the Neurorepair Process. Front. Cell Dev. Biol. 2021, 9, 665795.
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622.
- Hoogland, I.C.M.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic Inflammation and Microglial Activation: Systematic Review of Animal Experiments. J. Neuroinflammation 2015, 12, 114.
- Farooq, R.K.; Alamoudi, W.; Alhibshi, A.; Rehman, S.; Sharma, A.R.; Abdulla, F.A. Varied Composition and Underlying Mechanisms of Gut Microbiome in Neuroinflammation. Microorganisms 2022, 10, 705.
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836.
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191.
- Baquero, F.; Nombela, C. The Microbiome as a Human Organ. Clin. Microbiol. Infect. 2012, 18, 2–4.
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904.
- Jandhyala, S.M. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787.
- Mancini, A.; Campagna, F.; Amodio, P.; Tuohy, K.M. Gut:Liver:Brain Axis: The Microbial Challenge in the Hepatic Encephalopathy. Food Funct. 2018, 9, 1373–1388.
- Jiang, L.; Schnabl, B. Gut Microbiota in Liver Disease: What Do We Know and What Do We Not Know? Physiology 2020, 35, 261–274.
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200.
- Fukiya, S.; Arata, M.; Kawashima, H.; Yoshida, D.; Kaneko, M.; Minamida, K.; Watanabe, J.; Ogura, Y.; Uchida, K.; Itoh, K.; et al. Conversion of Cholic Acid and Chenodeoxycholic Acid into Their 7-Oxo Derivatives by Bacteroides intestinalis AM-1 Isolated from Human Feces. FEMS Microbiol. Lett. 2009, 293, 263–270.
- Trebicka, J.; Macnaughtan, J.; Schnabl, B.; Shawcross, D.L.; Bajaj, J.S. The Microbiota in Cirrhosis and Its Role in Hepatic Decompensation. J. Hepatol. 2021, 75, S67–S81.
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836.
- Albillos, A.; de Gottardi, A.; Rescigno, M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72, 558–577.
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379.
- Galland, L. The Gut Microbiome and the Brain. J. Med. Food 2014, 17, 1261–1272.
- Rocco, A.; Sgamato, C.; Compare, D.; Coccoli, P.; Nardone, O.M.; Nardone, G. Gut Microbes and Hepatic Encephalopathy: From the Old Concepts to New Perspectives. Front. Cell Dev. Biol. 2021, 9, 748253.
- Bellot, P.; García-Pagán, J.C.; Francés, R.; Abraldes, J.G.; Navasa, M.; Pérez-Mateo, M.; Such, J.; Bosch, J. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology 2010, 52, 2044–2052.
- Cirera, I.; Martin Bauer, T.; Navasa, M.; Vila, J.; Grande, L.; Taurá, P.; Fuster, J.; García-Valdecasas, J.C.; Lacy, A.; Suárez, M.J.; et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J. Hepatol. 2001, 34, 32–37.
- Muñoz, L.; José Borrero, M.; Ubeda, M.; Lario, M.; Díaz, D.; Francés, R.; Monserrat, J.; Pastor, Ó.; Aguado-Fraile, E.; Such, J.; et al. Interaction between intestinal dendritic cells and bacteria translocated from the gut in rats with cirrhosis. Hepatology 2012, 56, 1861–1869.
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148.
- Foster, J.A.; Neufeld, K.-A.M. Gut–Brain Axis: How the Microbiome Influences Anxiety and Depression. Trends Neurosci. 2013, 36, 305–312.
- El Aidy, S.; Dinan, T.G.; Cryan, J.F. Gut Microbiota: The Conductor in the Orchestra of Immune–Neuroendocrine Communication. Clin. Ther. 2015, 37, 954–967.
- Heiss, C.N.; Olofsson, L.E. The Role of the Gut Microbiota in Development, Function and Disorders of the Central Nervous System and the Enteric Nervous System. J. Neuroendocr. 2019, 31, e12684.
- De Lartigue, G.; de La Serre, C.B.; Raybould, H.E. Vagal Afferent Neurons in High Fat Diet-Induced Obesity; Intestinal Microflora, Gut Inflammation and Cholecystokinin. Physiol. Behav. 2011, 105, 100–105.
- Amodio, P. Hepatic Encephalopathy: Historical Remarks. J. Clin. Exp. Hepatol. 2015, 5, S4–S6.
- Rose, C.F.; Amodio, P.; Bajaj, J.S.; Dhiman, R.K.; Montagnese, S.; Taylor-Robinson, S.D.; Vilstrup, H.; Jalan, R. Hepatic En- cephalopathy: Novel Insights into Classification, Pathophysiology and Therapy. J. Hepatol. 2020, 73, 1526–1547.
- Campion, D.; Giovo, I.; Ponzo, P.; Saracco, G.M.; Balzola, F.; Alessandria, C. Dietary Approach and Gut Microbiota Modulation for Chronic Hepatic Encephalopathy in Cirrhosis. World J. Hepatol. 2019, 11, 489–512.
- Shawcross, D.L.; Sharifi, Y.; Canavan, J.B.; Yeoman, A.D.; Abeles, R.D.; Taylor, N.J.; Auzinger, G.; Bernal, W.; Wendon, J.A. Infection and Systemic Inflammation, Not Ammonia, Are Associated with Grade 3/4 Hepatic Encephalopathy, but Not Mortality in Cirrhosis. J. Hepatol. 2011, 54, 640–649.
- Rai, R.; Saraswat, V.A.; Dhiman, R.K. Gut Microbiota: Its Role in Hepatic Encephalopathy. J. Clin. Exp. Hepatol. 2015, 5, S29–S36.
- Hassouneh, R.; Bajaj, J.S. Gut Microbiota Modulation and Fecal Transplantation: An Overview on Innovative Strategies for Hepatic Encephalopathy Treatment. J. Clin. Med. 2021, 10, 330.
- Chen, Z.; Ruan, J.; Li, D.; Wang, M.; Han, Z.; Qiu, W.; Wu, G. The Role of Intestinal Bacteria and Gut–Brain Axis in Hepatic Encephalopathy. Front. Cell. Infect. Microbiol. 2021, 10, 595759.
- Bajaj, J.S. The Role of Microbiota in Hepatic Encephalopathy. Gut Microbes 2014, 5, 397–403.
- Kawai, T.; Akira, S. Signaling to NF-KB by Toll-like Receptors. Trends Mol. Med. 2007, 13, 460–469.
- Seo, Y.S.; Shah, V.H. The Role of Gut-Liver Axis in the Pathogenesis of Liver Cirrhosis and Portal Hypertension. Clin. Mol. Hepatol. 2012, 18, 337.
- Seki, E.; De Minicis, S.; Österreicher, C.H.; Kluwe, J.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. TLR4 Enhances TGF-β Signaling and Hepatic Fibrosis. Nat. Med. 2007, 13, 1324–1332.
- Labrenz, F.; Ferri, F.; Wrede, K.; Forsting, M.; Schedlowski, M.; Engler, H.; Elsenbruch, S.; Benson, S.; Costantini, M. Altered Temporal Variance and Functional Connectivity of BOLD Signal Is Associated with State Anxiety during Acute Systemic Inflammation. NeuroImage 2019, 184, 916–924.
- D’Mello, C.; Le, T.; Swain, M.G. Cerebral Microglia Recruit Monocytes into the Brain in Response to Tumor Necrosis Factorα Signaling during Peripheral Organ Inflammation. J. Neurosci. 2009, 29, 2089–2102.
- Chastre, A.; Bélanger, M.; Beauchesne, E.; Nguyen, B.N.; Desjardins, P.; Butterworth, R.F. Inflammatory Cascades Driven by Tumor Necrosis Factor-Alpha Play a Major Role in the Progression of Acute Liver Failure and Its Neurological Complications. PLoS ONE 2012, 7, e49670.
- Frontera, J.A.; Kalb, T. Neurological Management of Fulminant Hepatic Failure. Neurocrit. Care 2011, 14, 318–327.
- Butterworth, R.F. Neuroinflammation in Acute Liver Failure: Mechanisms and Novel Therapeutic Targets. Neurochem. Int. 2011, 59, 830–836.
- Jaeger, V.; DeMorrow, S.; McMillin, M. The Direct Contribution of Astrocytes and Microglia to the Pathogenesis of Hepatic Encephalopathy. J. Clin. Transl. Hepatol. 2019, 7, 352.
- Butterworth, R.F. Pathogenesis of Hepatic Encephalopathy and Brain Edema in Acute Liver Failure. J. Clin. Exp. Hepatol. 2015, 5, S96–S103.
- McMillin, M.; Grant, S.; Frampton, G.; Petrescu, A.D.; Williams, E.; Jefferson, B.; Thomas, A.; Brahmaroutu, A.; DeMorrow, S. Ele- vated Circulating TGFβ1 during Acute Liver Failure Activates TGFβR2 on Cortical Neurons and Exacerbates Neuroinflammation and Hepatic Encephalopathy in Mice. J. Neuroinflammation 2019, 16, 69.
- Cauli, O .; Rodrigo, R.; Piedrafita ,B .; Boix, J.; Felipo, V. Inflammation and Hepatic Encephalopathy: Ibuprofen Restores Learnig Ability in Rats with Portacaval Shunts. Hepatology 2007, 46, 514–519.
- Cagnin , A.In Vivo Imaging of Cerebral “Peripheral Benzodiazepine Binding Sites” in Patients with Hepatic Encephalopathy. Gut 2006, 55, 547–553.
- Ponziani, F.R.; Zocco, M.A.; D’Aversa, F.; Pompili, M.; Gasbarrini, A. Eubiotic Properties of Rifaximin: Disruption of the Traditional Concepts in Gut Microbiota Modulation. World J. Gastroenterol. 2017, 23, 4491.
- Mangas-Losada, A.; García-García, R.;Leone, P.; Ballester, M.P.; Cabrera-Pastor, A.; Urios, A.; Gallego, J.-J.; Martínez-Pretel, J.-J.; Giménez-Garzó, C.; Revert, F.; et al. Selective Improvement by Rifaximin of Changes in the Immunophenotype in Patients Who Improve Minimal Hepatic Encephalopathy. J. Transl. Med. 2019, 17, 293.
- Meng, D.; Yang, M.; Hu, L.; Liu, T.; Zhang, H.; Sun, X.; Wang, X.; Chen, Y.; Jin, Y.; Liu, R. Rifaximin Protects against Circa- dian Rhythm Disruption–Induced Cognitive Impairment through Preventing Gut Barrier Damage and Neuroinflammation. J. Neurochem. 2022, 163, 406–418.
- Li, H.; Xiang, Y.; Zhu, Z.; Wang, W.; Jiang, Z.; Zhao, M.; Cheng, S.; Pan, F.; Liu, D.; Ho, R.C.M. et al. Rifaximin-Mediated Gut Microbiota Regulation Modulates the Function of Microglia and Protects against CUMS-Induced Depression-like Behaviors in Adolescent Rat. J. Neuroinflammation 2021, 18, 254.
- Dalile, B.; VanOudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478.
- Vilstrup, H.; Amodio, P.; Bajaj,J .; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic Encephalopathy in Chronic Liver Disease: 2014 Practice Guideline by the American Association for the Study Of Liver Diseases and the European Association for the Study of the Liver: Vilstrup et Al. Hepatology 2014, 60, 715 735.
- Rodrigo, R.; Cauli, O.; Gomez-Pinedo, U.; Agusti, A.; Hernandez-Rabaza, V.; Garcia-Verdugo, J.; Felipo, V. Hyperammonemia Induces Neuroinflammation That Contributes to Cognitive Impairment in Rats with Hepatic Encephalopathy. Gastroenterology 2010, 139, 675–684.
- Andersson, A. K.; Rönnbäck, L.; Hansson, E. Lactate Induces Tumour Necrosis Factor-α, Interleukin-6 and Interleukin-1β Release in Microglial and Astroglial-Enriched Primary Cultures: Lactate Induces Glial Cell Cytokine Release. J. Neurochem. 2005, 93, 1327–1333.
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M. Linkage of Gut Microbiome with Cognition in Hepatic Encephalopathy. Am. J. Physiol.—Gastrointest. Liver Physiol. 2012, 302, G168–G175.
- Jia, L. Comparison of Probiotics and Lactulose in the Treatment of Minimal Hepatic Encephalopathy in Rats. WorldJ. Gastroenterol. 2005, 11, 908.
- Jain, L.; Sharma, B.C.; Srivastava, S.; Puri, S.K.; Sharma, P.; Sarin, S. Serum Endotoxin, Inflammatory Mediators, and Magnetic Resonance Spectroscopy before and after Treatment in Patients with Minimal Hepatic Encephalopathy: Inflammatory Mediators in Minimal Hepatic Encephalopathy. J. Gastroenterol. Hepatol. 2013, 28, 1187–1193.
- Elwir, S.; Rahimi, R.S. Hepatic Encephalopathy: An Update on the Pathophysiology and Therapeutic Options .J. Clin. Transl. Hepatol. 2017, 5, 142.
- Bahrami, T.; Yaghmaei, P.; Yousofvand, N. The Effects of Ibuprofen and 1,8 Cineol on Anxiety and Spatial Memory in Hyperammonemic Rats. Metab. Brain Dis. 2023, 38, 613–620.
- Cauli, O.; Rodrigo, R.; Piedrafita, B.; Llansola, M.; Mansouri, M.T.; Felipo, V. Neuroinflammation Contributes to Hypokinesia in Rats with Hepatic Encephalopathy: Ibuprofen Restores Its Motor Activity. J. Neurosci. Res. 2009, 87, 1369–1374.
- Ackerman, Z.; Cominelli, F.; Reynolds, T.B. Effect of Misoprostol on Ibuprofen-Induced Renal Dysfunction in Patients with Decompensated Cirrhosis: Results of a Double-Blind Placebo-Controlled Parallel Group Study. Am. J. Gastroenterol. 2002, 97, 2033–2039.
- Hawkey, C.J. Non-Steroidal Anti-Inflammatory Drug Gastropathy: Causes and Treatment. Scand. J. Gastroenterol .1996,31(Suppl. S220), 124–127.
- Wang, W.-W.; Zhang, Y.;Huang, X.B.; You, N.; Zheng, L.; Li, J. Fecal Microbiota Transplantation Prevents Hepatic Encephalopathy in Rats with Carbon Tetrachloride-Induced Acute Hepatic Dysfunction. World J. Gastroenterol. 2017, 23, 6983–6994.
- Mennigen, R.; Nolte, K.; Rijcken, E.; Utech, M.; Loeffler, B.; Senninger, N.; Bruewer, M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am. J. Physiol.—Gastrointest. Liver Physiol. 2009, 296, G1140–G1149.
- Albillos, A.; de la Hera, A. Multifactorial gut barrier failure in cirrhosis and bacterial translocation: Working out the role of probiotics and antioxidants. J. Hepatol. 2002, 37, 523–526.
- Ukena, S.N.; Singh, A.; Dringenberg, U.; Engelhardt, R.; Seidler, U.; Hansen, W.; Bleich, A.; Bruder, D.; Franzke, A.; Rogler, G.; et al. Probiotic Escherichia coli Nissle 1917 Inhibits Leaky Gut by Enhancing Mucosal Integrity. PLoS ONE 2007, 2, e1308.
- Dhiman, R.K.; Rana, B.; Agrawal, S.; Garg, A.; Chopra, M.; Thumburu, K.K.; Khattri, A.; Malhotra, S.; Duseja, A.; Chawla,Y.K. Probiotic VSL#3 Reduces Liver Disease Severity and Hospitalization in Patients with Cirrhosis: A Randomized, Controlled Trial. Gastroenterology 2014, 147, 1327–1337.e3.
- Stadlbauer, V.; Mookerjee, R.P.; Hodges, S.; Wright, G.A.K.; Davies, N.A .; Jalan, R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J. Hepatol. 2008, 48, 945–951.
- Horvath, A.; Leber, B.; Schmerboeck, B.; Tawdrous, M.; Zettel, G.; Hartl, A.; Madl, T.; Stryeck, S.; Fuchs, D.; Lemesch, S.;et al. Randomised clinical trial: The effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment. Pharmacol. Ther. 2016, 44, 926–935.
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; Puri, P.; Sterling, R.K.; Luketic, V.; Stravitz, R.T.; Siddiqui, M.S.; Fuchs, M.; et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment. Pharmacol. Ther. 2014, 39, 1113–1125.
- Cao, Q.; Yu, C.-B.; Yang,S.-G.;Cao, H.-C.; Chen, P.; Deng, M.; Li,L.-J. Effect of probiotic treatment on cirrhotic patients with minimal hepatic encephalopathy: A meta-analysis. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 9–16.
- Ponziani, F.R. Effect of rifaximin on gut microbiota composition in advanced liver disease and its complications. World J. Gastroenterol. 2015, 21, 12322.
- Hudson, M.; Schuchmann, M. Long-term management of hepatic encephalopathy with lactulose and/or rifaximin: A review of the evidence. Eur. J. Gastroenterol. Hepatol. 2019, 31, 434–450.
- Montagnese, S.; Russo, F.P.; Amodio, P.; Burra, P.; Gasbarrini, A.; Loguercio, C.; Marchesini, G.; Merli, M.; Ponziani, F.R.; Riggio, O.; et al. Hepatic encephalopathy 2018: A clinical practice guideline by the Italian Association for the Study of the Liver (AISF). Dig. Liver Dis. 2019, 51, 190–205.
- Bajaj, J.S.; Riggio, O. Drug therapy: Rifaximin1. Hepatology 2010, 52, 1484–1488.
- Sbahi, H.; Di Palma, J.A. Faecal microbiota transplantation: Applications and limitations in treating gastrointestinal disorders. BMJ Open Gastroenterol. 2016, 3, e000087.
- Shi, Y.-C.; Yang,Y.S. Fecal microbiota transplantation: Current status and challenges in China: FMT in China. JGH Open 2018, 2, 114–116.
- De Filipp, Z.; Bloom, P.P.; Soto, M.T.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen,Y.-B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050.
