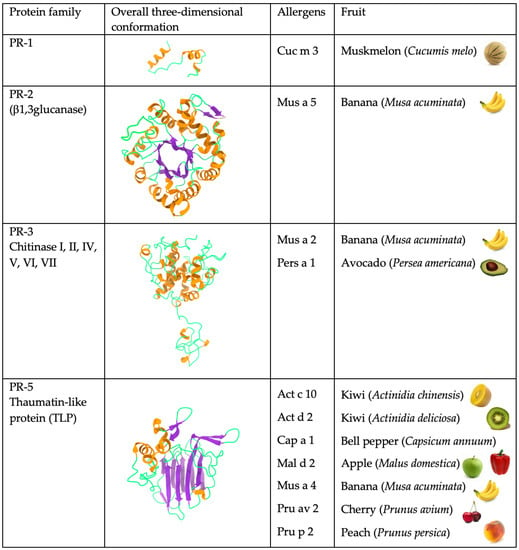
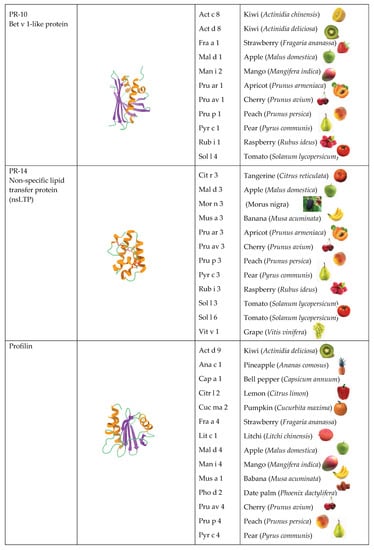
Most of the allergenic proteins from fruits identified belong to different families of pathogenesis-related (PR) proteins. These PR proteins have been classified in different families of structurally and functionally unrelated proteins, but the majority of all fruit allergens belong to three groups, in particular PR-5 thaumatin-like proteins (TLP), PR-10 Bet v 1-like proteins, and PR-14 non-specific lipid transfer proteins (nsTLP). The cross-reactivities between fruit allergens and homologous proteins from other vegetable, seed, and pollen sources account for different food-pollen syndromes. The allergens responsible for these food-pollen syndromes essentially consist of the pan-allergens nsLTPs, profilins, GRPs, β-1,3-glucanases, but also the seed storage proteins.
1. Introduction
Allergies to edible fruits have grown dramatically during the last years, due to increased fruit consumption and the introduction of many exotic fruits into people's eating habits. Edible fruits, especially fleshy fruits, contain allergenic proteins that are responsible for various allergic manifestations, ranging from a simple oral syndrome (OAS) to a more severe anaphylactic shock. In this respect, fruits from the Rosaceae family, and kiwi fruits from the Actinidiaceae family, have become a worrying source of food allergies largely distributed in many countries.
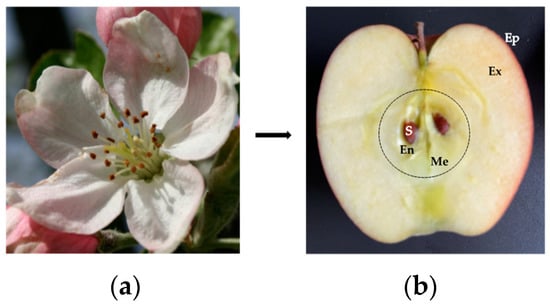
Botanically, fruits are derived from the transformation and development of the ovary from flower parts after pollination. Depending on the fruits, the transport of the pollen to the pistil is either self-pollinated, or carried out by insects (entomophilous plants) or wind (anemophilous plants). In fleshy fruits from the Rosaceae family, the edible part of the fruit consists of the mesocarp and the exocarp, which develop from the ripened ovary or carpels and the floral envelopes, respectively. The exocarp is limited by the skin or epicarp. The seeds, derived from the pollinated ovules, are located in the endocarp (Figure 1). In many fruits from the Rosaceae family, such as peach (Prunus persica), apricot (Prunus armeniaca), or plum (Prunus dulcis), the mesocarp and endocarp are sclerified and form a kernel. A similar organization occur in other fruits from the Solanaceae (tomato), Cucurbitaceae (pumpkin), Musaceae (banana) or Lythraceae (pomegranate) families.
Figure 1. (a) Apple flower showing the central pistil surrounded by the stamens. (b) Cut-section of the fruit showing the different parts of the apple fruit including the epicarp (Ep), the exocarp (Ex), the mesocarp (M) delineated by a black dashed line, and the sclerified endocarp (En) containing the seeds (S).
Besides the commonly consumed fleshy fruits, e.g., fruits from the Rosaceae family, a large diversity of edible fruits has been described, including the caryopses from Poaceae (maize, wheat, rice) which are, however, essentially made up of the seeds and are therefore considered more like seeds, or false fruits like strawberries (
Fragaria vesca), resulting from the development of the calyx accrescent from the flowers covered with an aggregate of achenes containing tiny seeds. Accordingly, defining edible fruits is not an easy task because, in common language, some genuine fruits (tomato, bell pepper, cucumber) are often considered as vegetables and, vice versa, some so-called fruits (rhubarb stalks) readily correspond to genuine vegetables
[1].
Most of the allergenic proteins from fruits identified so far belong to different families of PR-protein
[2]. PR proteins correspond to a disparate set of proteins, which differ in their structures and properties, and are all involved in various processes of plant defense against phytopathogens and, more generally, in all the stress situations that plants have to face. PR proteins have been classified in seventeen distinct families of structurally and functionally unrelated proteins
[3], but the majority of all fruit allergens belong to three families, in particular PR-5 thaumatin-like proteins (TLP), PR-10 Bet v 1-like proteins, and PR-14 non-specific lipid transfer proteins (nsTLP) (
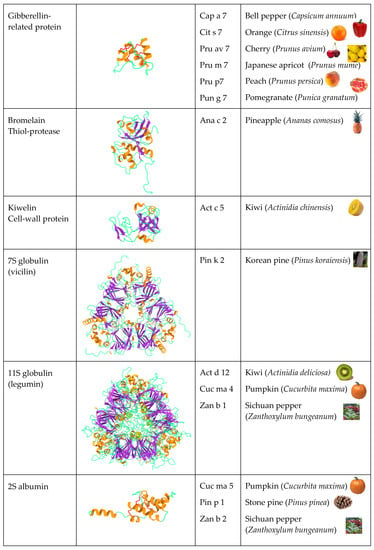
Figure 2). Other PR-protein families, such as PR-2 β1,3-glucanase proteins, PR-3 chitinases I, II, IV-VII, and PR-8 chitinases III, also contain some allergenic proteins from fruits. However, other important fruit allergens are not related to the PR-protein families, such as the profilins and the newly emerging group of gibberellin-regulated proteins (GBR-protein). Although not restricted to fruits, the small knottin-folded defensins occur as food allergens susceptible to cross-reaction with their well-identified pollen allergen counterparts. In addition, a few allergens, including Cuc m 3 of the PR-1 family, bromelain (Ana c 2) and kiwelin (Act c 5), belong to a single fruit or a restricted number of fruits. Finally, proteins that belong to the three classes of seed storage proteins from higher plants, 2S albumins, 7S globulins (vicilin), and 11S globulins (legumin)
[4], should also be retained as possible potential fruit allergens resulting from the unintended consumption of the seeds of kernels
[5]. Many of these fruit allergens represent widely distributed pan-allergens, which are responsible for IgE-binding cross-reactivity often associated with some cross-allergenicity.
Figure 2.
Overview of the different structural scaffolds and distributions of the different families of fruit allergens.
2. Cross-Reactivity of Fruit Allergens
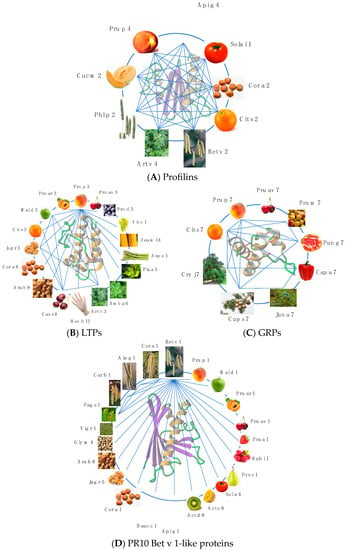
Most of the fruit allergens consist of pan-allergens widely distributed in different plant organs, which are thus responsible for cross-reactivities associated or not to cross-allergenicities between fruits, vegetables, and pollens. All these fruit allergens exhibit closely related amino acid sequences and quasi-identical folds. Fruit and pollen profilins display a high degree of cross-reactivity (
Figure 3A)
[6][43]. Depending on the relatedness between their amino acid sequences, their overall fold, and their IgE-binding epitope community, nsLTP allergens from Rosaceae fruits, especially Pru p 3, display pronounced cross-reactivities with other nsLTPs from vegetable, seed and pollen sources (
Figure 3B)
[7][8][9][11,15,27]. Due to the widespread distribution of Bet v 1-like proteins closely related to the white birch pollen Bet v 1 allergen in various tree pollens, vegetables, and seeds, extensive IgE-binding cross-reactivity between the pollen of various trees and foods are frequently observed (
Figure 3D)
[10][19]. Although less investigated as compared to profilins and PR10 Bet v 1-like proteins, the recently identified GRP allergens fall apparently into the same category of highly cross-reactive allergens (
Figure 3C).
Figure 3. (A). Cross-reactivity between profilins from fruit (Cit s 2, Cuc m 2, Pru p 4, Sola l 1), vegetable (Api g 4), seed (Cor a 2), and pollen (Art v 4, Bet v 2, Phl p 2) sources. (B). Cross-reactivity between LTPs from fruit (Cit s 3, Mal d 3, Pru ar 3, Pru av 3, Pru d 4, Pru p 3, Vit v 1), vegetable (Asp o 1), seed (Ara h 9, Cas s 8, Cor a 1, Jug r 3, Zea m 14), pollen (Amb a 6, Art v 3, Pla a 3), and latex (Hev b 12) sources. (C). Cross-reactivity between GRPs from fruit (Cap a 7, Cit s 7, Pru av 7, Pru m 7, Pru p 7, Pun g 7) and pollen (Cry j 7, Cup s 7, Jun a 7) sources. (D). Cross-reactivity between PR10 bet v 1-like proteins from fruit (Act c 8, Act d 8, Fra a 1, Mal d 1, Pru ar 1, Pru av 1, Pru p 1, Pyr c 1, Rub I 1, Sol a 4), vegetable (Api g 1, Dauc c 1), seed (Ara h 8, Cor a 1, Gly m 4, Jug r 5, Vig r 1), and pollen (Aln g 1, Bet v 1, Carb 1, Cor a 1, Fag s 1) sources. IgE-binding cross-reactivities are indicated by blue lines.
The cross-reactivities between fruit allergens and homologous proteins from other vegetable, seed, and pollen sources account for different food-pollen syndromes. The allergens responsible for these food-pollen syndromes essentially consist of the pan-allergens nsLTPs, profilins, GRPs, β-1,3-glucanases, but also the seed storage proteins
[4]. Most often these pollen-food syndromes result in a benign oral allergy syndrome (OAS) but can, less frequently, lead to a more severe systemic reaction, especially with the peach nsLTP Pru p 3
[9][11][27,75].
However, it should be noted that many IgE-binding cross-reactivities measured in vitro by IgE-binding activity tests, inhibition tests of the IgE-binding activity and, more rarely, by the direct activation of previously sensitized basophil cells (BAT), have apparently no clinical significance, and the consumption of fruit or vegetable allergens does not induce any reaction in sensitized individuals
[11][75].