Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Peter I. Lelkes and Version 3 by Dean Liu.
Electrical Stimulation (ES) bioreactors are defined here as any bioreactor capable of providing some type of ES to cells and organoids cultured within the device. Although there are several approaches to providing ES to cells, such as capacitive coupling and inductive stimulation, the most common method is direct stimulation. Here we describe the benefits of electrical stimulation for organoid culture.
- differentiation
- biomimetic
- stem cell
- pluripotent
- embryoid body
1. Organoid Electrical Stimulation
The application of ES aids in the differentiation and maturation of organoids of excitable tissues. For example, stem cell-derived cardiomyocytes cannot fully mature under standard in vitro culture conditions as they lack the enhanced morphology, electrophysiology, calcium handling, contractility, and metabolism seen in mature cardiomyocytes in vivo [1][84]. Electrical stimulation is one method that has been used to enhance cardiomyocyte maturation in 3D organoids. Using cardiac embryoid bodies/organoids, one study used a custom bioreactor consisting of carbon rods embedded into the side of PDMS (polydimethylsiloxane) wells (see Figure 1A) [2][85]. The organoids were subjected to electrical stimulation on days 23–30 of differentiation using a 5 V/cm amplitude and 2 ms pulses at a frequency of either 0.5, 1, or 2 Hz. Overall, ES led to an increase in cardiac gene expression and cell–cell connection, as can be seen in the data summarized in Figure 1. In addition, as the frequency increased, the authors reported an adaptation to beating at higher frequencies. For 2 Hz, after stopping ES, the organoids continued spontaneous contractions at or close to 2 Hz, whereas non-stimulated organoids exhibited a slower spontaneous contraction. Furthermore, conditioning organoids at 2 Hz allowed them to survive 3 Hz stimulation, whereas non-conditioned organoids saw reduced survival at 3 Hz ES. In another study, increasing the stimulation frequency from 1 Hz to 2 Hz led to increases in cardiac troponin expression and sarcomere organization in cardiac organoids formed from hiPSC-derived cardiomyocytes [3][86].
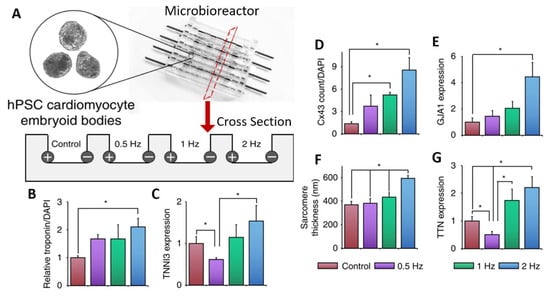
Figure 1. PDMS microbioreactor for electrical stimulation. (A) Schematic of microbioreactor set-up showing carbon rod electrodes embedded in PDMS. A cross-sectional view shows electrodes at the edges of PDMS wells with room for organoids in the center. Cells were subject to ES for 7 days at specified frequencies. (B–G) Quantification of various markers for cardiac differentiation and maturation measured by immunostaining for Troponin T (B), Connexin43 (D), sarcomere count (F), qPCR for Troponin I (C), Gap Junction Protein Alpha 1 (E), or Titin (G). All error bars show the standard error of the mean (SEM), * p < 0.05. Adapted with permission from Eng et al. [2][85].
While these studies sought to determine whether ES would enhance the maturation of cardiac organoids, ES has also been used to induce early-stage cardiac differentiation. For example, Ma et al. used an IonOptix C-Pace commercial stimulation system [4][87] to initiate ES after seven days of organoid formation and observed beating cardiac organoids in as little as two days after ES initiation (compared to 7 days without ES) [5][88]. After 2 weeks of culture with ES, the spontaneous flux of calcium ions was increased compared to the control group, as observed using an engineered GCaMP calcium indicator under the control of a cardiac troponin reporter. With ES, the proportion of cells expressing cardiac troponin and Nkx2-5 cells was increased, and many genes for ion channels and cardiac transcription were upregulated, as shown via the use of RNA-seq analysis. The authors concluded that electrical stimulation alone, i.e., without the use of any external chemical signaling, could improve early cardiac differentiation.
ES bioreactors have also been used to enhance the culture of neural organoids. Aiming to stimulate cortical brain organoids cultured from primary mouse cortical neurons, Zhang et al. designed a system of interdigitated electrodes, as seen in Figure 2 [6][89]. This system was used to apply ES to both wild-type(WT) and neuregulin 1 knock-out (NRG1-KO) neural organoids. A comparison was made between the WT and NRG1-KO lines with and without electrical stimulation. Exposure to ES led to an improvement in the number and length of neurites and increased the expression of markers for synaptogenesis, including NCAM and PSD95, in the NRG1-KO organoids, showing the ability of ES to rescue the phenotype and promote the development of dysfunctional neurons (see Figure 2 for more details). Sefton et al. studied the effects of electrical stimulation on the formation and differentiation of neurospheres made from mouse neural precursor cells [7][90]. Using electrodes made from platinum wires, they found that in vitro stimulation increased both cell survival and the size of neurospheres formed. Furthermore, electrical stimulation led to an increased fraction of cells displaying neuronal and oligodendrocyte markers, while the fraction of cells exhibiting astrocytic markers declined. This suggests that electrical stimulation may influence the fate determination of neural cells in their early stages.
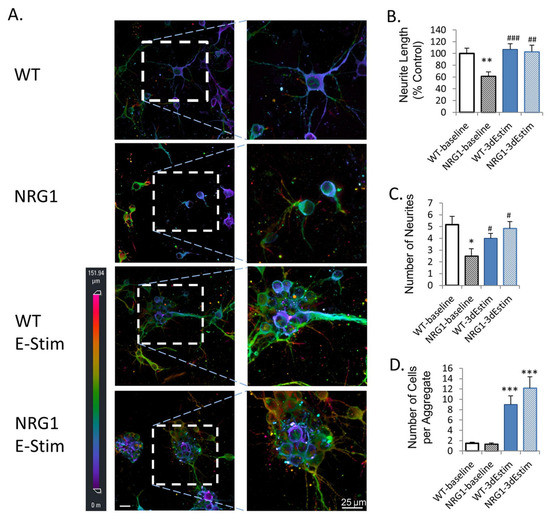
Figure 2. Effects of ES on primary neural organoids. (A) MAP2 immunofluorescence imaged using 3D confocal imaging. Conditions are WT (wild type), NRG1 (neuregulin 1 knock-out), WT ES, and NRG1 with ES. All cells are primary mouse prefrontal cortex neurons. (B) Quantified neurite length. (C) Number of neurons. (D) Number of cells per aggregate. * p < 0.05 vs. wild-type, baseline; ** p < 0.01 vs. wild-type, baseline; *** p < 0.001 vs. wild-type, baseline; # p < 0.05 vs. NRG1-KO, baseline; ## p < 0.01 vs. NRG1-KO, baseline; ### p < 0.001 vs. NRG1-KO, baseline. Error bars indicate SEM. Reproduced with permission from Zhang et al. [6][89].
2. Commercial vs. Custom-Made Systems
Multiple commercial devices have been used for the electrical stimulation of cells and organoids; to the best of our knowledge, the most common system is the Culture Pacing System (C-Pace) by IonOptix [4][87]. This system includes a series of custom tissue culture plates utilizing carbon electrodes that extend from the plate lids down to the culture surface. This also includes the signal generator for creating the ES pulses, which have been used to provide ES to the custom electrodes. Specifically, the IonOptix system has been used in numerous studies using ES culture organoids [3][5][8][9][10][86,88,91,92,93] due to its ease of use as an out-of-the-box solution for providing ES to cell/organoid cultures. However, some researchers prefer to develop fully custom ES setups, some of which were mentioned previously in this paper. Reasons for this may include the ability to further customize the system, including the use of different electrode materials [11][94], different culture vessels [12][95], and more specific control over the stimulation parameters [13][96]. In addition, custom-designed systems may allow for the incorporation of features not found in commercial systems, such as the addition of media perfusion [14][97] or the combination of electrical and mechanical stimulation [15][98]. Another factor is cost, as many custom-made systems can be made in-house for relatively little money. As discussed above, the most basic ES systems mainly involve only electrodes being inserted into tissue culture plates, which may be accomplished for a very low cost.
Table 1. Selected studies utilizing electrically stimulated bioreactors for organoid culture. hiPSC: human induced pluripotent stem cells; mESC: mouse embryonic stem cells; hESC: human embryonic stem cells.
| Reference | Cell Source | Goal | Bioreactor Setup | Outcomes |
|---|---|---|---|---|
| Eng et al. [2][85]. | hESCs and hiPSC. | Cardiac organoid differentiation/maturation. | Custom bioreactor with carbon rods in PDMS microchambers. | An increase in frequency leads to increased cardiac gene expression and better beating adaptation. |
| Yoshida et al. [3][86]. | hiPSC-CMs. | Cardiac organoid maturation. | Organic carbon electrodes in poly(vinyl) alcohol hydrogel chambers. | ES leads to enhanced cardiac troponin expression and sarcomere formation. |
| Ma et al. [5][88]. | hiPSCs with GCaMP reporter. | Early induction of cardiomyocyte differentiation. | IonOptix C-Pace system. | Organoid contractions were seen after 2 days with ES compared to 7 days without ES. |
| Zhang et al. [6][89]. | Primary mouse cortical neurons. | Enhanced Neural Organoid. | 3D interdigitated electrodes coated with polypyrrole. | ES of organoids leads to enhanced neurite outgrowth and synaptogenesis and rescues the phenotype of NRG1-KO neurons. |
| Sefton et al. [7][90]. | Primary neural precursor cells. | Formation and differentiation of neural organoid. | Agarose salt bridge stimulation. | ES increases survival and spheroid size. It also increases neuronal expression while reducing astrocyte expression. |
| Ahadian et al. [9][92]. | mESCs. | Cardiac organoid differentiation. | IonOptix C-Pace system. | ES enhanced cardiac gene expression and the beating area of organoids. |
