Sphingomyelin (SM) and its metabolites are crucial regulators of tumor cell growth, differentiation, senescence, and programmed cell death. With the rise in lipid-based nanomaterials, engineered lipidic nanomaterials inspired by SM metabolism, corresponding lipid targeting, and signaling activation have made fascinating advances in cancer therapeutic processes.
1. Introduction
Human mortality is becoming increasingly dominated by cancer, which has long posed a significant public health concern
[1]. According to the latest estimates by the World Health Organization (WHO)’s International Agency for Research on Cancer (IARC), the number of new cancer cases reported worldwide have risen to 19.29 million, with 9.96 million deaths reported by the year of 2020
[2]. For decades, scientists have been seeking effective cancer therapeutic targets and developing innovative cancer treatment strategies to defeat cancer. Over the past few decades, combining nanotechnology, materials science, and biotechnology has accelerated the development of the innovative nanomedicine field for cancer treatment
[3]. Due to the unique appeal of nanomaterials for drug delivery, cancer diagnosis and imaging, and immune vaccine development, interest in applying nanotechnology to cancer treatment has grown, and a considerable level of technical success has been achieved
[4][5][4,5]. Notable examples include small superparamagnetic iron oxide nanoparticles, which have been widely used as magnetic resonance imaging (MRI) contrast agents, and the successful introduction of the lipid nanoparticle COVID-19 mRNA vaccine
[6][7][6,7]. Lipidic nanomaterials are nanoparticles consisting of lipid-like substances, with their diameters typically being between 10–200 nm
[8]. As one of the most popular platforms for cancer treatment, they have demonstrated tremendous therapeutic potential across a wide range of treatment modalities. The natural origin of lipids ensures an ultra-high biocompatibility and degradability, making them superior to other materials like polymers due to their low probability of causing lipid-induced toxicity
[8]. Moreover, they also exhibit attractive multifunctional properties, including an encapsulation capability for either hydrophilic or hydrophobic therapeutics, as well as surface properties, such as charge and targeting ligand modifications, that can be easily modified by altering the lipid composition or by attaching antibodies
[9][10][9,10]. This flexibility allows for precise drug delivery and targeted therapy, making lipidic nanomaterials promising candidates for innovative cancer therapeutic strategies.
The emergence of lipidomic and lipid quantification in recent years has provided large-scale qualitative and quantitative studies of lipid compounds in organisms, providing insights into the functions and changes of lipid compounds under physiopathological conditions, and has also significantly contributed to the development of lipidic nanomaterials
[11][12][13][11,12,13]. There is growing evidence in that when SM levels are expressed abnormally, it increases the cancer risk
[14][15][14,15]. Among them, biological studies on the metabolism and function of sphingomyelin (SM) have revealed that effector molecules in the SM metabolic pathway contribute significantly to cell signaling, especially in regulating tumor cell growth, differentiation, senescence, and survival
[16][17][18][16,17,18]. SM is integral for the membrane, and is an essential structural molecule in the cell membrane. Related enzymes, such as sphingomyelinase (SMase) and sphingomyelin synthase (SMS), strongly regulate its abundance
[19]. A critical role for SM content and metabolic enzyme activity in signaling pathway transduction and therapy must be considered. Furthermore, SM metabolites have diverse biological functions across various cancerous processes
[20]. For example, ceramide (Cer), produced by the SMase-mediated hydrolysis of SM, is closely associated with cell death, senescence, and cellular arrest
[21][22][21,22]. Upon further metabolism, Cer can be converted to a pro-survival metabolite called sphingosine-1-phosphate (S1P), which is an anti-apoptotic molecule
[23][24][23,24]. Thus, the dynamic transformation processes associated with SM metabolism are critical for mediating the fate of cancer cells.
Currently, an increasing number of investigators are focusing on the design of lipidic nanomaterials based on SM metabolism, initiating preclinical trials for different types of tumors to evaluate the potential application of therapeutic strategies utilizing SM, Cer, and S1P
[25][26][25,26].
2. Synthesis and Metabolism of SM
2.1. Structure and Distribution of SM
In the 1880s, Johann Ludwig Wilhelm Thudichum
[27] discovered and named the first sphingolipid SM, which is a phospholipid that is mainly found in vertebrates, accounting for 2% to 15% of total phospholipids, respectively
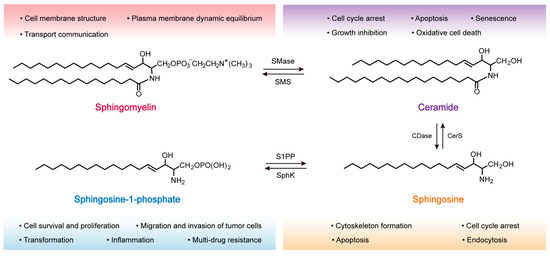
[27]. Each molecule of SM is composed of a sphingosine (Sph) molecule, a fatty acid, and phosphorylcholine polar head group, and is characterized by a hydrophilic head and a hydrophobic tail made of two fatty acid chains, allowing them to form a lipid bilayer, the chemical structure of which is shown in
Figure 1 (top left)
[28][29][28,29]. The hydrophobic tail of SM is typically 16 to 24 carbons in length. At the same time, the sphingoid backbone can be either dihydrosphingosine, sphingosine, or 4-hydroxysphinganine, thus conferring heterogeneity to the SM molecule
[30]. Various studies have shown that SM is present in most intracellular compartments, including the Golgi apparatus (which produces most of the SM molecules) and the plasma membrane (PM) (
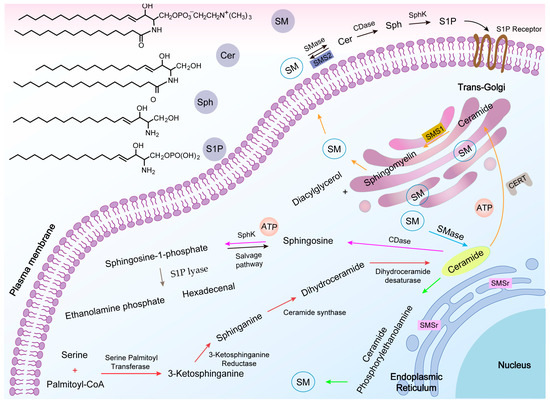
Figure 2)
[16]. SM is also highly enriched in the outer leaflet of the PM and is the major component of the PM
[31]. Exceptionally, erythrocytes and neural tissue have a higher SM content
[20]. It has been estimated that the proportion of SM in the PM of fibroblasts can account for 40% to 90% of the total SM in the cell, respectively
[32].
Figure 1. Chemical structure formula of sphingomyelin and its metabolites. The biological roles of the relevant signaling molecules are labeled under different colored fonts. Sphingomyelinase (SMase), sphingomyelin synthase (SMS), ceramidase (CDase), ceramide synthase (CerS), sphingosine kinase (SphK), and sphingosine-1-phosphate phosphatase (S1PP).
Figure 2. Sphingomyelin-related metabolic pathways. The relevant chemical structures are shown (above left). Ceramide (Cer) de novo synthesis starts from the endoplasmic reticulum (ER), and once synthesized, can be transferred to the trans-Golgi via the ceramide transfer protein (CERT), or be reorganized via a salvage pathway (red arrow). The action of sphingomyelin synthase 1 (SMS1) on Cer leads to the production of sphingomyelin (SM) (orange arrow). In response to the stimulus of apoptosis, phospholipid disorganization moves the isolated SM from the outer leaflet to the cytoplasmic side of the plasma membrane so that sphingomyelinase (SMase) can act on them to produce apoptotic Cer, with the opposite process occurring via sphingomyelin synthase (SMS) (black arrow). Second, Cer can be produced via the SMase (blue arrow). SM can also be produced by converting Cer via sphingomyelin synthase-associated protein (SMSr) (green arrow). Sphingosine (Sph) is produced by the hydrolysis of Cer by ceramidase (CDase), which is then phosphorylated by sphingosine kinase (SphK) to produce sphingosine-1-phosphate (S1P) (pink arrow). The presence of S1P lyase (SPL) also allows S1P to produce ethanolamine phosphate and hexadecenal (brown arrow).
2.2. SM Synthesis
SM is synthesized by two major isoforms of SMS, SMS1 (located in the trans-Golgi) and SMS2 (located in the trans-Golgi and PM)
[33]. SMS2 in the PM is the only enzyme capable of synthesizing SM, and may have the unique function of directly maintaining SM content in the PM. It is unknown where the primary site of SM synthesis is located, but it occurs either on the luminal side of the trans-Golgi or in the extracellular leaflet of the PM (orange arrow in
Figure 2)
[34][35][34,35]. Cer is the substrate for this reaction, and this molecule is synthesized in the endoplasmic reticulum (ER). Thus, Cer must be transferred from its site of synthesis to the Golgi via a mediator termed the ceramide transfer protein (CERT) (a non-vesicular mechanism). The transfer of a phosphocholine headgroup from phosphatidylcholine to Cer to generate SM is catalyzed by SMS1, along with the generation of diacylglycerol (DAG)
[36]. CERT is a cytoplasmic protein with two unique domains: the START (StAR-related lipid-transfer) domain for Cer recognition and the PH (pleckstrin homology) domain for phosphatidylinositol binding
[36][37][36,37]. Unlike SMS1, SMS2 is not reliant on CERT-mediated Cer molecules to synthesize SM due to its unique cellular location, and is the only type of SMS present in the PM. Its most prominent mechanism is likely in converting local Cer to SM
[38]. Sphingomyelin synthase-related protein (SMSr) is an enzyme present in the ER that is capable of converting Cer to ceramide phosphorylethanolamine (CPE), followed by the head group being exchanged or methylated to produce SM (green arrow in
Figure 2)
[31][39][31,39]. As CPE synthesizes extremely slowly, SM is produced in much lower amounts than when SM is synthesized in the Golgi, and it is commonly believed that SMSr regulates the balance of Cer in the ER to prevent excessive accumulation of Cer.
2.3. SM Metabolism
The metabolites of SM include Cer, Sph, and S1P, which are an essential class of biologically active signaling molecules
[40]. Cer and Sph are important components of the stress response regulatory system, mediating cell cycle arrest and inducing apoptosis. However, S1P exhibits the opposite effect, promoting cell growth, invasion, and survival
[41]. The synthesis and catabolism of these metabolites constitute a dynamic equilibrium relationship that judges the cell fates (e.g., cell proliferation, differentiation, or apoptosis). The regulation of these interconnected cell fates results from interactions and mutual equilibria between regulatory molecules within the cell. This subtle relationship between Cer, Sph, and S1P has been described as a “sphingomyelin rheostat”
[21][22][21,22].
3. Role of SM in the Development of Tumors
Active molecules of the SM-related pathways play a crucial role in tumorigenesis and progression, and alterations in their related signaling play a vital role in the induction of cancer cell death or survival (
Table 1).
Table 1.
The role of active molecules in SM-related pathways in tumorigenesis and development.
|
Active Molecule
|
Pathway
|
Function or Activity
|
Cancer
|
Refs.
|
|
Sphingosine kinase
|
Akt/NF-κB
|
Cancer progression and chemoresistance
|
Colon
|
[42][74]
|
| |
S1P/S1PR1
|
Inflammation and angiogenesis
|
Breast
|
[43][75]
|
| |
PI3k/Akt/FOXO3a
|
Apoptosis resistance
|
Breast
|
[44][76]
|
| |
S1P/Stat3/AKT
|
Proliferation
|
Colon
|
[45][77]
|
| |
E-cad
|
Tumorigenesis and metastasis
|
Breast
|
SW480
[46][78]
|
|
|
| [ | 72 | ][134]
|
|
S1P/ERK/CD44
|
Chemoresistance
|
Colon
|
[47][79]
|
| |
|
SNs_PEG
|
77 ± 3
|
|
PANC-1,
|
[91][160]
|
S1P/AKT/GSK-3β
|
HIF-1α stabilization
|
Glioblastoma
|
[48][80]
|
|
Lipid–porphyrin conjugates
|
180 ± 10
|
|
Kyse-30
|
[92][161]
|
Sphingomyelinase
|
|
|
SMLs@PDA
|
229.5 ± 26.3
|
↑ Sensitivity
|
Glioblastoma
|
[49][ |
81 |
]
|
|
| [ | 93 | ] | [162]
|
|
|
| |
C6-NBD-SM Liposomes
↑ |
71 ± 3
Resistance
|
Melanoma
|
[ |
|
CCRF-CEM
50][82]
|
|
| [ | 94 | ] | [163]
|
|
|
Induce apoptosis
|
|
CPNPs
|
| Colon |
|
[51 |
20
][83]
|
| |
| MCF-7 |
|
[78][142]
|
|
|
Induce apoptosis and resistance
|
Ovarian
|
|
C6 ceramide
|
| [ |
~80
| 52][84]
|
| |
| B16, WM-115 |
|
[95][145]
|
Acid ceramidase
|
|
↑ Proliferative ↓ Sensitivity
|
Melanoma
|
[53] |
|
TRI-Gel
|
|
Subcutaneous injection
|
Lewis lung carcinoma
| [54][85,86]
|
| |
|
↓ Sensitivity
|
Prostate
|
[55][87]
|
| |
|
↑ Radioresistant
|
Glioblastoma
|
[55][87]
|
|
Sphingosine kinase 2
|
Mcl-1
|
↑ Cell survival
|
Leukemia
|
[56][88]
|
|
Sphingosine-1-phosphate lyase
|
p53 and p38
|
↑ Apoptosis
|
Colon
|
[57][89]
|
|
Sphingomyelin synthase
|
TGF- b1
|
↑ Migratory, invasion
|
Breast
|
[58][90]
|
| |
Overexpression of SMS1
|
↓ Cell death
|
Lymphatic
|
[59][91]
|
|
Sphingomyelinase
|
CD95
|
↑ Apoptosis
|
Lymphatic
|
[60][92]
|
| |
p53
|
↑ Apoptosis
|
Lung
|
[61][93]
|
|
Sphingosine
|
Cdk4
|
↓ Cell proliferation
|
Intestinal adenoma
|
[62][94]
|
|
Ceramide
|
CerS6/C16-ceramide activated
|
↑ Apoptosis
|
Lung
|
[63][95]
|
| |
High cytotoxicity in p53
|
↑ Apoptosis
|
Breast
|
[64][96]
|
↑, increase; ↓, decrease.
4. SM Metabolism-Based Lipidic Nanomaterials for Cancer Therapy
4.1. SM-Based Lipidic Nanomaterials for Cancer Therapy
Various chemotherapeutic agents, small molecule inhibitors, and microRNAs (miRNAs) have all been used for cancer treatment in the clinic, but often have a poor therapeutic efficacy due to their low in vivo utilization and off-target
[65][127]. To improve their clinical translation, various nanocarriers have been designed to achieve precise targeting and low side effects, enhanced permeability, and retention effects
[66][128]. SM nanosystems have been shown to be promising carriers for effectively delivering anticancer drugs. Recently, Wang et al.
[67][129] developed an SM-derived camptothecin (CPT) (SM-CPT) liposome nanotherapeutic platform, in which the hydroxyl group of SM is partially suffixed with CPT in self-assembly, driven by the amphiphilic nature of SM in aqueous media. This enhances lactone stability and triggers a cytotoxic T lymphocyte (CTL) response to activating anti-tumor immunity. Coupling this with a PD-L1/PD-1 blockade eradicated more than 80% of MC38 colon tumors. Based on the excellent ability of SM nanosystems to bind to different types of drugs, Bouzo et al.
[68][130] proposed the chemical modification of the natural ligands of guanylyl cyclase (GCC) receptors expressed in metastatic colorectal cancer tumors, which were then anchored to SM nanosystems, and finally loaded with the anticancer drug etoposide (Etp) for combination therapy, a strategy that has previously shown strong potential for the treatment of metastatic colorectal cancer cells. Medina et al.
[69][131] increased the homing ability of pancreatic ductal adenocarcinoma (PDAC) tumors for pancreatic tumor imaging by encapsulating iron nanoparticles and the indocyanine green (ICG) in liposomes composed of cationic SM, while embedding RA-96 Fab fragments at the surface of the liposomal nanoparticles. This may be a suitable drug delivery tool for enhancing positive therapeutic outcomes in PDAC patients. Another novel active release system is the encapsulation of cisplatin and ICG in magnetic SM liposomes for their release in response to stress cellular SMase. Individual iron particles and large clusters of iron particle structural domains can be observed in TEM images upon iron and SMase treatment and are clearly distinguishable from the untreated control liposomes. Intracellular release studies were subsequently performed with Texas red membrane-labeled liposomes loaded with calreticulin, and CLSM images demonstrated a high diffusion of calreticulin under alternating magnetic field (AMF) treatment. This design allowed the system to only open at the appropriate time within the lesion, showing an improved therapeutic efficacy in murine squamous cell carcinoma tumors
[70][132]. RNA-based gene therapy is a prospective option in the fight against cancer, but the efficiency of biological delivery requires further optimization. Farimah et al.
[71][133] developed a gene therapy nanodrug capable of interfering with tumor growth and migration using a biocompatible SM nanosystem to effectively deliver encoded
TP53TG1 plasmids to HCT-116 colorectal cells as treatment. Another SM-based nanosystem (SNs) incorporating a cationic lipid stearylamine (ST) has been developed that is able to support miRNA conjugation by establishing electrostatic interactions (SNs–ST)
[72][134].
4.2. Cer-Based Lipidic Nanomaterials for Cancer Therapy
Cer-mediated anticancer effects have been demonstrated in various cancers (pancreatic, breast, and gastric), affecting T-cell signaling and inducing apoptosis in cancer cells
[73][74][75][137,138,139]. However, the cellular impermeability and hydrophobicity of Cer greatly limits its practical application. Several delivery vehicles have been developed to increase Cer delivery and enhance its pharmacological effects, such as calcium phosphate nanocomposite particles, nanoemulsions, liposomes, and others
[76][77][78][79][140,141,142,143]. Li et al.
[80][144] developed C6-Cer-containing nanoliposomes (LipC6) to overcome this limitation for improving the efficacy of immunotherapy in patients with liver cancer.
4.3. S1P-Based Lipidic Nanomaterials for Cancer Therapy
S1P is a lipid member of the SM metabolic pathway that facilitates tumor progression. To address the lack of effective targets for GBM and the low accumulation of drugs in the diseased area from the blood–brain-tumor barrier (BBTB), Liu et al.
[81][150] developed a liposome-based GBM drug delivery system, S1P/JS-K/Lipo, which utilized S1P as its active lipid ligand. This enabled the active penetration of nitric oxide (NO) prodrugs (JS-K, O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl) piperazin-1-yl] diazen-1-ium-1,2-diolate) into the BBTB to produce tiny NO bubbles for killing tumor cells. When S1P/JS-K/Lipo (equivalent to 2 × 10
−3 mg kg
−1 body weight of S1P and 1 mg kg
−1 body weight of JS-K) was intravenously administered to mice, significant inhibitions of U87MG-RFP-tumor growth was observed. The system was also demonstrated to adequately interact specifically with the highly expressed S1PRs on GBM cells for an efficient targeted delivery. The FDA-approved drug FTY720 (fingolimod) impairs acid-induced osteosarcoma cell survival and migration by reducing S1P levels. It is currently in the experimental phase as an immunomodulatory agent, and may be useful in designing nanotherapeutic agents
[82][151]. To date, various types of S1P signaling modulators have been developed, including S1P agonists, S1P antagonists, and SphK inhibitors
[83][152]. Their positive impact on cancer therapy (including, but not limited to increased sensitivity to chemotherapy and improved oncologic outcomes) has been described in the literature
[84][85][86][153,154,155]. Among them includes the sphingolipid phosphatase SGPP1, an antagonist of S1P signaling which can improve the radiosensitivity of miR-95 overexpressed mice
[87][156]. Additionally, restraining S1P synthesis by inhibiting SphK activity can be used to heighten the response of cancer cells to cytotoxic treatments, and these studies will be a strong basis for future nanomedicine design
[88][89][157,158].
Finally, recent lipidic nanomaterials designed based on SM metabolism for cancer therapy were summarized, and their related parameters are listed in
Table 2.
Table 2.
SM metabolism-based lipidic nanomaterials for cancer therapy.