Polysaccharides play a crucial role in medicine, pharmacy, and cosmetology, as well as in the production of biofuels and biomaterials. Among microbial biopolymers, microbial levan, a fructose polysaccharide, holds significant promise due to its high productivity and chemical diversity. Levan exhibits a wide range of properties, including film-forming ability, biodegradability, non-toxicity, self-aggregation, encapsulation, controlled release capacity, water retention, immunomodulatory and prebiotic activity, antimicrobial and anticancer activity, as well as high biocompatibility. These exceptional properties position levan as an attractive candidate for nature-based materials in food production, modern cosmetology, medicine, and pharmacy.
- levan
- biopolymers
- polysaccharide
- Bacillus subtilis
1. Biosynthesis of Levan
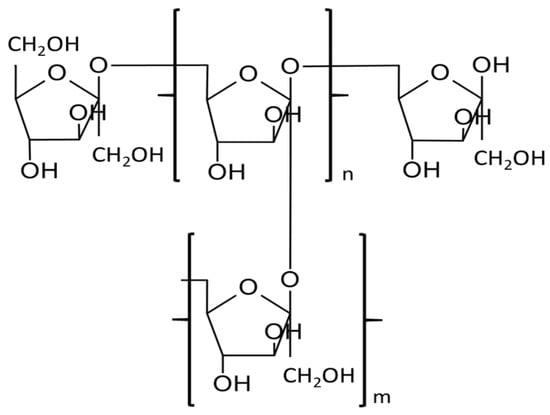
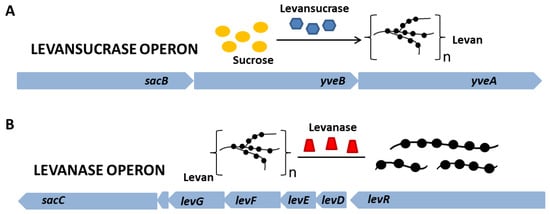
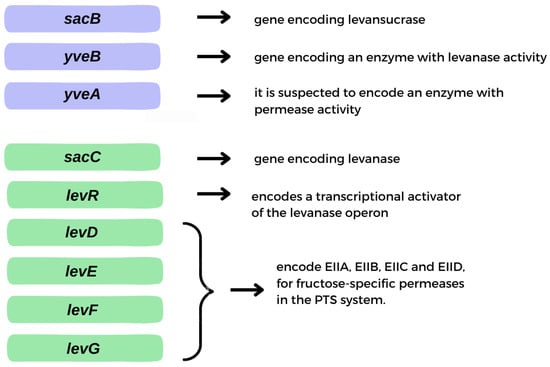
2. Microbial Preparation of Levan
| Scale | Microorganism | Sucrose Concentration [g/L] | Cultivation Conditions | Levan Concentration [g/L] | Literature | ||
|---|---|---|---|---|---|---|---|
| Laboratory | Leuconostoc citreum | BD1707 | 172.0 | 112 h, 26 °C, 200 rpm, pH 6.12 | 34.86 | [65] | [40] |
| Tanticharoenia sakaeratensis | 200.0 | 35 h, 37 °C, 250 rpm | 24.70 | [66] | [41] | ||
| B. subtilis | AF17 | 162.5 | 20 h, 30 °C, 150 rpm, pH 7.0 | 7.90 | [67] | [42] | |
| B.subtilis | MT453867 | 80.0 | 54 h, 37 °C, 150 rpm, pH 5.0 | 33.00 | [68] | [43] | |
| Brenneria goodwinii | 500.0 | 12 h, 35 °C, 8000 rpm, pH 6.0 | 185.00 | [50] | [25] | ||
| 76.00 | |||||||
| [ | |||||||
| 73 | |||||||
| ] | |||||||
| [ | |||||||
| 48 | |||||||
| ] | |||||||
| Substrate/By-Product used | Microorganism | Sucrose Concentration [%] | Levan Concentration [g/L] | Literature | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molasses | Halomonas | sp. AAD6 | 48–51 | 12.40 | [61] | [36] | ||||||||
| B. licheniformis | NS032 | 49.40 | 53.20 | [78] | [53] | |||||||||
| B. lentus | V8 | 54 | 45.34 | [75] | [50] | |||||||||
| Sugar beet juice and syrup | Z. mobilis | 113 | 65 | 9.60 | [79] | [54] | ||||||||
| The pulp after squeezing orange juice | B. atrophaeus | 12–27 | 24.20 | [63] | [38] | |||||||||
| Clostridium acetobutylicum | 28.00 | 72 h, 20 °C, - *, pH 6.0 | - * | [51] | [ | |||||||||
| Distillers’ rye stock | Z. mobilis | * | 26 | 25.17] | ||||||||||
| [ | 80 | ] | [ | 55] | Leuconostoc mesenteroides | 90.0 | 24 h, 30 °C, - *, pH 5.0 | - * | [52] | [27] | ||||
| Date syrup | Microbacterium laevaniformans | * | 10.48 | [81] | [56 | Sphingobium chungbukense | 10.00 | 24 h, 37 °C, - *, pH 5–10 | - * | [54] | [29] | |||
| ] | ||||||||||||||
| B. phenoliresistens | * | 8.12 | [76] | [51] | Gluconobacter japonicus | LMG 1417 | 720.00 | 24 h, 28 °C, 180 rpm, pH 6.8 | 157.90 | |||||
| Sugar cane syrup | [ | 55 | ] | [30] | ||||||||||
| Z. mobilis | * | 15.46 | [ | 82] | [57] | Peanibacillus polymyxa | 124.00 | 96 h, 30 °C, 250 rpm, pH 7.2 | 68.00 | [56] | [31] | |||
| Baklava syrup | Z. mobilis | * | 8.90 | [83] | [58] | Azotobacter chroococcum | DSM 2286 + | Gluconobacter | japonicus | LMG 1417 | 809.6 | 48 h, 45 °C, - *, pH 6.2 | 387.40 | |
| Buckwheat sourdough bread | G. albidus | TMW 2.1191; | Kozakia baliensis | NBRC 16680 | [57] | [32] | ||||||||
| 8.86 ± 21.71; 50.26 ± 25.21 | 13.91; 12.65 | [ | 64 | ] | [39] | Saccharomyces | cereviasie | 50.00 | 48 h, 30 °C, pH 6.5 | 15.00 | [58] | [33] | ||
| Bioreactor | B. subtilis | M | 100.0 | 150 L bioreactor, 24 h, 30 ℃, 200 rpm, pH 7.0, 0.5 vvm |
47.00 | [69] | [44] | |||||||
| B. subtlis | B58 | 130.0 | 16 L bioreactor, 16 h, 37 ℃, 400 rpm, pH 7.0, 1 vvm |
26.65 | [70] | [45] | ||||||||
| Z. mobilis | B-14023 | 299.1 | 42.3 h, 28 ℃, pH 6.0 | 40.20 | [59] | [34] | ||||||||
| E. herbicola | 50.0 | 5 L bioreactor, 48 h, 25 ℃, 200 rpm, 0.2 vvm |
4.50 | [60] | [35] | |||||||||
| B. subtilis AF17 | 162.5 | 30 °C, 72 h, 150 rpm, pH 7.0 | 7.90 | [67] | [42] | |||||||||
| Halomonas smyrnensis | AAD6 | T | 20.00 | 10 L bioreactor 37 °C, 200 rpm, 0.1 vvm, |
18.06 | [70] | [45] | |||||||
| Bacillus | amyloliquefaciens | 20.00 | 5 L bioreactor, 37 °C, 48 h, 300 rpm, pH 6.0, 6 vvm |
102.00 | [71] | [46] | ||||||||
| Pichia pastoris | 160.00 | 1,5 L bioreactor, 59 h, 28 °C, 200–1200 rpm |
72.90 | [72] | [47] | |||||||||
| Saccharomyces | cerevisiae | 191.00 | 50 L bioreactor, 48 h, 30 °C, 300 rpm, pH 5.5, 0.5 vvm |
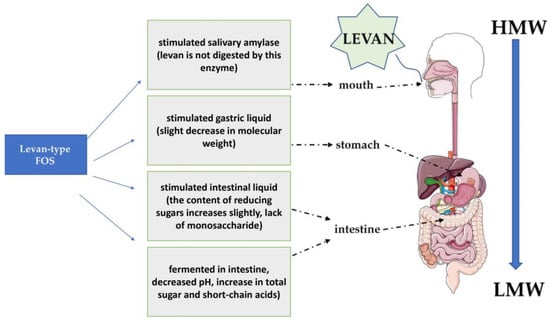
3. Properties and Application of Levan
References
- Greig-Smith, R.; Steel, T. Levan: A New Bacterial Gum from Sugar. J. Soc. Chem. Ind. Scottish Sect. 1902, 21, 1381.
- Gupta, S.K.; Das, P.; Singh, S.K.; Akhtar, M.S.; Meena, D.K.; Mandal, S.C. Microbial Levan, an Ideal Prebiotic and Immunonutrient in Aquaculture. World Aquac. 1990, 42, 61–64.
- Combie, J. Properties of Levan and Potential Medical Uses. Polysaccharides Drug Deliv. Pharm. Appl. 2006, 934, 13–263.
- Domżał-Kędzia, M.; Lewińska, A.; Jaromin, A.; Weselski, M.; Pluskota, R.; Łukaszewicz, M. Fermentation Parameters and Conditions Affecting Levan Production and Its Potential Applications in Cosmetics. Bioorg. Chem. 2019, 93, 102787.
- Goncalves, B.C.M.; Baldo, C.; Celligoi, M.A.P.C. Levan and Levansucrase—A Mini Review. Int. J. Sci. Technol. Res. 2015, 4, 100–104.
- Mardo, K.; Visnapuu, T.; Gromkova, M.; Aasamets, A.; Viigand, K.; Vija, H.; Alamäe, T. High-Throughput Assay of Levansucrase Variants in Search of Feasible Catalysts for the Synthesis of Fructooligosaccharides and Levan. Molecules 2014, 19, 8434–8455.
- González-Garcinuño, Á.; Tabernero, A.; Domínguez, Á.; Galán, M.A.; Martin del Valle, E.M. Levan and Levansucrases: Polymer, Enzyme, Micro-Organisms and Biomedical Applications. Biocatal. Biotransformation 2017, 36, 233–244.
- Freitas, F.; Reis, M.A.M.; De Lisboa, U.N.; Lisboa, U. De Polysaccharides; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–24.
- Leloup, L.; Driessen, A.J.M.; Freudl, R.; Chambert, R.; Petit-Glatron, M.F. Differential Dependence of Levansucrase and α-Amylase Secretion on SecA (Div) during the Exponential Phase of Growth of Bacillus Subtilis. J. Bacteriol. 1999, 181, 1820–1826.
- Daguer, J.P.; Geissmann, T.; Petit-Glatron, M.F.; Chambert, R. Autogenous Modulation of the Bacillus Subtilis SacB-LevB-YveA Levansucrase Operon by the LevB Transcript. Microbiology 2004, 150, 3669–3679.
- Rey, M.W.; Ramaiya, P.; Nelson, B.A.; Brody-Karpin, S.D.; Zaretsky, E.J.; Tang, M.; Lopez de Leon, A.; Xiang, H.; Gusti, V.; Clausen, I.G.; et al. Complete Genome Sequence of the Industrial Bacterium Bacillus Licheniformis and Comparisons with Closely Related Bacillus Species. Genome Biol. 2004, 5, r77.
- Pereira, Y.; Petit-Glatron, M.F.; Chambert, R. YveB, Encoding Endolevanase LevB, Is Part of the SacB-YveB-YveA Levansucrase Tricistronic Operon in Bacillus Subtilis. Microbiology 2001, 147, 3413–3419.
- Jensen, S.L.; Diemer, M.B.; Lundmark, M.; Larsen, F.H.; Blennow, A.; Mogensen, H.K.; Nielsen, T.H. Levanase from Bacillus Subtilis Hydrolyses β-2,6 Fructosyl Bonds in Bacterial Levans and in Grass Fructans. Int. J. Biol. Macromol. 2016, 85, 514–521.
- Méndez-Lorenzo, L.; Porras-Domínguez, J.R.; Raga-Carbajal, E.; Olvera, C.; Rodríguez-Alegría, M.E.; Carrillo-Nava, E.; Costas, M.; Munguía, A.L. Intrinsic Levanase Activity of Bacillus Subtilis 168 Levansucrase (SacB). PLoS ONE 2015, 10, e0143394.
- Shih, I.L.; Chen, L.D.; Wang, T.C.; Wu, J.Y.; Liaw, K.S. Tandem Production of Levan and Ethanol by Microbial Fermentation. Green Chem. 2010, 12, 1242–1247.
- Marvasi, M.; Visscher, P.T.; Casillas Martinez, L. Exopolymeric Substances (EPS) from Bacillus Subtilis: Polymers and Genes Encoding Their Synthesis. FEMS Microbiol. Lett. 2010, 313, 1–9.
- Martin, I.; Debarbouille, M.; Klier, A.; Rapoport, G. Induction and Metabolite Regulation of Levanase Synthesis in Bacillus Subtilis. J. Bacteriol. 1989, 171, 1885–1892.
- Martin, I.; Débarbouillé, M.; Ferrari, E.; Klier, A.; Rapoport, G. Characterization of the Levanase Gene of Bacillus Subtilis Which Shows Homology to Yeast Invertase. MGG Mol. Gen. Genet. 1987, 208, 177–184.
- Martin-Verstraete, I.; Débarbouillé, M.; Klier, A.; Rapoport, G. Levanase Operon of Bacillus Subtilis Includes a Fructose-Specific Phosphotransferase System Regulating the Expression of the Operon. J. Mol. Biol. 1990, 214, 657–671.
- Débarbouillé, M.; Martin-Verstraete, I.; Arnaud, M.; Klier, A.; Rapoport, G. Positive and Negative Regulation Controlling Expression of the Sac Genes in Bacillus Subtilis. Res. Microbiol. 1991, 142, 757–764.
- Wanker, E.; Huber, A.; Schwab, H. Purification and Characterization of the Bacillus Subtilis Levanase Produced in Escherichia coli. Appl. Environ. Microbiol. 1995, 61, 1953–1958.
- Raga-Carbajal, E.; Carrillo-Nava, E.; Costas, M.; Porras-Dominguez, J.; López-Munguía, A.; Olvera, C. Size Product Modulation by Enzyme Concentration Reveals Two Distinct Levan Elongation Mechanisms in Bacillus Subtilis Levansucrase. Glycobiology 2016, 26, 377–385.
- Shih, I.-L.; Yu, Y.-T.; Shieh, C.-J.; Hsieh, C.-Y. Selective Production and Characterization of Levan by Bacillus Subtilis (Natto) Takahashi. J. Agric. Food Chem. 2005, 53, 82118215.
- Euzenat, O.; Guibert, A.; Combes, D. Production of Fructo-Oligosaccharides by Levansucrase from Bacillus Subtilis C4. Process Biochem. 1997, 32, 237–243.
- Liu, Q.; Yu, S.; Zhang, T.; Jiang, B.; Mu, W. Efficient Biosynthesis of Levan from Sucrose by a Novel Levansucrase from Brenneria Goodwinii. Carbohydr. Polym. 2017, 157, 1732–1740.
- Gao, S.; Qi, X.; Hart, D.J.; Gao, H.; An, Y. Expression and Characterization of Levansucrase from Clostridium Acetobutylicum. J. Agric. Food Chem. 2017, 65, 867–871.
- Ishida, R.; Sakaguchi, K.; Matsuzaki, C.; Katoh, T.; Ishida, N.; Yamamoto, K.; Hisa, K. Levansucrase from Leuconostoc Mesenteroides NTM048 Produces a Levan Exopolysaccharide with Immunomodulating Activity. Biotechnol. Lett. 2016, 38, 681–687.
- Kirtel, O.; Menéndez, C.; Versluys, M.; Van den Ende, W.; Hernández, L.; Toksoy Öner, E. Levansucrase from Halomonas Smyrnensis AAD6T: First Halophilic GH-J Clan Enzyme Recombinantly Expressed, Purified, and Characterized. Appl. Microbiol. Biotechnol. 2018, 102, 9207–9220.
- Lee, S.Y.; Shin, W.-R.; Sekhon, S.S.; Lee, J.-P.; Kim, Y.-C.; Ahn, J.-Y.; Kim, Y.-H. Molecular Docking Analysis and Biochemical Evaluation of Levansucrase from Sphingobium Chungbukense DJ77. ACS Comb. Sci. 2018, 20, 414–422.
- Hövels, M.; Kosciow, K.; Kniewel, J.; Jakob, F.; Deppenmeier, U. High Yield Production of Levan-Type Fructans by Gluconobacter Japonicus LMG 1417. Int. J. Biol. Macromol. 2020, 164, 295–303.
- Liyaskina, E.V.; Rakova, N.A.; Kitykina, A.A.; Rusyaeva, V.V.; Toukach, P.V.; Fomenkov, A.; Vainauskas, S.; Roberts, R.J.; Revin, V.V. Production and Characterization of the Exopolysaccharide from Strain Paenibacillus Polymyxa 2020. PLoS ONE 2021, 16, e0253482.
- Hövels, M.; Kosciow, K.; Deppenmeier, U. Characterization of a Novel Endo-Levanase from Azotobacter Chroococcum DSM 2286 and Its Application for the Production of Prebiotic Fructooligosaccharides. Carbohydr. Polym. 2021, 255, 117384.
- Franken, J.; Brandt, B.A.; Tai, S.L.; Bauer, F.F. Biosynthesis of Levan, a Bacterial Extracellular Polysaccharide, in the Yeast Saccharomyces Cerevisiae. PLoS ONE 2013, 8, e77499.
- Silbir, S.; Dagbagli, S.; Yegin, S.; Baysal, T.; Goksungur, Y. Levan Production by Zymomonas Mobilis in Batch and Continuous Fermentation Systems. Carbohydr. Polym. 2014, 99, 454–461.
- Keith, J.; Wiley, B.; Ball, D.; Arcidiacono, S.; Zorfass, D.; Mayer, J.; Kaplan, D. Continuous Culture System for Production of Biopolymer Levan Using Erwinia Herbicola. Biotechnol. Bioeng. 1991, 38, 557–560.
- Küçükaşik, F.; Kazak, H.; Güney, D.; Finore, I.; Poli, A.; Yenigün, O.; Nicolaus, B.; Öner, E.T. Molasses as Fermentation Substrate for Levan Production by Halomonas sp. Appl. Microbiol. Biotechnol. 2011, 89, 1729–1740.
- Seibel, J.; Moraru, R.; Götze, S.; Buchholz, K.; Na’amnieh, S.; Pawlowski, A.; Hecht, H.J.; Secchi, M.; Castellani, V.; Collina, E.; et al. Selenium Enriched Green Tea Increase Stability of Lactobacillus Casei and Lactobacillus Plantarum in Chitosan Coated Alginate Microcapsules during Exposure to Simulated Gastrointestinal and Refrigerated Conditions. Int. J. Biol. Macromol. 2020, 21, 406–411.
- González-Garcinuño, Á.; Tabernero, A.; Sánchez-Álvarez, J.M.; Galán, M.A.; Martin del Valle, E.M. Effect of Bacteria Type and Sucrose Concentration on Levan Yield and Its Molecular Weight. Microb. Cell Fact. 2017, 16, 91.
- Ua-Arak, T.; Jakob, F.; Vogel, R.F. Influence of Levan-Producing Acetic Acid Bacteria on Buckwheat-Sourdough Breads. Food Microbiol. 2017, 65, 95–104.
- Han, J.; Feng, H.; Wang, X.; Liu, Z.; Wu, Z. Levan from Leuconostoc Citreum BD1707: Production Optimization and Changes in Molecular Weight Distribution during Cultivation. BMC Biotechnol. 2021, 21, 14.
- Aramsangtienchai, P.; Kongmon, T.; Pechroj, S.; Srisook, K. Enhanced Production and Immunomodulatory Activity of Levan from the Acetic Acid Bacterium, Tanticharoenia Sakaeratensis. Int. J. Biol. Macromol. 2020, 163, 574–581.
- Bouallegue, A.; Casillo, A.; Chaari, F.; Cimini, D.; Corsaro, M.M.; Bachoual, R.; Ellouz-Chaabouni, S. Statistical Optimization of Levan: Influence of the Parameter on Levan Structure and Angiotensin I-Converting Enzyme Inhibitory. Int. J. Biol. Macromol. 2020, 158, 945–952.
- Gamal, A.A.; Abbas, H.Y.; Abdelwahed, N.A.M.; Kashef, M.T.; Mahmoud, K.; Esawy, M.A.; Ramadan, M.A. Optimization Strategy of Bacillus Subtilis MT453867 Levansucrase and Evaluation of Levan Role in Pancreatic Cancer Treatment. Int. J. Biol. Macromol. 2021, 182, 1590–1601.
- Ragab, T.I.M.; Malek, R.A.; Elsehemy, I.A.; Farag, M.M.S.; Salama, B.M.; Abd EL-Baseer, M.A.; Gamal-Eldeen, A.M.; El Enshasy, H.A.; Esawy, M.A. Scaling up of Levan Yield in Bacillus Subtilis M and Cytotoxicity Study on Levan and Its Derivatives. J. Biosci. Bioeng. 2019, 127, 655–662.
- Hamid, K.R.A.; Elsayed, E.A.; Enshasy, H.A.E.; Esawy, M.; Malek, R.A. Bioprocess Optimization for Levan Production by Bacillus Subtilis B58. J. Sci. Ind. Res. (India) 2018, 77, 386–393.
- Gu, Y.; Zheng, J.; Feng, J.; Cao, M.; Gao, W.; Quan, Y.; Dang, Y.; Wang, Y.; Wang, S.; Song, C. Improvement of Levan Production in Bacillus Amyloliquefaciens through Metabolic Optimization of Regulatory Elements. Appl. Microbiol. Biotechnol. 2017, 101, 4163–4174.
- Ávila-Fernández, Á.; Montiel, S.; Rodríguez-Alegría, M.E.; Caspeta, L.; López Munguía, A. Simultaneous Enzyme Production, Levan-Type FOS Synthesis and Sugar by-Products Elimination Using a Recombinant Pichia Pastoris Strain Expressing a Levansucrase-Endolevanase Fusion Enzyme. Microb. Cell Fact. 2023, 22, 18.
- Ko, H.; Bae, J.H.; Sung, B.H.; Kim, M.J.; Kim, C.H.; Oh, B.R.; Sohn, J.H. Efficient Production of Levan Using a Recombinant Yeast Saccharomyces Cerevisiae Hypersecreting a Bacterial Levansucrase. J. Ind. Microbiol. Biotechnol. 2019, 46, 1611–1620.
- Sarilmiser, H.K.; Ates, O.; Ozdemir, G.; Arga, K.Y.; Oner, E.T. Effective Stimulating Factors for Microbial Levan Production by Halomonas Smyrnensis AAD6T. J. Biosci. Bioeng. 2015, 119, 455–463.
- Abou-taleb, K.A.; Abdel-Monem, M.O.; Yassin, M.H.; Draz, A.A.; Blaiotta, G. Production, Purification and Characterization of Levan Polymer from Bacillus Lentus V8 Strain. Br. Microbiol. Res. J. 2015, 5, 22–32.
- Moussa, T.A.A.; Al-Qaysi, S.A.S.; Thabit, Z.A.; Kadhem, S.B. Microbial Levan from Brachybacterium Phenoliresistens: Characterization and Enhancement of Production. Process Biochem. 2017, 57, 9–15.
- Erkorkmaz, B.A.; Kırtel, O.; Ateş Duru, Ö.; Toksoy Öner, E. Development of a Cost-Effective Production Process for Halomonas Levan. Bioprocess Biosyst. Eng. 2018, 41, 1247–1259.
- Gojgic-Cvijovic, G.D.; Jakovljevic, D.M.; Loncarevic, B.D.; Todorovic, N.M.; Pergal, M.V.; Ciric, J.; Loos, K.; Beskoski, V.P.; Vrvic, M.M. Production of Levan by Bacillus Licheniformis NS032 in Sugar Beet Molasses-Based Medium. Int. J. Biol. Macromol. 2019, 121, 142–151.
- Bekers, M.; Linde, R.; Danilevich, A.; Kaminska, E.; Upite, D.; Vigants, A.; Scherbaka, R. Sugar Beet Diffusion Juice and Syrup as Media for Ethanol and Levan Production by Zymomonas Mobilis. Food Biotechnol. 1999, 13, 107–119.
- Bekers, M.; Grube, M.; Vulfa, L.; Upite, D.; Kaminska, E.; Scherbaka, R.; Vigants, A.; Danilevich, A. Stillage as a Source of Growth Promoting Biofactors and a Stimulator of Levan and Extracellular Levansucrase Synthesis for Zymomonas Mobilis. Food Technol. Biotechnol. 2002, 40, 305–310.
- Bekers, M.; Grube, M.; Vulfa, L.; Upite, D.; Kaminska, E.E.; Scherbaka, R.; Vigants, A.; Danilevich, A.; de Oliveira, M.R.; da Silva, R.S.S.F.; et al. Microbial Production of Levan Using Date Syrup and Investigation of Its Properties. Int. J. Nutr. Food Eng. World Acad. Sci. Eng. Technol. 2007, 13, 107–119.
- de Oliveira, M.R.; da Silva, R.S.S.F.; Buzato, J.B.; Celligoi, M.A.P.C. Study of Levan Production by Zymomonas Mobilis Using Regional Low-Cost Carbohydrate Sources. Biochem. Eng. J. 2007, 37, 177–183.
- Omeroglu, M.A.; Gonul-Baltaci, N.; Arslan, N.P.; Adiguzel, A.; Taskin, M. Microbial Conversion of Waste Baklava Syrup to Biofuels and Bioproducts. Biocatal. Agric. Biotechnol. 2022, 42, 102364.
- Mummaleti, G.; Sarma, C.; Kalakandan, S.K.; Gazula, H.; Sivanandham, V.; Anandharaj, A. Characterization of Levan Produced from Coconut Inflorescence Sap Using Bacillus Subtilis and Its Application as a Sweetener. LWT 2022, 154, 112697.
- Bersaneti, G.T.; Pan, N.C.; Baldo, C.; Celligoi, M.A.P.C. Co-Production of Fructooligosaccharides and Levan by Levansucrase from Bacillus Subtilis Natto with Potential Application in the Food Industry. Appl. Biochem. Biotechnol. 2018, 184, 838–851.
- de Paula, V.C.; Pinheiro, I.O.; Lopes, C.E.; Calazans, G.M.T. Microwave-Assisted Hydrolysis of Zymomonas Mobilis Levan Envisaging Oligofructan Production. Bioresour. Technol. 2008, 99, 2466–2470.
- Zhang, W.; Xu, W.; Ni, D.; Dai, Q.; Guang, C.; Zhang, T.; Mu, W. An Overview of Levan-Degrading Enzyme from Microbes. Appl. Microbiol. Biotechnol. 2019, 103, 7891–7902.
- Cai, G.; Wu, D.; Li, X.; Lu, J. Levan from Bacillus Amyloliquefaciens JN4 Acts as a Prebiotic for Enhancing the Intestinal Adhesion Capacity of Lactobacillus Reuteri JN101. Int. J. Biol. Macromol. 2020, 146, 482–487.
- Cheng, R.; Cheng, L.; Zhao, Y.; Wang, L.; Wang, S.; Zhang, J. Biosynthesis and Prebiotic Activity of a Linear Levan from a New Paenibacillus Isolate. Appl. Microbiol. Biotechnol. 2021, 105, 769–787.
- Shimizu, N.; Abea, A.; Ushiyama, T.; Toksoy Öner, E. Effect of Temperature on the Hydrolysis of Levan Treated with Compressed Hot Water Fluids. Food Sci. Nutr. 2020, 8, 2004–2014.
- Xu, M.; Pan, L.; Zhou, Z.; Han, Y. Structural Characterization of Levan Synthesized by a Recombinant Levansucrase and Its Application as Yogurt Stabilizers. Carbohydr. Polym. 2022, 291, 119519.
- Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules 2019, 24, 3770.
- Gan, L.; Jiang, G.; Yang, Y.; Zheng, B.; Zhang, S.; Li, X.; Tian, Y.; Peng, B. Development and Characterization of Levan/Pullulan/Chitosan Edible Films Enriched with ε-Polylysine for Active Food Packaging. Food Chem. 2022, 388, 132989.
- Koşarsoy Ağçeli, G. A New Approach to Nanocomposite Carbohydrate Polymer Films: Levan and Chia Seed Mucilage. Int. J. Biol. Macromol. 2022, 218, 751–759.
- Kim, K.S.H.; Chung, C.B.; Kim, Y.H.; Kim, K.S.H.; Han, C.S.; Kim, C.H. Cosmeceutical Properties of Levan Produced by Zymomonas Mobilis. Int. J. Cosmet. Sci. 2006, 28, 231.
- Lewińska, A.; Domżał-Kędzia, M.; Kierul, K.; Bochynek, M.; Pannert, D.; Nowaczyk, P.; Łukaszewicz, M. Targeted Hybrid Nanocarriers as a System Enhancing the Skin Structure. Molecules 2021, 26, 1063.
- Kim, T.; Kim, K.; Ryo, K.; Lee, O.; Kim, T. Cosmetic Composition Containing Levan Having Cell Proliferation, Skin-Moisturizing and Irritation-Alleviating Effects. Patent JP2003277225A, 2 October 2003.
- Masayo, F.; Takayuki, T. Beautifully Whitening Agent 2006. Patent JP2006052146A, 23 February 2006.
- Pantelić, I.; Lukić, M.; Gojgić-Cvijović, G.; Jakovljević, D.; Nikolić, I.; Lunter, D.J.; Daniels, R.; Savić, S. Bacillus Licheniformis Levan as a Functional Biopolymer in Topical Drug Dosage Forms: From Basic Colloidal Considerations to Actual Pharmaceutical Application. Eur. J. Pharm. Sci. 2020, 142, 105109.
- Sezer, A.D.; Kazak Sarılmışer, H.; Rayaman, E.; Çevikbaş, A.; Öner, E.T.; Akbuğa, J. Development and Characterization of Vancomycin-Loaded Levan-Based Microparticular System for Drug Delivery. Pharm. Dev. Technol. 2017, 22, 627–634.
- Cinan, E.; Cesur, S.; Erginer Haskoylu, M.; Gunduz, O.; Toksoy Oner, E. Resveratrol-Loaded Levan Nanoparticles Produced by Electrohydrodynamic Atomization Technique. Nanomaterials 2021, 11, 2582.
- Kim, S.J.; Bae, P.K.; Chung, B.H. Self-Assembled Levan Nanoparticles for Targeted Breast Cancer Imaging. Chem. Commun. 2015, 51, 107–110.
- Richa, R.; Roy Choudhury, A. Exploration of Polysaccharide Based Nanoemulsions for Stabilization and Entrapment of Curcumin. Int. J. Biol. Macromol. 2020, 156, 1287–1296.
- Patel, P.; Agrawal, Y. Preparation and In-Vitro Evaluation of Levan Micelles: A Polyfructan Based Nano-Carrier for Breast Cancer Targeted Delivery. Drug Deliv. Lett. 2019, 9, 97–107.
- Xu, W.; Peng, J.; Ni, D.; Zhang, W.; Wu, H.; Mu, W. Preparation, Characterization and Application of Levan/Montmorillonite Biocomposite and Levan/BSA Nanoparticle. Carbohydr. Polym. 2020, 234, 115921.
- Demirci, T.; Hasköylü, M.E.; Eroğlu, M.S.; Hemberger, J.; Toksoy Öner, E. Levan-Based Hydrogels for Controlled Release of Amphotericin B for Dermal Local Antifungal Therapy of Candidiasis. Eur. J. Pharm. Sci. 2020, 145, 105255.
- Hamada, M.A.; Hassan, R.A.; Abdou, A.M.; Elsaba, Y.M.; Aloufi, A.S.; Sonbol, H.; Korany, S.M. Bio_Fabricated Levan Polymer from Bacillus Subtilis MZ292983.1 with Antibacterial, Antibiofilm, and Burn Healing Properties. Appl. Sci. 2022, 12, 6413.
- Osman, A.; Lin, E.; Hwang, D.S. A Sticky Carbohydrate Meets a Mussel Adhesive: Catechol-Conjugated Levan for Hemostatic and Wound Healing Applications. Carbohydr. Polym. 2023, 299, 120172.
- Hundschell, C.S.; Jakob, F.; Wagemans, A.M. Molecular Weight Dependent Structure and Polymer Density of the Exopolysaccharide Levan. arXiv 2019, arXiv:1909.07737.
- de Siqueira, E.C.; Rebouças, J.d.S.; Pinheiro, I.O.; Formiga, F.R. Levan-Based Nanostructured Systems: An Overview. Int. J. Pharm. 2020, 580, 119242.
- Makino Tadashi, I.; Masayuki Yamada, K.; Jun-ichi, K. Fast Dissolving Tablet and Production. Patent US005501861A, 5 March 1996.
- Kang, S.A.; Jang, K.-H.; Seo, J.-W.; Kim, K.H.; Kim, Y.H.; Rairakhwada, D.; Seo, M.Y.; Lee, J.O.; Do Ha, S.; Kim, C.-H.; et al. Levan: Applications and Perspectives. In Microbial Production of Biopolymers and Polymer Precursors; Caister Academic Press: Norwich, UK, 2009; pp. 145–161.
- Elvassore, N.; Bertucco, A.; Caliceti, P. Production of Insulin-Loaded Poly(Ethylene Glycol)/Poly(L-Lactide) (PEG/PLA) Nanoparticles by Gas Antisolvent Techniques. J. Pharm. Sci. 2001, 90, 1628–1636.
- Bahadori, F.; Eskandari, Z.; Ebrahimi, N.; Bostan, M.S.; Eroğlu, M.S.; Oner, E.T. Development and Optimization of a Novel PLGA-Levan Based Drug Delivery System for Curcumin, Using a Quality-by-Design Approach. Eur. J. Pharm. Sci. 2019, 138, 105037.
- González-Garcinuño, Á.; Tabernero, A.; Marcelo, G.; Sebastián, V.; Arruebo, M.; Santamaría, J.; Martín del Valle, E. Differences in Levan Nanoparticles Depending on Their Synthesis Route: Microbial vs Cell-Free Systems. Int. J. Biol. Macromol. 2019, 137, 62–68.
- Charoenwongpaiboon, T.; Wangpaiboon, K.; Septham, P.; Jiamvoraphong, N.; Issaragrisil, S.; Pichyangkura, R.; Lorthongpanich, C. Production and Bioactivities of Nanoparticulated and Ultrasonic-Degraded Levan Generated by Erwinia Tasmaniensis Levansucrase in Human Osteosarcoma Cells. Int. J. Biol. Macromol. 2022, 221, 1121–1129.
