Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Min Hwei Ng.
MicroRNAs are short, single-stranded ribonucleic acids expressed endogenously in the body to regulate gene expression at the post-translational level, with exogenous microRNA offering an attractive approach to therapy. Among the myriad microRNA candidates involved in controlling bone homeostasis and remodeling, microRNA 21 (miR21) is the most abundant.
- microRNA 21
- osteogenesis
- bone resorption
- bone homeostasis
- exogenous miR21
1. Introduction
Bone is a type of mineralized connective tissue that constantly undergoes remodeling to maintain the structural integrity of the skeletal system (i.e., bone homeostasis) and to adapt to mechanical stress or changes in the body’s needs throughout life by replacing damaged or old bone. Bone remodeling primarily consists of three phases, i.e., resorption, reversal, and bone formation [1[1][2],2], through a complex interplay of many factors. It primarily involves the interaction of two distinct bone cells, i.e., osteoblasts and osteoclasts.
1.1. Bone Remodeling and Homeostasis
Bone remodeling is an adaptation process of bone to external stimuli and the environment, and it begins with the bone resorption phase. Bone resorption is a process of mineralized bone removal via osteoclasts, and is guided by the receptor activator of the nuclear kappa-B ligand (RANKL), the receptor activator nuclear factor kappa-B (RANK), and osteoprotegerin (OPG). RANKL and OPG are primarily secreted by stromal cells, osteoblasts, and osteocytes, while RANK is expressed on the surfaces of osteoclasts and their precursors. The binding of RANKL to RANK activates NF-KB, which in turn upregulates c-Fos and NFATc1 in a series of processes that induce the differentiation of osteoblasts into mature osteoclasts [3]. Osteoclasts are multinucleated cells derived from a mononuclear lineage, and are compacted with Golgi complexes, mitochondria, and transport vesicles of lysosomal enzymes. Osteoclasts attach themselves to the bone, then release acid phosphatase and cathepsin K to break down the bone by proteolysis and acidification of the bone matrix and HA [4]. OPG competes with RANK for RANKL to avoid over-excessive resorption by inducing apoptosis of osteoclasts. Thus, the RANK/OPG ratio is crucial to determine the rate of bone resorption.
In the subsequent phase, known as reversal, osteoclasts reabsorb the bone surface for the purpose of new bone formation. Termination of the osteoclasts leaves the remaining collagen fragments exposed. The bone lining cell removes the fragments and forms a thin layer of new bone matrix to distinguish the old from the new [5].
This is then followed by the bone formation phase, or osteogenesis, which involves the (i) differentiation and (ii) maturation of osteoblasts (osteoblastogenesis), the (iii) synthesis of the bone matrix, its (iv) mineralization, and the eventual (v) formation of mature bone tissue. Osteoblastogenesis begins with the differentiation of mesenchymal stem cells (MSCs) residing in the periosteum or migrating from the surrounding tissues, such as the bone marrow into osteoprogenitor cells, also known as pre-osteoblasts, in response to triggers such as growth factors (e.g., BMPs, TGF-beta, IGF, FGF), cytokines, signaling proteins (e.g., Wnt, Notch, Shh proteins), and hormones (parathyroid hormone). Osteoprogenitor cells then undergo further differentiation into osteoblasts under the influence of specific signaling molecules and transcription factors, including Runx2 (Runt-related transcription factor 2) and Osterix, which drive the expression of genes involved in bone formation. Osteoblasts then synthesize and secrete the unmineralized organic matrix of bone, also known as osteoid, which mainly consists of collagen type I fibers, osteopontin, and osteocalcin. The osteoid provides the framework for mineralization and acts as a scaffold for the deposition of calcium and other minerals. The mineralization of the osteoid begins with the osteoblast forming specialized membrane-bound vesicles called matrix vesicles within their cytoplasm containing enzymes such as alkaline phosphatase, ions such as calcium and phosphate, and other molecules necessary for mineralization. Small amorphous mineral clusters serving as nucleation sites for the formation of hydroxyapatite crystals are released into the extra cellular space where they continue to grow, align and become integrated with the collagen fibers of the osteoid. Calcium and phosphate ions from the bloodstream are deposited onto the collagen scaffold, forming hydroxyapatite crystals. Mineralization by osteoblasts can be achieved either via intramembranous ossification or endochondral ossification mechanisms. In intramembranous ossification, bone is directly formed by mesenchymal stem cells differentiating into osteoblasts, which is followed by secretion of the bone matrix (osteoid) and mineralization. Endochondral ossification, which is more common, is a process by which mesenchymal stem cells first differentiate into chondroblasts, thus secreting a cartilaginous matrix, and then invasion by osteoblasts replaces the cartilage with the mineralized bone matrix [4,5][4][5]. As the bone matrix becomes mineralized, some of the osteoblasts become embedded within the matrix and differentiate into osteocytes. Osteocytes are the most abundant cells in mature bone tissue and play crucial roles in both maintaining bone health and responding to mechanical stimuli.
The delicate balance between bone resorption by osteoclasts and bone formation by osteoblasts is essential to ensure that the skeletal system remains strong and healthy. This balance is maintained by a complex interplay of hormones, growth factors, and cellular signaling pathways that modulate the activity of osteoclasts and osteoblasts. Various interactions of osteoclasts and osteoblasts have been studied to date, and most are orchestrated by the RANKL/RANK/OPG and Wnt signaling pathways [3]. Aside from signaling pathways, endocrine secretion hormones also contribute to bone remodeling by coupling osteoclastogenesis and osteoblastogenesis. Examples include growth hormones, insulin growth factors, glucocorticoids, sex hormones (estrogen and androgen), growth factors, prostaglandins, and cytokines [2].
1.2. The Coupling Mechanism between Osteoblasts and Osteoclasts
The coupling mechanism between osteoblasts and osteoclasts is crucial for maintaining bone remodeling homeostasis by balancing bone resorption and formation. This process is regulated by several mediators, including EFNB2-EPHB4, FAS-FASL, and NRP1-SEMA3A. During bone resorption, osteoblasts secrete TGF-β and IGF-1, which induce osteoblastic activity. Osteoblasts also secrete M-CSF, RANKL, and WNT5A, which promote osteoclastic formation [6]. EFNB2, expressed on the osteoclast cell surface, forms a bond with EFNB4 on the osteoblast surface to mediate bidirectional signal transduction between the two cells. EPBH2-mediated EPHB4 activation promotes osteoblastogenesis while EPHB4-induced EFNB2 activation interrupts C-Fos/NFATC signaling pathway, and thus working in reverse fashion from osteoblast to osteoclast to reduce osteoclast activity [7]. FASL is secreted in response to a paracrine signal to decrease osteoclast activity, whereas osteoblasts secrete FAS to increase osteoclast activity [8]. SEMA3A, produced by the osteoblast cell lineage, inhibits RANKL-induced osteoclast reactions and promotes osteoblast activity [9]. M-CSF, secreted by osteoblasts, is an important factor for cell proliferation, binding to C-FMS on the surface of osteoclasts to maintain the coupling mechanism. RANKL, highly expressed in osteoblasts, binds to RANK to initiate osteoclast differentiation, and OPG negatively regulates this process by inhibiting osteoclastogenesis by binding to RANKL [10]. WNT is also essential in bone remodeling, and WNT5A expressed in the osteoblast cell lineage binds to ROR2 on the osteoclast surface. WNT5A enhances bone resorption through the MAPK pathway [11].
1.3. Role of MicroRNA in Bone Homeostasis
MicroRNAs (miRNAs) are single-stranded non-coding RNAs consisting of 19 to 24 nucleotides. They modulate gene activity by binding to or degrading the target messenger RNA (mRNA), hence inhibiting the translation of mRNA into protein. As a result, the expression of specific proteins is suppressed while the upregulation of target proteins associated with the inhibited proteins is simultaneously orchestrated. This indirect mechanism enables miRNAs to facilitate the upregulation of certain proteins by inhibiting the expression of their negative regulators. This interplay leads to negative regulation of gene expression. As a result, specific gene expressions are downregulated or upregulated. In short, microRNAs play an essential role in cell proliferation and differentiation, apoptosis, the metabolism of fat, and resistance to stress [12].
MicroRNA plays a prominent role in both osteoblast and osteoclast differentiation by regulating bone formation through multiple pathways, involving a cascade of signaling pathways [12]. miR20 has been shown to upregulate the osteogenesis of human bone mesenchymal cells (hBMSCs) by downregulating peroxisome proliferator-activated receptor- (PPARỳ) and BMP activin membrane-bound inhibitor (BAMBI) signaling. According to the results, osteoblast markers BMP2, BMP4, Runx2, Osx, OCN, and OPN were elevated [12]. A different study showed that the downregulation of miR-133 and miR-135 inversely upregulates the Runx2 and Smad 5 osteogenesis gene regulators. miR-133 and miR-135 can bind to the 3′ untranslated region (UTR) of CTGF mRNA, resulting in the downregulation of connective tissue growth factor (CTGF) expression [13]. The downregulation of CTGF by miR-133 and miR-135 can potentially affect the balance between bone formation and bone resorption, leading to an influence on osteogenic differentiation and bone mineralization [14]. The action of miR 346 on T cell factor/lymphoid enhancer factor (TCF/LEF), a transcription factor of the Wnt/Catenin pathway, should also be noted. The Wnt/B-catenin pathway has been shown to enhance the activity of ALP in undifferentiated BMSCs to promote osteogenesis [15,16][15][16]. Downregulation of miR-31 was shown to suppress RANKL and induce the formation of osteoclasts to induce bone resorption [12]. Another example is that miR15b positively regulates osteoblasts by targeting Smurf1 to express Runx2 expression. miR 15b is a specific miR expressed in osteoblasts which binds to the mRNA of Smurf1 through complementary base pairing, specifically recognizing the 3′ untranslated region (UTR) of Smurf1 mRNA. This binding leads to the downregulation of Smurf1 expression. Smurf1 is an E3 ubiquitin that targets Runx2, the master regulator of osteoblast differentiation, and degrades it [17]. In terms of the expression of Runx2, when miR-15b targets and downregulates the expression of Smurf1, it indirectly leads to an increase in the expression of Runx2. Both positive and negative coordination of osteogenesis are regulated by miR through transcription factors. MicroRNAs can exhibit a dual role in gene regulation. While they typically inhibit the expression of specific proteins, in some cases, this inhibition leads to the upregulation of other proteins, resulting in a positive regulatory effect. Conversely, when microRNAs inhibit the expression of certain proteins and consequently alter or diminish their effects, this is referred to as negative regulation of the mechanism. These findings suggest that exogenous microRNA supplementation may be a possible therapeutic approach to overcome bone-related disorders.
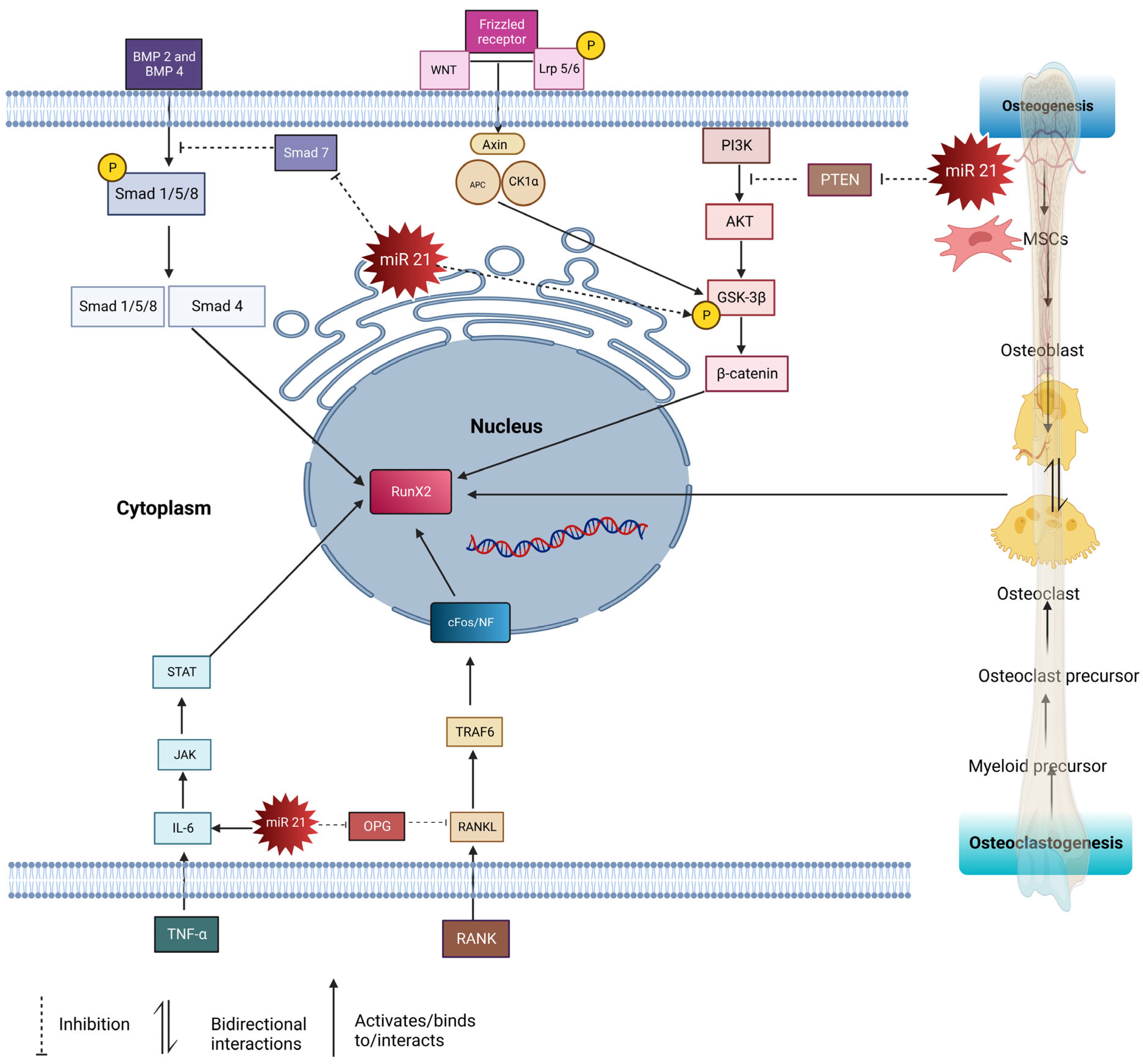
2. Role of MicroRNA 21 in Bone Homeostasis
The discovery of microRNA in Caenorhabditis elegans (C. elegan) and humans, along with the study of their regulatory functions, explained gene expressions and genomics as an entirely new concept [18]. The discovery of miRNAs and their regulatory functions has shed light on the complexity and fine-tuning of gene expression. Previously, gene regulation was primarily attributed to transcription factors and other DNA-binding proteins. Lin-4 was discovered in C. elegans as a short non-coding microRNA that regulates gene expression [1]. Let-7 was the second microRNA discovered, and this was followed by many novel microRNAs that were identified in flies, worms, vertebrates, and plants [19]. These findings have led to remarkable progress toward the study of diverse microRNAs. miR21 was one of the earliest to be identified and studied due to its role in health and diseases [20]. Essentially, miR21 regulates cell growth, migration, and invasion, and is also expressed in immune modulator cells, B and T cells, monocytes, macrophages, and dendritic cells (DCs) [21]. In general, miR21 suppresses the target mRNA’s gene of interest by binding to its 3′ UTRs. This binding leads to the degradation of the mRNA and inhibits its translation. This cascade of reactions can result in the upregulation or downregulation of specific gene expressions, which, in turn, has positive or negative effects on osteogenic differentiation and mineralization. miR21 plays a role in promoting osteogenesis by safeguarding Runx2, which regulates the synthesis of other proteins related to bone formation. Additionally, miR21 serves as a crucial regulator for inducing RunX2 [22]. Recent updates have stated that miR21 may act with either pro-inflammatory or anti-inflammatory effects in any healthy or pathological environment. This condition depends on the microenvironment; complex signaling pathways; signaling radiated by immune cells; and extracellular signals such as 12-O-Tetradecanoylphorbol-13-acetate (TPA)/Phorbol 12 myristate 13 acetate (PMA), lipopolysaccharides (LPS), interleukin 6 (IL6), tumor growth factor (TGF)/bone morphogenetic proteins (BMP), and many more. The end results of these signals stimulate the role of miR21 as a negative or positive regulator of an inflammation environment at the transcriptional or post-transcriptional level [23]. It has been labeled as a cancer-promoting microRNA, or “oncomiR”, and has since been a target for diagnostic or prognostic markers and therapeutic candidates (anti-microRNA therapy) [24]. However, it is still not clearly understood. This miR21 is released as a biomarker of tumor formation, as it is widely transported in the exosomes. In addition, miR21 itself elicits an inflammation reaction to escalate tumor progression or to orchestrate a general immune response. Despite this association, there is also a rising body of evidence that miR21 plays an integral role in osteogenesis, and, thereby, there have been attempts to develop microRNA therapy for treatment of bone loss [25].3. Empirical Evidence of MicroRNA-21 in Regulating Bone Homeostasis
Smieszek transfected the MC3T3 cell line, derived from Mus Musculus mice, to investigate the effect of miR21 on inducing osteoblast proliferation [32][26]. The MG63 cell line was incorporated with the Ti-SrHA-21 scaffold by Geng to study the effect of miR21 on osteogenesis [29][27]. The 4B12 cell line, derived from the young calvaria of a mouse, is primarily used to analyze the differentiation of TRAP-positive multinucleated cells into osteoclasts. Smieszek utilized this cell line to compare the effects of miR21 on both bone formation and bone sorption. Besides using MSCs and cell lines from mice, CD4+ T cells extracted from the mouse spleen were also employed to measure the effect of miR21 on promoting osteogenesis [32][26]. Wu concluded that T cells play a role in the osteoclast mechanism, and found that miR21 promoted the secretion of RANKL by activated T cells, leading to the promotion of osteoclast activity and thereby increasing osteoblast activity [29][27]. Xu analyzed the regulation of the miR21/STAT3 signal on odontoblast differentiation of dental pulp stem cells (DPSCs) in an inflammation microenvironment constructed by TNF-a [26][28]. The study assessed the role of miR21 in orthodontic bone development by measuring the dentin matrix acidic phosphoprotein (DMP1) and dental sialophosphoprotein (DSPP) proteins. Overall, most in vitro studies were conducted to investigate the effect of miR21, along with its scaffold, directly on cell lines or primary cells from animals to demonstrate that miR21 positively regulates osteogenic differentiation and mineralization by promoting the expression of key osteogenic factors, such as ALP, RUNX2, OPN, and OSX. The rates of bone formation and bone resorption were measured by RT-PCR, Western blot, and ELISA analysis. Immunostaining, ALP, and alizarin staining were carried out to detect the presence of minerals. In vivo studies provide more significant outcomes that are more relevant to future human subjects. The mineralization rate and bone formation or healing rate were measured using imaging techniques, RT PCR, and Western blot analysis. Various staining methods are used to determine the rates of bone healing and mineralization. Improved movement ability of animal subjects after treatment or surgery indicated successful bone growth and healing in some studies. Wang and Li demonstrated the novel contribution of miR21 by comparing its effects in wild-type mice with those in knock-out mice [22,28][22][29]. Endogenous miR21 expression in wild-type mice BMSCs after osteoinduction was analyzed, revealing that these cells naturally express miR21. Thise study also compared the effect of endogenous miR21 expression with that of exogenously introduced miR21. Wild-type mice treated with miR21 mimic showed enhanced new bone formation due to miR21 overexpression. Li also conducted an in vivo study to evaluate miR21′s osteogenic properties [28][29].4. Pathways Regulated by miR-21 in Bone Homeostasis
4.1. Smad Pathway
Smad is an intracellular signaling protein molecule composed of Smad 1–9 members. These molecules react by phosphorylating transforming growth factor-beta (TGF β) or bone morphogenic protein (BMP) [31][30]. Transforming growth factor-beta (TGF-β) and bone morphogenic proteins (BMPs) BMP 2, BMP 4, and BMP 7 are crucial in osteoblast differentiation. Both TGF β and BMP have significant roles in bone development. BMP binds to the type 1 receptor and activates the Smad pathway by phosphorylating Smad 1/5/8, which then forms complexes with Smad 4. This complex is then translocated into the nucleus and acts on transcription factors such as RunX2 and Osx. RunX2 plays a crucial role in regulating MSCs to differentiate in osteoblast lineage; it is known as the master gene in regulating osteoblast cell formations. Osx is involved in osteogenesis by inducing bone matrix formation and initiating the differentiation of MSCs into an osteogenic lineage [31][30]. RunX2 and Osx induce pre-osteoblast cells to differentiate into osteoblasts [33][31]. Additionally, miR21 modulates the expression of target genes involved in bone-forming cells’ osteoblast proliferation and differentiation. miR21 regulates the Smad signaling pathway by targeting and suppressing the expression of Smad 7, a negative regulator of the osteogenesis pathway. Thus, this leads to the activation of the Smad 1/5/8 pathway to control bone formation. The synergistic effect of strontium-substituted hydroxyapatite and miR21 in improving bone remodeling and osseointegration was demonstrated by Geng through both in vitro and in vivo studies [29][27]. In the study, Ti-SrHA (titanium-strontium hydroxyapatite) loaded with miR21 was implanted in rabbits. The Ti-SrHA-miR21 provided better surface adhesion, promoting increased cell proliferation compared to the non-coated implant. The nanostructured and hydrophilic properties of this Ti-SrHA combination allowed for uniform distribution of the nanocapsules of miR21. Endogenously added miR21 increased the osteoblast cell proliferation and the ALP expression compared to the non-treated group. The imaging analyses using SEM, MicroCT, and X-ray projected accelerated mineralization in Ti-SrHA-miR21 compared to Ti, Ti-miR21, and Ti-SrHa. The participation of SrHa increased the osteoblast proliferation rate as well, but was not as efficient as the participation of miR21. This proved that Ti-SrHA-miR21 led to rapid bone healing. The paper concluded that COL-I, RUNX2, OPN, OPG, and OCN, which are osteogenesis-related genes, were significantly upregulated as a synergic effect of Ti-SrHA, along with miR21. This finding suggests the probable participation of miR21 in the Smad pathway, as was demonstrated in a previous study by Li [16,29][16][27].4.2. RANKL/RANK/OPG Pathway
Nuclear factor -k β (NF-k β) ligand (RANKL), tumor necrosis factor (TNF), and macrophage colony-stimulating factor (M-CSF) are osteoclast-stimulating factors [34][32]. RANKL is a transmembrane protein found as a membrane-bound, secreted protein resulting from proteolytic cleavage, and is expressed by synovial cells or secreted by activated T cells. RANKL interacts with RANK further by activating TRAF6, leading to cascade’s mitogen-activated protein kinase (MAPKs), ERK, p38, JNK (c-Jun N terminal kinase), and AKT (protein kinase B). T cell-produced RANKL stimulates osteoclast formation through c-Fos. In contrast, osteoprotegerin (OPG) is a decoy receptor that binds to RANKL and inhibits osteoclastogenesis through the nuclear factor of activated T cells (NFATc1), NFATc1 stimulates OSX, an essential transcription factor for osteoblasts [33][31]. Smieszek’s paper investigated MC3T3 pre-osteoblast cells, MC3T3 miR21-inhibited cells and the 4BI2 pre-osteoclast precursor cell line. MC3T3/4B12, the merge between pre-osteoclast and osteoblast cell lines, resulted in increased tartrate-resistant acid phosphatase (TRAP), matrix metalloproteinase (MMP9), and Cathepsin K (Ctsk) genes, which are actively involved in osteoclastogenesis. MC3T3inh21 cells reduced Coll-1, OCL, OPN, and RunX2, the key regulators of osteogenesis. The inhibition of miR21 upregulates the osteoclast-suppressor-programmed cell death protein 4 (PDCD4), and, vice versa, downregulates osteoclasts. Thus, this explains the significance of miR21 in osteogenesis. The coupling role of (OPN) in osteoblast differentiation facilitates the attachment of osteoclasts at the resorbed matrix region, which is emphasized in thise study. OPN plays a crucial role in the early stage of bone remodeling by differentiating osteoblast cells. During paracrine interaction between osteoblast cells, OPN stimulates the attachment of osteoclasts and the release of RANKL [2].4.3. STAT3 Pathway
Bone healing is initiated by an inflammatory mechanism, followed by bone formation processes. This inflammatory reaction is initiated by lymphocytes; monocytes; neutrophils; macrophages; and secretions such as IL1-IL6, TNF, and many more. Janus kinase (JAK)/signal transducer and activator of transcription (STAT) are primarily involved in cell metabolism and differentiation in inflamed microenvironments. JAKs belongs to the protein tyrosine kinase (PTK) family, and its primary role is to act as a STAT [35][33]. JAK induces the phosphorylation of STAT, which then migrates to the nucleus and binds to target genes to induce MSCs for osteoblast differentiation [31][30]. In bone homeostasis, IL-6 is produced by osteoblast cells to promote bone resorption by osteoclasts. IL-6 may negatively regulate osteogenesis through SHP2/MEK/ERK and SHP2/p13K/Akt2, or may positively regulate osteogenesis through the JAK/STAT3 pathway [36][34]. JAK/STAT3 is a pathway activated by a series of cytokinins, and it can also be regulated by miR21 by suppressing PTEN/PDCD4. Vice versa, JAK/STAT3 activation can also induce the expression of miR21 in an inflammation microenvironment [37][35].4.4. PTEN-PI3K/Akt Signaling Pathway
Phosphatase and tensin homolog (PTEN) play crucial roles in bone formation by regulating the phosphoinositide 3-kinases/protein Kinase B (P13/Akt) pathway. PTEN is a bilipid protein phosphatase targeted to phosphatidylinositol-3,4,5-triphosphate (PIP3), a product of phosphatidylinositol 3 kinases (P13K). The inhibition of PTEN, as well as P13K, activates the downstream activators pyruvate dehydrogenase kinase 1(PDK1), AKT/PKN, and G proteins Rac1/cdc42, leading to cell growth and proliferation [38][36]. PTEN is well known for its function in osteogenesis through the AKT/P13K pathway. Interestingly, miR21 has the potential to regulate PTEN to modulate both osteoblastogenesis and osteoclastogenesis.4.5. WNT/β- Catenin Pathway
P13K also activates AKT, which enhances pGSK3b and plays a role through the canonical WNT signaling pathway. Canonical WNT/β- catenin binds to LPS 5/6 (low-density lipoprotein receptor-related protein). Followed by the binding of WNT receptors to frizzled (FZD), this forms a ternary complex resulting in the phosphorylation of WNT. This was followed by the assembly of disheveled (Dlv), which induces the phosphorylation of Lrp5/6. This resulted in the inhibition of the AXIN complex protein, which is composed of glycogen synthase kinase 3 beta (GSK-3β), adenomatous polyposis coli (APC), and casein kinase I (CK1). AXIN is responsible for destructing β-catenin. Thus, Dvl blocks the phosphorylation of Axin by (GSK-3β), which is an essential factor for the phosphorylation of β- catenin. This then causes the accumulation of β-catenin in the cytoplasm, eventually migrating into the nucleus and regulating the gene expression of RunX 2, thus increasing the formation of osteoblast precursor cells [39][37]. Besides this, miR21 also promotes the phosphorylation of the glycogen synthase kinase three beta (GSK-3β), which accumulates beta-catenin in the cytoplasm, then targets TCF3, a gene which enhances osteogenesis [21]. The mentioned pathways (Smad, RANKL/RANK/OPG, STAT, and beta-catenin) are interconnected, and play important roles in bone homeostasis. The Smad pathway is involved in bone formation and is regulated by miR-21. In this pathway, miR-21 targets and suppresses the expression of Smad 7, a negative regulator of osteogenesis. By inhibiting Smad 7, miR-21 promotes the activation of the Smad 1/5/8 pathway, which enhances bone formation. The RANKL/RANK/OPG pathway is crucial for regulating the balance between bone resorption (osteoclast activity) and bone formation (osteoblast activity). miR21 influences this pathway by regulating the expression of key factors. miR21 can upregulate the expression of RANKL, a protein that stimulates osteoclast formation, and can downregulate the expression of OPG, a protein that inhibits osteoclast formation. This imbalance between RANKL and OPG promotes osteoclast activity, leading to increased bone resorption. The STAT pathway is involved in various cellular processes, including bone metabolism. miR21 is known to play a role in this pathway. By targeting specific genes or regulators within the STAT pathway, miR21 can influence the signaling and transcriptional activity of STAT proteins, which may impact bone homeostasis. The β-catenin pathway is a critical signaling pathway involved in bone development, maintenance, and regeneration. It regulates the differentiation and activity of osteoblasts, which are responsible for bone formation. MiR-21 has been found to interact with this pathway, although the exact mechanisms are still being investigated. It may regulate the expression of certain genes or modulate the activity of key proteins within the beta-catenin pathway, impacting bone remodeling processes. In summary, miR-21 regulates multiple pathways involved in bone homeostasis, including the Smad, RANKL/RANK/OPG, STAT, and β- catenin pathways. These pathways interact and collectively contribute to the regulation of osteoblastogenesis, osteoclastogenesis, and bone remodeling. miR21′s modulation of these pathways can have significant effects on bone health and may play a role in conditions such as osteoporosis or bone-related disorders (Figure 1).
Figure 1. The pathways involved in osteogenesis and osteoclastogenesis regulated by miR21: RANKL/OPG, PTEN/AKT/P13K, BMP2/SMAD 1/5/8, TNFα/IL-6/JAK/STAT3, WNT/βCatenin. PTEN/AKT/P13K pathway: miR21 suppresses PTEN, thus activating the PI3K-AKT-GSK3β pathway, promoting β-catenin in the nucleus to modulate the expression of osteogenesis-related genes. TNFα/IL-6/JAK/STAT3 pathway: IL 6 activates miR21, resulting in the STAT pathway being upregulated and increasing osteogenesis. RANKL/OPG pathway: RANK ligand binds to RANKL receptors and activates TRAF6 cascades, leading to the activation of osteoclasts. miR21 inhibits the secretion of OPG, and the imbalance ratio of OPG/RANKL upregulates osteoclastogenesis. WNT/βCatenin pathway: WNT binds to Fz and Lrp5/6, causing Dvl to phosphorylate Lrp5/6, leading to the accumulation of β-catenin, which then translocates to the nucleus to induce osteogenesis. miR21 phosphorylation upregulates GSK-3β, thus increasing the accumulation of β-catenin. Smad pathway: BMPs bind to type 1 receptor and activate the Smad pathway by phosphorylating Smad 1/5/8, forming complexes with Smad 4. This complex translocates into the nucleus and acts on transcription factors such as RunX 2 to induce osteogenesis. miR21 inhibits Smad 7, thus upregulating the Smad 1/5/8 pathways.
5. miR21 in Therapeutic Applications
Pharmaceutical companies are actively exploring alternative therapeutic molecules as substitutes for existing chemically composed drugs. These molecules need to fulfill the medical requirements in terms of pharmacokinetic availability, properties, safety, and efficacy. Presently, numerous miRNA molecules are undergoing clinical trials, and there is a significant body of literature, consisting of around 600 published articles, focusing on miRNA-based therapeutics. The first miRNA molecule to enter clinical trials was Miravirsen, which is currently in phase II trials across multiple countries [7]. These studies aim to assess the role of exogenous miR21, with or without a carrier, in new bone formation, potentially enhancing the effectiveness of the current therapeutic approaches for bone healing and formation. Sun’s study consistently highlights the role of nanocapsulated miR21 in the healing of osteoporotic fractures, demonstrating its potential to accelerate bone healing in osteoporotic patients. Yang also acknowledged the promising therapeutic effect of miR21 on the facilitation of rapid bone formation. In Yang’s research, miR21 was encapsulated with chitosan and administered via injection in gel form to an osteoporotic model. The efficient release of miR21 stimulated bone repair in the osteoporotic model. Yang conducted experiments on canine and rat bone defect models to investigate the influence of miR21 on new bone formation, suggesting that miR21 regulates the PTE/PI3K pathway for osteogenesis. The study validated these effects through both in vivo and in vitro assessments. In the canine model, an osteoperiosteal defect model was introduced and treated with miR21 incorporated into β-tricalcium phosphate (β-TCP), resulting in a remarkable increase in bone formation compared to the non-treated group. This further emphasizes the positive impact of miR21 on bone healing. Geng’s research focused on studying the effects of titanium coated with strontium-substituted hydroxyapatite, incorporating miR21 into bone healing using a rabbit model. The findings indicated that a combination of SrHA, miR21, and titanium promoted bone mineralization and strength. The consistent evidence of mineralization leading to new bone formation resulting from the addition of endogenous miR21 suggests its broad potential for implementation in therapeutic approaches for the treatment of bone-related disorders [15,29,30][15][27][38].
6. miR-21 in the Coupling Mechanism
miR21 is induced towards osteoclastogenesis by RANKL secretion. In cases of inflammation, IL6 can result in the overexpression of miR21, which activates STAT3 and inhibits OPG production, ultimately promoting RANKL gene activation. Conversely, inhibiting miR21 would hinder STAT3 activation, thereby disrupting the OPG/RANKL pathway. The contribution of miR21 to the coupling mechanism is entirely reliant on the inducing factor. The maintenance of bone remodeling homeostasis depends on various hormones, factors, and signaling molecules. RANKL binds to RANK, leading to the activation of TRAFs and a cascade of ERK, p38, JNK, and P13K [43][39]. This process triggers c-Fos, upregulates the expression of miR21 gene, and downregulates PDCD4 protein levels. PDCD4 is a tumor suppressor that is involved in cell proliferation and progression. As a result of RANKL binding, NFATc1 and BMM are expressed to initiate osteoclast differentiation. NFATc1 acts as a co-factor with AP-1, composed of Fos/Jun proteins, to bind to osteoclast-specific markers such as TRAP and cathepsin. PDCD4 also affects the transcription factor AP-1, which regulates cellular differentiation, proliferation, and apoptosis. Studies show that miR21 has binding sites for transcription factors such as Ap-1 and PU.1. PU.1 is a lineage-specific transcription factor that regulates various cell lineages, including osteoclasts. OPN, which plays a critical role in osteoclastogenesis, is regulated by PU.1. Transcription factors such as c-Fos and PU.1 increase miR21 expression through Ap-1 and upregulate OPN, leading to a shift in bone homeostasis to bone resorption [44][40].
References
- Van Rooij, E. The Art of MicroRNA Research. Circ. Res. 2011, 108, 219–234.
- Allen, M.R.; Burr, D.B. Bone Modeling and Remodeling. In Basic and Applied Bone Biology; Academic Press: Cambridge, MA, USA, 2013; pp. 75–90.
- Boyce, B.F.; Xing, L. The RANKL/RANK/OPG Pathway. Epidemiol. Pathophysiol. Accessory 2007, 5, 98–194.
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396.
- Kenkre, J.S.; Bassett, J.H.D. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327.
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. 2020. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073.
- Zhao, C.; Irie, N.; Takada, Y.; Shimoda, K.; Miyamoto, T.; Nishiwaki, T.; Suda, T.; Matsuo, K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006, 4, 111–121.
- Yan, X.; Liu, Z.; Chen, Y. Regulation of TGF- b signaling by Smad7 Overview of TGF- b Signaling Pathways. Acta Biochim. Biophys. Sin. 2009, 41, 263–272.
- Hayashi, M.; Nakashima, T.; Taniguchi, M.; Kodama, T.; Kumanogoh, A.; Takayanagi, H. Osteoprotection by semaphorin 3A. Nature 2012, 485, 69–74.
- Mizuno, A.; Kanno, T.; Hoshi, M.; Shibata, O.; Yano, K.; Fujise, N.; Kinosaki, M.; Yamaguchi, K.; Tsuda, E.; Murakami, A.; et al. Transgenic mice overexpressing soluble osteoclast differentiation factor (sODF) exhibit severe osteoporosis. J. Bone Miner. Metab. 2002, 20, 337–344.
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-Osteoclast Interactions. Connect. Tissue Res. 2018, 59, 99–107.
- Sriram, M.; Sainitya, R.; Kalyanaraman, V.; Dhivya, S.; Selvamurugan, N. Biomaterials mediated microRNA delivery for bone tissue engineering. Int. J. Biol. Macromol. 2015, 74, 404–412.
- Wildman, B.J.; Godfrey, T.C.; Rehan, M.; Chen, Y.; Afreen, L.H.; Hassan, Q. MICROmanagement of Runx2 Function in Skeletal Cells. Curr. Mol. Biol. Rep. 2019, 5, 55–64.
- Mitchelson, K.R. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. World J. Biol. Chem. 2015, 6, 162–208.
- Sun, X.; Li, X.; Qi, H.; Hou, X.; Zhao, J.; Yuan, X.; Ma, X. MiR-21 nanocapsules promote early bone repair of osteoporotic fractures by stimulating the osteogenic differentiation of bone marrow mesenchymal stem cells. J. Orthop. Transl. 2020, 24, 76–87.
- Li, X.; Guo, L.; Liu, Y.; Su, Y.; Xie, Y.; Du, J.; Zhou, J.; Ding, G.; Wang, H.; Bai, Y.; et al. MicroRNA-21 promotes osteogenesis of bone marrow mesenchymal stem cells via the Smad7-Smad1/5/8-Runx2 pathway. Biochem. Biophys. Res. Commun. 2017, 493, 928–933.
- Shimazu, J.; Wei, J.; Karsenty, G. Smurf1 Inhibits Osteoblast Differentiation, Bone Formation, and Glucose Homeostasis. Cell Rep. 2016, 15, 27–35.
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854.
- Kumarswamy, R.; Volkmann, I.; Thum, T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011, 8, 706–713.
- Hill, M.; Tran, N. Global miRNA to miRNA Interactions: Impacts for miR-21. Trends Cell Biol. 2021, 31, 3–5.
- Meng, Y.-B.; Li, X.; Li, Z.-Y.; Zhao, J.; Yuan, X.-B.; Ren, Y.; Cui, Z.-D.; Liu, Y.-D.; Yang, X.-J. microRNA-21 promotes osteogenic differentiation of mesenchymal stem cells by the PI3K/β-catenin pathway. J. Orthop. Res. 2015, 33, 957–964.
- Li, H.; Yang, F.; Wang, Z.; Fu, Q.; Liang, A. MicroRNA-21 promotes osteogenic differentiation by targeting small mothers against decapentaplegic 7. Mol. Med. Rep. 2012, 12, 1561–1567.
- Sheedy, F.J. Turning 21: Induction of miR-21 as a Key Switch in the Inflammatory Response. Front. Immunol. 2015, 6, 19.
- Feng, Y.-H.; Tsao, C.-J. Emerging role of microRNA-21 in cancer (Review). Biomed. Rep. 2016, 5, 395–402.
- Van Wijnen, A.J.; van de Peppel, J.; van Leeuwen, J.P.; Lian, J.B.; Stein, G.S.; Westendorf, J.J.; Oursler, M.-J.; Im, H.-J.; Taipaleenmäki, H.; Hesse, E.; et al. MicroRNA Functions in Osteogenesis and Dysfunctions in Osteoporosis. Curr. Osteoporos. Rep. 2013, 11, 72–82.
- Smieszek, A.; Marcinkowska, K.; Pielok, A.; Sikora, M. The Role of miR-21 in Osteoblasts—Osteoclasts. Cells 2020, 9, 479.
- Geng, Z.; Wang, X.; Zhao, J.; Li, Z.; Ma, L.; Zhu, S.; Liang, Y.; Cui, Z.; He, H.; Yang, X. The synergistic effect of strontium-substituted hydroxyapatite and microRNA-21 on improving bone remodeling and osseointegration. Biomater. Sci. 2018, 6, 2694–2703.
- Xu, K.; Xiao, J.; Zheng, K.; Feng, X.; Zhang, J.; Song, D.; Wang, C.; Shen, X.; Zhao, X.; Wei, C.; et al. MiR-21/STAT3 Signal Is Involved in Odontoblast Differentiation of Human Dental Pulp Stem Cells Mediated by TNF-α. Cell. Reprogram. 2018, 20, 107–116.
- Wang, S.; Liu, Z.; Wang, J.; Ji, X.; Yao, Z.; Wang, X. miR-21 promotes osteoclastogenesis through activation of PI3K/Akt signaling by targeting Pten in RAW264.7 cells. Mol. Med. Rep. 2020, 21, 1125–1132.
- Wu, L.; Su, Y.; Lin, F.; Zhu, S.; Wang, J.; Hou, Y.; Du, J.; Liu, Y.; Guo, L. MicroRNA-21 promotes orthodontic tooth movement by modulating the RANKL/OPG balance in T cells. Oral Dis. 2020, 26, 370–380.
- Zou, M.-L.; Chen, Z.-H.; Teng, Y.-Y.; Liu, S.-Y.; Jia, Y.; Zhang, K.-W.; Sun, Z.-L.; Wu, J.-J.; Yuan, Z.-D.; Feng, Y.; et al. The Smad Dependent TGF-β and BMP Signaling Pathway in Bone Remodeling and Therapies. Front. Mol. Biosci. 2021, 8, 593310.
- Al-Bari, A.A.; Al Mamun, A. Current advances in regulation of bone homeostasis. FASEB BioAdvance 2020, 2, 668–679.
- Sanpaolo, E.R.; Rotondo, C.; Cici, D.; Corrado, A.; Cantatore, F.P. JAK/STAT pathway and molecular mechanism in bone remodeling. Mol. Biol. Rep. 2020, 47, 9087–9096.
- Mu, Y.; Yang, L.; Li, C.; Qing, W. Role of Inflammatory Factors in Regulation of Osteogenesis in Tissue-Engineered Bone. In Osteogenesis and Bone Regeneration; IntechOpen: Rijeka, Croatia, 2019.
- Wu, Y.; Song, Y.; Xiong, Y.; Wang, X.; Xu, K.; Han, B.; Bai, Y.; Liming, Z.; Zhang, Y.; Zhou, L. MicroRNA-21 (Mir-21) Promotes Cell Growth and Invasion by Repressing Tumor Suppressor PTEN in Colorectal Cancer. Cell. Physiol. Biochem. 2017, 43, 945–958.
- Blanco-Aparicio, C.; Renner, O.; Leal, J.F.; Carnero, A. PTEN, more than the AKT pathway. Carcinogenesis 2007, 28, 1379–1386.
- Bisson, S.-K.; Ung, R.-V.; Mac-Way, F. Role of the Wnt/β-Catenin Pathway in Renal Osteodystrophy. Int. J. Endocrinol. 2018, 2018, 5893514.
- Yang, C.; Liu, X.; Zhao, K.; Zhu, Y.; Hu, B.; Zhou, Y.; Wang, M.; Wu, Y.; Zhang, C.; Xu, J.; et al. miRNA-21 promotes osteogenesis via the PTEN/PI3K/Akt/HIF-1α pathway and enhances bone regeneration in critical size defects. Stem Cell Res. Ther. 2019, 10, 65.
- Kong, L.; Smith, W.; Hao, D. Overview of RAW264.7 for osteoclastogensis study: Phenotype and stimuli. J. Cell. Mol. Med. 2019, 23, 3077–3087.
- Sugatani, T.; Vacher, J.; Hruska, K.A. A microRNA expression signature of osteoclastogenesis. Blood 2011, 117, 3648–3657.
More
