Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Vasile Valeriu Lupu and Version 2 by Camila Xu.
Coronavirus disease 2019 (COVID-19) is a complex infectious disease caused by the SARS-CoV-2 virus, and it currently represents a worldwide public health emergency. The pediatric population is less prone to develop severe COVID-19 infection, but children presenting underlying medical conditions, such as diabetes mellitus, are thought to be at increased risk of developing more severe forms of COVID-19.
- children
- COVID-19
- diabetes mellitus
- SARS-CoV-2
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first isolated and identified, in Wuhan, China, in 2019 [1]. Many studies suggested that viruses, like enteroviruses (Coxsackie virus B), rotavirus, mumps virus and cytomegalovirus, are potential triggers of Type 1 diabetes mellitus (T1DM) in children and young adults [2]. Currently, SARS-CoV-2 infection also appears to be a potential trigger for the development of diabetes mellitus type 1 and 2 in children, which represents the most frequent chronic metabolic disorder within the pediatric population. In their study, Graff et al. [3] found that among 454 patients identified with SARS-CoV-2 infection, those with diabetes were more prone for admission (aOR, 6.6; p = 0.04), and, moreover, data also showed that diabetes mellitus and other cardiovascular comorbidities were identified as major risk factors regarding outcome and mortality in patients with COVID-19. ACE2 is a receptor expressed in various organs, including both exocrine and endocrine tissues of the pancreas. In a manner similar to SARS-CoV, the virus responsible for the 2003 pandemic, SARS-CoV-2 binds to ACE2 receptors through its spike protein. Since the emergence of the SARS-CoV-2 pandemic, diabetes has been acknowledged as a risk factor associated with increased morbidity and mortality in patients with COVID-19. Moreover, recent evidence indicated that COVID-19 may result in poorer outcomes among individuals with pre-existing diabetes, potentially leading to the development of diabetic ketoacidosis (DKA) [4].
Clinical manifestations of COVID-19 are correlated with age according to the data available in the literature [5]. Pediatric patients with no important medical history are susceptible to COVID-19 but usually have a milder course compared to adults [6]. COVID-19 can exhibit a pronounced clinical progression involving acute respiratory distress syndrome (ARDS), accompanied by a localized and systemic surge of cytokines, which can potentially lead to rapid clinical deterioration and failure of multiple organs. Compared to adults who develop respiratory symptoms that can evolve into ARDS, most children do not have respiratory diseases but can develop a life-threatening multisystem inflammatory syndrome (MIS-C). MIS-C is a multi-system inflammatory syndrome that affects individuals aged <21 years with a history of SARS-CoV-2 infection within 4–6 weeks prior to the onset of symptoms presenting with fever, laboratory evidence of inflammation and multisystem (>2) organ involvement, with cardiac and gastrointestinal being the most frequent issues and no alternative plausible diagnoses [7]. In children, MIS-C exhibits similarities to Kawasaki disease, such as febrile illness with inflammation of the blood vessels and possible subsequent consequences of coronary artery aneurysm, conjunctivitis, rash and congestion of the oropharynx [8]. Neurological manifestations, such as headache, meningism, skin hyperesthesia and altered consciousness, were also described in MIS-C [9]. Nevertheless, these findings are non-specific and are found in a vast area of other pediatric infectious diseases [10].
The defects in insulin action and/or secretion are present in diabetes mellitus, which sums up a group of chronic metabolic diseases that imply elevated blood glucose values, mentioning that insulin action and/or secretion disorder may coexist in the same patient [11]. Diabetes can be classified as type 1 diabetes (insulin-dependent diabetes mellitus) and type 2 diabetes (non-insulin-dependent).
1.1. T1DM
Type 1 diabetes mellitus (T1DM) occurs when the beta cells in Langerhans pancreatic islets are gradually destroyed by the immune system, leading to a decrease in the body’s insulin production capacity, eventually resulting in no insulin production [12]. If insulin is lacking, the production of hepatic glucose increases and the use of insulin decreases. This leads to a significant breakdown of adipose tissue, ketonemia and successive diabetic ketoacidosis that can be life threatening if not treated [13].
The diagnosis of type 1 diabetes is established based on the association between clinical manifestations, such as polydipsia, polyuria, weight loss, the laboratory findings and the autoantibody positivity, and other clinical signs can include abdominal pain, vomiting, shortness of breath and an altered general status. Diabetes can sometimes be misdiagnosed as asthma (because of Kussmaul respiration) or as an acute abdomen, but these errors are becoming rare with the help of laboratory testing [14]. In the absence of important comorbidities, T1DM and its treatment are known to have multiple complications, the two major ones being diabetic ketoacidosis and hypoglycemia.
Acute hyperglycemia leads to diabetic ketoacidosis, which is an acute life-threatening metabolic complication of diabetes with a mortality rate of 0.5 percent and with almost 40 percent of DKA cases at presentation. Cerebral edema represents the most frequent cause of death among the diabetic population. For the already known cases of diabetes, the rate of this complication is 1 to 8 percent per year. DKA is managed with immediate hospitalization, insulin replacement and rehydration [15][16][15,16].
On the other hand, hypoglycemia is a T1DM complication of insulin treatment. Its symptoms are the result of a fall in blood glucose, leading to a range of neurogenic and neuroglycopenic symptoms, including emotional instability and tremor. In critical cases, seizures and unconsciousness may be present, and there are suspicions regarding permanent cognitive sequelae caused by repeated hypoglycemic episodes, which are to be confirmed [17][18][17,18]. This condition was reported to exist in approximately 19–37 percent of children and adolescents with the tendency of reduction in the prevalence over time [19]. When severe hypoglycemia occurs, urgent treatment is required and can be effective with the administration of glucagon intravenously, intramuscularly or subcutaneously [20].
Studies describe that SARS-CoV-2 infects [21] pancreatic cells, leading to replication and alteration of the β-cell function precisely and via impairment of insulin secretion. Moreover, SARS-CoV-2 triggers autoimmunity [22], meaning that both autoantibody-positive insulin-dependent and autoantibody-negative diabetes can develop during an infection with the novel coronavirus [23].
1.2. T2DM
Type 2 diabetes mellitus (T2DM) is a complex, chronic metabolic disease that consists of hyperglycemia as a result of insulin resistance and a variable grade of impairment in insulin secretion due to β-cell dysfunction (lipotoxicity, inborn genetic defect or acquired from glucose toxicity or other mechanisms) [24].
The frequency of type 2 diabetes has increased significantly around the world over the past 20 years [25]. In the pediatric populations, T2DM accounts for less than 50% of all diabetes cases, and there are significant ethnic differences in prevalence all around the world. After adjustment for other demographic characteristics, Pham et al. demonstrated, in their study, that the probability of having type 2 diabetes was double among the Asian population, 65% more likely among Black people, and 17% more likely among people of mixed/other ethnicities [26][27][26,27]. The constantly increasing incidence of type 2 diabetes mellitus among children and adolescents is also becoming a source of concern to all those involved in the care of diabetic children [28].
Usually, the debut of T2DM occurs during puberty in high-risk obese adolescents having insulin resistance, knowing that the data regarding the prevalence of T2DM among children under 10 years of age are very limited [29]. Clinically, it presents characteristics of metabolic syndrome, such as arterial hypertension, hyperlipidemia, acanthosis nigricans, fatty liver disease and polycystic ovary disease [26]. The complications of T2DM are of great importance. Arterial hypertension and imbalance of lipids have an impact on the early development of retinopathy, nephropathy and increase the risk of future cardiovascular disease, which is a major cause of morbidity and mortality in adults with T2DM. These comorbidities occur early and progress at an alarming rate in children and adolescents, even in those with efficient metabolic control. Unfortunately, in comparison to the adult population, treatment options for children are limited. Needless to mention, an early initiation of treatment with metformin alongside lifestyle modifications and physical activity for all youth with T2D remains imperative and leads to better compliance [30][31][30,31].
In addition, measures, like social distancing, online schooling, increased intake of high-calorie foods, isolation with consequent reduction in physical exercises, leading to a worsening of preexistent obesogenic factors in a population, and health disparities with temporization of seeking medical care, may have all led towards an increased incidence of the disease as the pandemic developed [19].
During the COVID-19 pandemic, diabetic children encountered difficulties regarding dietary habits, physical activity, insulin dose adjustment and, more rarely, access to insulin supply due to the challenging lifestyle changes [32]. Moreover, a significant increase in severe ketoacidosis was discovered in diabetic pediatric cases during the same interval of time [33].
2. Diabetes Mellitus and COVID-19 in Children
2.1. T1DM and COVID-19
From the beginning of the pandemic, it was quickly discovered that pre-existing T1DM represented one of the high risk factors for developing severe COVID-19 and related complications. On the other hand, SARS-CoV-2 has been suggested as a potential inducer of new-onset pediatric T1DM [34]. Data available in the literature at this moment suggest that severe acute respiratory syndrome coronaviruses (SARS-CoV-2, for instance) can enter in islet cells via angiotensin-converting enzyme-2 (ACE-2) receptors and cause reversible β-cell damage and transient hyperglycemia. Also, the discovery of SARS-CoV-2 in pancreatic tissue samples taken from deceased individuals, along with the evidence of decreased pancreatic function in both the pediatric and adult population with COVID-19, implies that this virus might harm the β cells of the pancreas and trigger the onset of T1DM through direct infection, inflammatory response and interactions with the renin-angiotensin system (Figure 1). It was proposed that several pathways may be involved, and β cells can be infected through several transmembrane receptors beyond ACE-2, such as neuropilin 1 (NRP-1), transferrin receptor (TFRC) and FES Upstream Region (FURIN). Also, immunofluorescent studies demonstrated a stronger expression of NRP-1 in β cells compared to α cells, and inhibition of this receptor with a specific antagonist reduced the uptake of SARS-CoV-2, suggesting its important role in viral entry. Downstream effects appear to involve the activation of key signaling pathways, such as c-Jun N-terminal kinase (JNK) and p21-activated kinase (PAK), that lead to apoptosis and impaired insulin excretion. Moreover, according to Tang et al., SARS-CoV-2 can lead to a transdifferentiation of β cells into α and acinar cells via the signaling pathway, involving the phosphorylation of protein kinase R (PKR) and eukaryotic translation initiation factor 2 (eIF2), which, in turn, determines integrated stress responses and cellular conversion [35][36][37][38][35,36,37,38].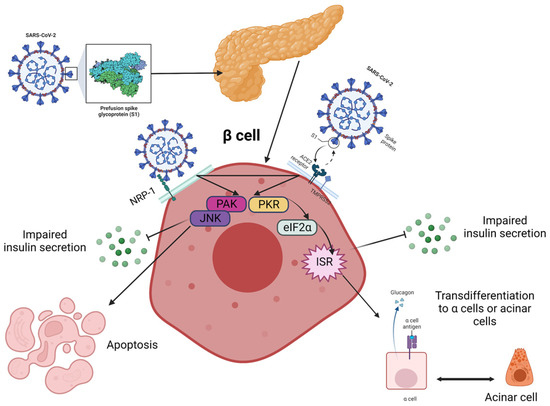
Figure 1. Proposed pathways for the fate of β cells post-COVID-19 are currently being investigated, although it remains unclear how the virus is transmitted to the pancreas and islets. Neurophilin-1 (NRP-1) has higher expression in β cells compared to angiotensin-converting enzyme 2 (ACE2) and could play a critical role in the infection. The virus stimulated β cells via p21-activated kinase (PAK) and c-Jun N-terminal kinase (JNK) and triggered the transformation of β cells into glucagon-producing α-cells or trypsin-producing acinar cells, leading to decreased insulin secretion. This transformation is facilitated through the PKR-eIF2a-mediated integrated stress response (IKR), and these two pathways may interact with each other. The eIF2 is the eukaryotic translation initiation factor 2, and PKR stands for protein kinase R. Created with Biorender.com, adapted after [38].
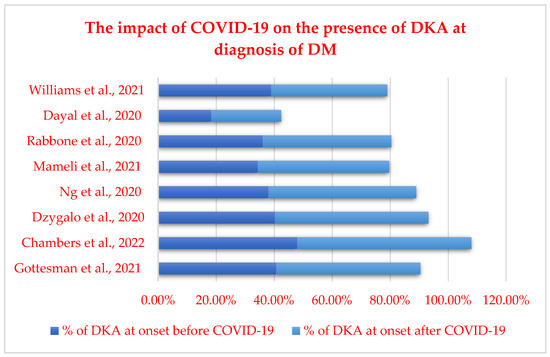
2.2. T2DM and COVID-19
Statistically, the prevalence of T2D among the pediatric population has significantly risen in recent years [60][61][60,61]. The World Health Organization (WHO) reports that in America, Europe and the Eastern Mediterranean, roughly 50% of the population is classified as overweight or obese, with lower rates in Africa and Asia [62]. The COVID-19 pandemic and its consequent circumstances, such as the movement restrictions and the repeated lockdown measures, could have played a substantial role in increasing the number of these patients in the world [63]. The mechanisms that may explain this phenomenon are described in Figure 3.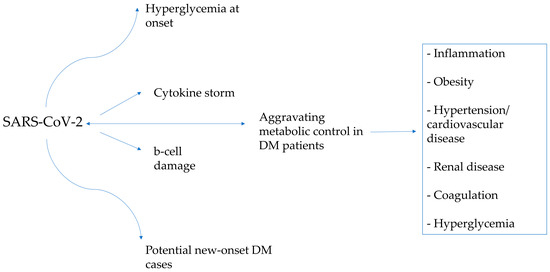
Figure 3.
The interplay between diabetes and COVID-19 and how they affect each other.
