The presence of oily wastewater poses a significant threat to both the ecological environment and public health. In order to solve this problem, the design and preparation of an efficient oil–water separation membrane is very important. Metal–organic frameworks (MOFs) are currently a promising material for oil–water separation due to their tunable wettability, adjustable pore size and also low density, high porosity, and high surface area. Therefore, MOFs-based membranes show great potential in the field of oil–water separation.
- :oil–water separation
- MOFs
- wettability
- membrane
- mechanism
1. Introduction
2. The Mechanism of Oil–Water Separation
2.1. Basic Theory of Wettability
Wettability, a macroscopic representation of the interaction between a liquid and a solid material, is primarily determined by the surface morphology and chemical composition of the solid material. The contact angle between a liquid droplet’s edge and the surface of the material is used to calculate the wettability of a liquid on a solid surface. Traditionally, materials were considered lyophobic when the contact angle was greater than 90° and lyophilic when the contact angle was less than 90°. However, recent research has suggested that the intrinsic wetting threshold to differentiate between hydrophobic and hydrophilic surfaces should be 65° [33][32]. Additionally, materials are considered superlyophobic when the contact angle is more than 150° and superlyophilic when the contact angle is less than 5°. These materials with superlyophilic or superlyophobic properties are referred to as special wetting materials [34,35,36,37][33][34][35][36]. Materials with selectivity for water and oil, as determined by their opposite wettability properties, can be used to separate oil–water mixtures. In air, the contact angle of a droplet on an ideal smooth solid surface can be expressed by Young model (Figure 21A), and the contact angle (θ) can be calculated by Young Equation (1):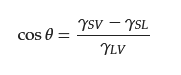 where γSV, γSL, and γLV denote the surface tension between the solid/gas, solid/liquid, and liquid/gas interfaces, respectively.
where γSV, γSL, and γLV denote the surface tension between the solid/gas, solid/liquid, and liquid/gas interfaces, respectively.
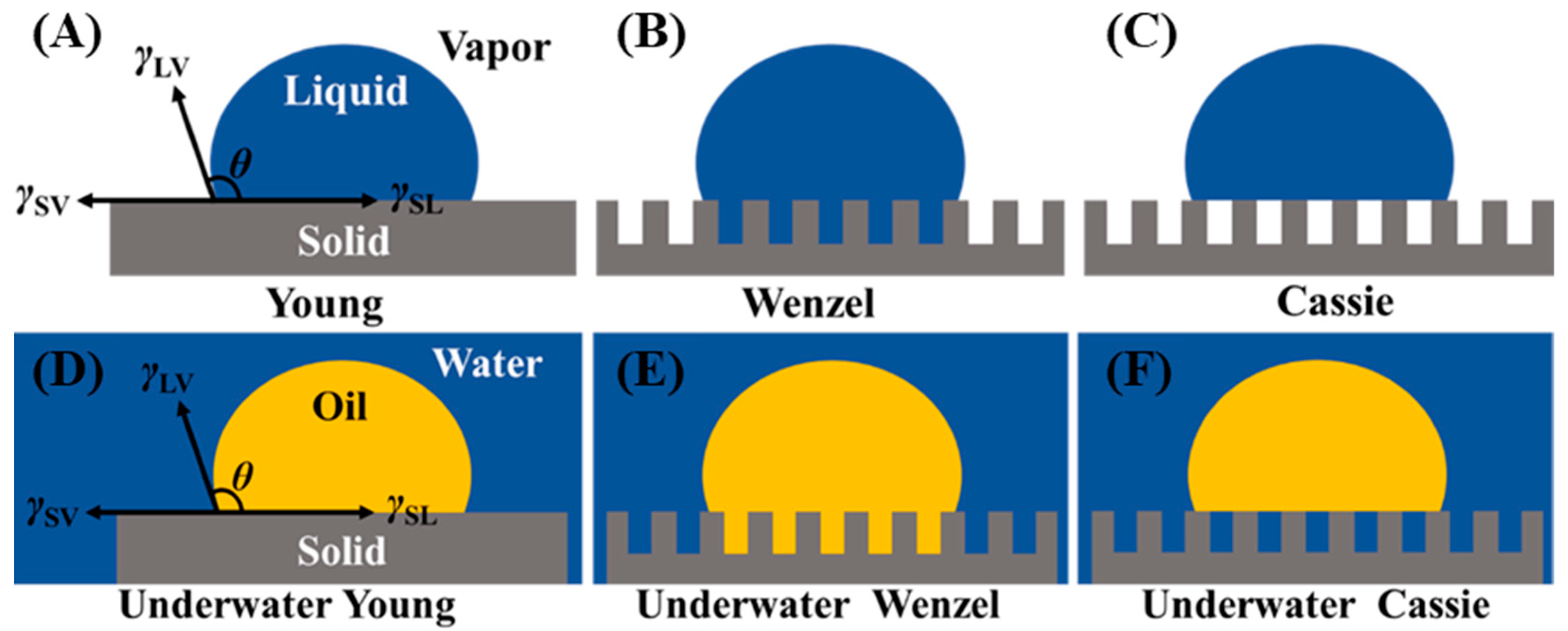
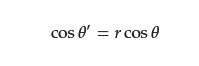 Among them, θ′ is the contact angle of droplets on the rough surface, and r is the roughness factor of the solid surface, which is defined as the ratio of the actual solid–liquid contact area to the apparent contact area of the rough surface. For the rough surface, r > 1, when θ < 90°, that is, when the material is lyophilic, the rough structure can make the material more lyophilic; when θ > 90°, that is, when the material is lyophobic, the rough structure makes the material more lyophobic. The contact angle of the droplets on this surface, however, may be determined using the Cassie model when air is trapped in the space between the droplets and the rough structure, forming a solid/liquid/gas three-phase interface (Figure 21C).
Among them, θ′ is the contact angle of droplets on the rough surface, and r is the roughness factor of the solid surface, which is defined as the ratio of the actual solid–liquid contact area to the apparent contact area of the rough surface. For the rough surface, r > 1, when θ < 90°, that is, when the material is lyophilic, the rough structure can make the material more lyophilic; when θ > 90°, that is, when the material is lyophobic, the rough structure makes the material more lyophobic. The contact angle of the droplets on this surface, however, may be determined using the Cassie model when air is trapped in the space between the droplets and the rough structure, forming a solid/liquid/gas three-phase interface (Figure 21C).
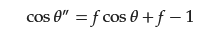 where θ″ is the contact angle of droplets on the solid–gas multiphase surface, and f is the ratio of the solid–liquid interface contact area to the total contact area (solid–liquid contact area and gas–liquid contact area).
The Young model, Wenzel model, and Cassie model can not only analyze the wettability of droplets on solid surfaces in the air but also effectively describe the oil–water–solid system (Figure 21D–F). The oil contact angle (θOW) on an ideal underwater smooth surface for the oil–water–solid three-phase system can be expressed as:
where θ″ is the contact angle of droplets on the solid–gas multiphase surface, and f is the ratio of the solid–liquid interface contact area to the total contact area (solid–liquid contact area and gas–liquid contact area).
The Young model, Wenzel model, and Cassie model can not only analyze the wettability of droplets on solid surfaces in the air but also effectively describe the oil–water–solid system (Figure 21D–F). The oil contact angle (θOW) on an ideal underwater smooth surface for the oil–water–solid three-phase system can be expressed as:
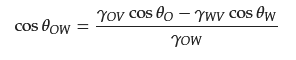 where θO and θW are the contact angles of oil and water droplets in air, respectively; γOV, γWV, and γOW are the interfacial tensions at the oil–vapor, water–vapor, and oil–water interfaces, respectively. Therefore, if γOVcosθO < γWVcosθW, an underwater oleophobic surface can be obtained; when γOVcosθO > γWVcosθW, an underwater oleophilic surface can be obtained. Most hydrophilic surfaces are underwater oleophobic because the surface tension of water is significantly greater than that of oils or other organic liquids. Hydrophobic/oleophilic surfaces at the solid–air–liquid interface always show lipophilicity at the solid–water–oil interface. Similar to the Wenzel and Cassie models in air, the rough surface can enhance the wetting property of materials underwater environment. Based on the aforementioned research, it is possible to construct the superhydrophobic-superoleophilic and superhydrophilic-underwater superoleophobic materials utilized for oil–water separation by controlling their surface chemical composition and creating the materials’ microstructures.
where θO and θW are the contact angles of oil and water droplets in air, respectively; γOV, γWV, and γOW are the interfacial tensions at the oil–vapor, water–vapor, and oil–water interfaces, respectively. Therefore, if γOVcosθO < γWVcosθW, an underwater oleophobic surface can be obtained; when γOVcosθO > γWVcosθW, an underwater oleophilic surface can be obtained. Most hydrophilic surfaces are underwater oleophobic because the surface tension of water is significantly greater than that of oils or other organic liquids. Hydrophobic/oleophilic surfaces at the solid–air–liquid interface always show lipophilicity at the solid–water–oil interface. Similar to the Wenzel and Cassie models in air, the rough surface can enhance the wetting property of materials underwater environment. Based on the aforementioned research, it is possible to construct the superhydrophobic-superoleophilic and superhydrophilic-underwater superoleophobic materials utilized for oil–water separation by controlling their surface chemical composition and creating the materials’ microstructures.
2.2. Separation of Free Oil–Water Mixture
One of the crucial factors influencing the effectiveness of materials for oil–water separation is the penetration pressure (∆Pc), which is the highest pressure applied to the surface before the liquid penetrates the membrane pore. For pores with cylindrical geometry, ∆Pc can be determined by the Young–Laplace Equation (5):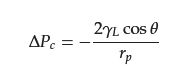 where γL denotes the surface tension of the liquid, θ is the contact angle of the liquid in a plane, and rp denotes the pore radius. In air, if θ > 90°, ∆Pc > 0, indicating that a certain external force is generated on the material surface to prevent the liquid from passing through the porous material, and an external pressure must be applied to pass through.
The penetration pressure of oil-in-water ∆PcW can be calculated from Equation (6) for a three-phase solid–water–oil contact in an aqueous environment as follows:
where γL denotes the surface tension of the liquid, θ is the contact angle of the liquid in a plane, and rp denotes the pore radius. In air, if θ > 90°, ∆Pc > 0, indicating that a certain external force is generated on the material surface to prevent the liquid from passing through the porous material, and an external pressure must be applied to pass through.
The penetration pressure of oil-in-water ∆PcW can be calculated from Equation (6) for a three-phase solid–water–oil contact in an aqueous environment as follows:
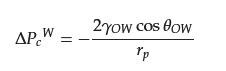 where γOW denotes the water/oil interfacial tension, and θOW denotes the underwater oil contact angle. When θOW > 90°, ∆PcW > 0, indicating that a certain external force is generated on the material surface to prevent the oil phase from entering the pore space, and the larger θOW is, the larger the value of ∆PcW is.
where γOW denotes the water/oil interfacial tension, and θOW denotes the underwater oil contact angle. When θOW > 90°, ∆PcW > 0, indicating that a certain external force is generated on the material surface to prevent the oil phase from entering the pore space, and the larger θOW is, the larger the value of ∆PcW is.
2.3. Separation of Oil–Water Emulsion
For emulsion separation, there are two main effects currently known: the “pore-size sieving” effect and the emulsion-breaking effect, which are shown in Figure 42. The “pore-size sieving” effect refers to the separation of oil-in-water emulsions using hydrophilic membranes with pore sizes smaller than the particle size of the emulsion. Under the influence of gravity, the oil droplets are intercepted, allowing the water phase to pass through. Similarly, water-in-oil emulsions can be separated using oleophilic membranes with pore that are smaller than the emulsion’s particle size [39][37]. However, the need for small pore sizes in the “pore-size sieving” effect often results in reduced separation flux, leading to the development of the emulsion-breaking effect. This process involves three main steps: (1) the emulsified droplets are captured by an agglomerated medium under hydrodynamic or other external forces; (2) the droplets combine to form larger droplets through wetting and shear collisions; and (3) the larger droplets are separated from the surface of the agglomerated medium through gravity and buoyancy [40][38]. By combining the “pore-size sieving” effect and emulsion breaking, emulsions can be separated with relatively larger membrane pore sizes, thus improving the separation flux and efficiency.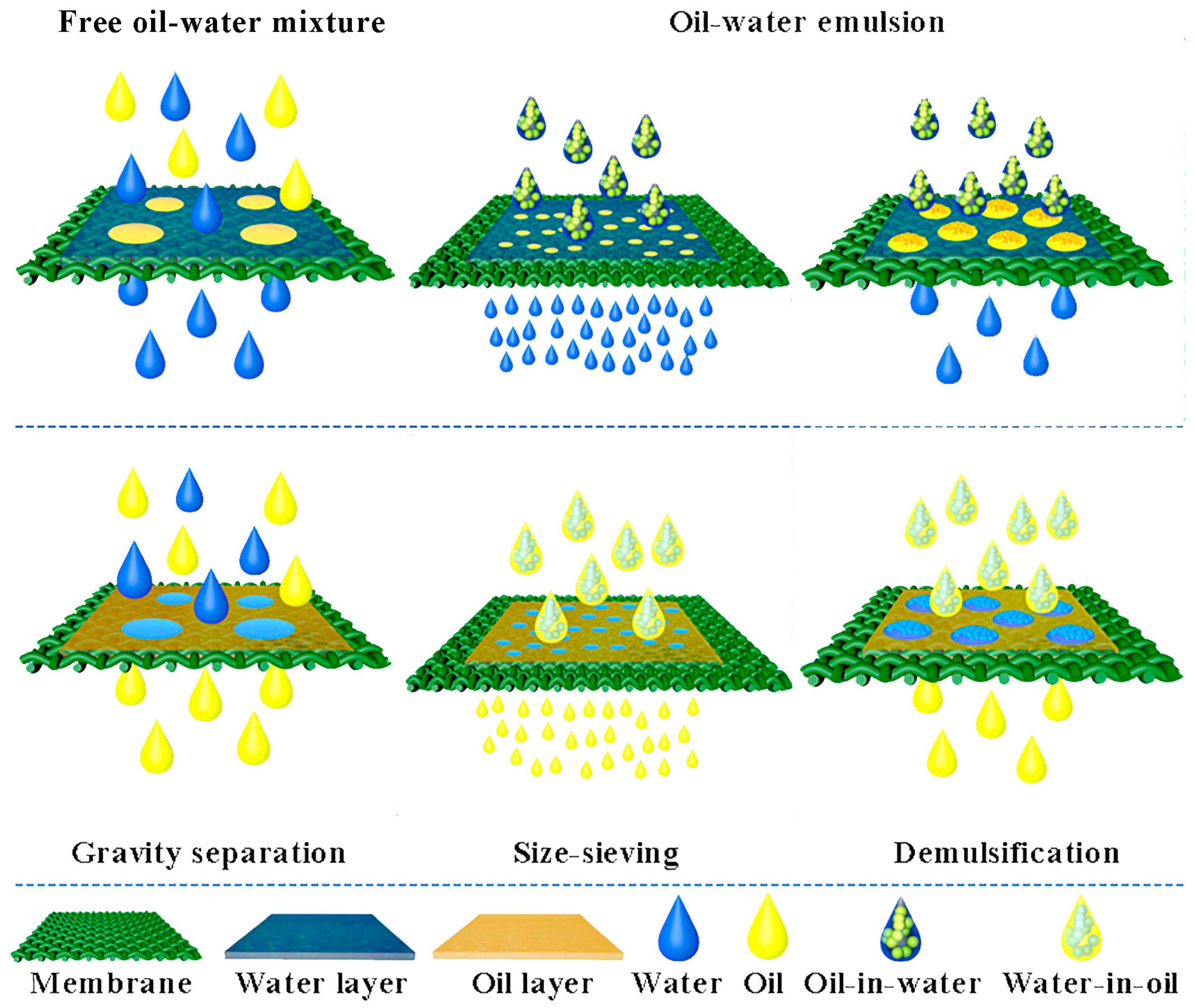
3. Methods to Prepare MOFs-Based Materials for Oil–Water Separation
3.1. In Situ Growth Method
3.1.1. Substrate Pre-Modification Method
3.1.2. Precursor Sacrifice Method
Utilizing some metal resources on the membrane as the precursor, MOFs could be obtained on the membrane directly with the subsequent conversion reaction. Metal oxides and hydroxides are the most used metal precursors in this case because they are easy to react with some acidic organic ligands to form the target MOF.3.1.3. Crystal Seed Growth
The crystal seed growth method is a process in which MOF crystals are nucleated and grown separately. The substrate is first covered with a crystal seed layer in this procedure. Then, the substrate with the seed layer is placed in a solution containing metal ions and organic ligands, allowing for the secondary growth of MOFs. As an example, a ZIF-8 seed solution was synthesized, and the substrate was immersed in it. This resulted in the growth and crystallization of ZIF-8 on the substrate through a secondary growth process under high-temperature conditions. Finally, further modification with PVA resulted in the formation of a PVA/ZIF-8-coated substrate, which was capable of separating both oil-in-water and water-in-oil emulsions [45][40].3.2. Deposition Method
3.2.1. Direct Deposition
The direct deposition method involves the direct synthesis of MOF composite membranes by placing the substrate into a mixture of metal ions and organic ligands. For example, a PP/ZIF-8 composite membrane can be prepared by simply adding polypropylene (PP) non-woven fabric into a mixed solution of zinc nitrate and 2-methylimidazole at room temperature, and it has the ability to separate free oil–water mixtures [46][41].3.2.2. Electrochemical Deposition
The electrochemical deposition method, similar to the direct deposition method, involves the rapid growth of MOFs on the substrate through the use of metal cations and organic ligands in the presence of an applied voltage. For example, after only 200 s of applied voltage, Co2+ and 2-methylimidazole react and accelerate to produce electrochemical deposition that aggregates onto the copper mesh, resulting in the formation of a uniform and dense ZIF-67 film [48][42]. This method offers advantages such as a short process time, uniform growth, and a high deposition rate.3.2.3. Layer-by-Layer Self-Assembly
The layer-by-layer (LBL) self-assembly method is a distinctive approach that involves alternating the deposition of metal ions and organic ligands instead of directly mixing them. The substrate can either be modified or left unmodified. The key factors that influence the membrane’s performance are the number of alternating depositions and the concentration of precursors. In a study by Gao et al., UiO-66-NH2-coated PP membranes with varying thicknesses were prepared using the LBL method. The PP membrane was first soaked in a solution of Zr4+ ions at high temperature, followed by immersion in a solution of 2-aminoterephthalic acid. By repeating this alternating process, UiO-66-NH2-coated PP membranes with different thicknesses were obtained [49][43]. Although the LBL method allows for the controlled synthesis of MOFs membranes, it is a time-consuming process.3.2.4. Filtration Deposition
The filtration deposition method is a technique for creating functional membranes by depositing solid suspensions on a substrate using vacuum filtration. For example, Zhu et al. first synthesized UiO-66-NH2 powder using Zr4+ and 2-NH2-benzenedicarboxylate by the solvent thermal method, added it to chitosan solution, and attached it to a cellulose membrane through vacuum filtration to obtain a superhydrophilic and underwater superoleophobic membrane, which can effectively separate oil-in-water emulsions and has excellent corrosion resistance [50][44].3.2.5. Spin-Coating
The spin-coating method involves coating a substrate with a suspension of MOFs using a spinning instrument. For instance, ZIF-8 nanoparticles were evenly deposited on a polyacrylonitrile (PAN) fiber membrane using the spin-coating method to create a biomimetic inverse desert beetle ZIF-8/PAN composite nanofiber membrane that can effectively separate oil-in-water emulsions [52][45]. The spin-coating method is simple to operate, but it can be challenging to achieve a uniform distribution of MOFs with a strong attachment to the substrate.3.3. Blending Membrane Method
In contrast to the above methods of coating MOFs onto the membrane surface, the blending membrane method focuses on creating a composite membrane in which the MOFs are dispersed within the membrane materials. This can be achieved through two main approaches: electrospinning and phase inversion.3.3.1. Electrospinning
Electrospinning is a process in which droplets are charged and transformed into nanometer-sized fibers through stretching and solidification. The precursor solution is extruded by adjusting the applied voltage and the receiving distance, and the solution is continuously ejected from the tip of the droplet and gathers on the receiver to form a film when the electrostatic repulsion force overcomes the surface tension.3.3.2. Phase Inversion
Phase inversion is a method of preparing homogeneous polymer solutions, which are then transformed into a three-dimensional macromolecular network gel structure by adding a non-solvent. The resulting structure is solidified into a film, making it a widely used method for the preparation of mixed matrix membranes (MMMs).4. The Classification of MOFs Used for Oil–Water Separation
4.1. ZIF Series
ZIFs, also known as zeolite imidazolate frameworks, are constructed from transition metals (zinc, cobalt, indium, etc.) with tetrahedral coordination geometry and imidazole-based organic ligands. ZIF series is one of the most extensively used MOFs in the field of oil–water separation. The effectiveness of oil–water separation using ZIFs-based membranes is found in the pore-size sieving effect, where different substrate sizes are suitable for separating different types of oil–water mixtures. T4.2. UiO Series
The UiO (Universitetet I Oslo) series of MOFs are known for their versatility and widespread use in various fields. Among the UiO series, UiO-66 and its derivatives have gained popularity for their effectiveness in oil–water separation. These MOFs have a three-dimensional microporous structure composed of a regular octahedron (Zr6O4(OH)4) containing Zr ions that are connected to 12 organic ligands of terephthalic acid (BDC). The structure consists of central pore cages in the shape of octahedrons and eight smaller tetrahedral corner cages.4.3. MIL Series
MIL series, also known as Lavahir skeleton series materials, are generally composed of trivalent metal ions (such as aluminum, chromium, iron, etc.) and carboxylic acid ligands. In addition to the extensive adsorption properties of MOFs, Fe-based MOFs can often endow the materials with photo-Fenton catalytic properties.4.4. Other MOFs
In addition to the ZIF, UiO, and MIL series, other MOFs such as HKUST-1, PCN series materials (containing multiple cub octahedral nanopore cages and forming a pore cage-pore channel-like topology in space), and Cu-MOFs have recently been developed for use in oil–water separation application.5. Conclusions
Most studies using MOF-based oil–water separation membranes are somewhat flawed, and the following efforts are still needed to solve the environmental problems of wastewater: (1) The long-term performance of MOFs-based membranes has been neglected, especially in continuous oil–water separation processes. Researchers can therefore focus on ways to improve the long-term durability of the membranes; (2) Most MOFs materials are unstable in wet environments due to weak coordination between their own metals and organic ligands. How to improve the stability of MOFs without affecting their structural properties remains to be investigated. (3) The specific wastewater environment is very complex and may contain organic molecules, heavy metals, microorganisms, etc. MOFs-based membranes need to be more stable and fouling resistant in the complex components. Based on this, there is a need for researchers to create materials with better stability and self-cleaning properties. (4) MOFs-based membranes should be used in a wider range of applications, and they must also be able to treat actual wastewater, from municipal wastewater to industrial wastewater, broadening their practical applications. (5) In addition, there is still a lack of longitudinal comparisons of data on the separation efficiency, mechanical and chemical stability, and pollution resistance of MOF-based membranes based on the separation of complex oil–water mixtures.
References
- Beyer, J.; Trannum, H.C.; Bakke, T.; Hodson, P.V.; Collier, T.K. Environmental effects of the deepwater horizon oil spill: A review. Mar. Pollut. Bull. 2016, 110, 28–51.
- Adetunji, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98.
- Monteiro, C.B.; Oleinik, P.H.; Leal, T.F.; Marques, W.C.; Nicolodi, J.L.; Lopes, B. Integrated environmental vulnerability to oil spills in sensitive areas. Environ. Pollut. 2020, 267, 115238.
- Murawski, S.A.; Kilborn, J.P.; Bejarano, A.C.; Chagaris, D.; Donaldson, D.; Hernandez, F.J.; MacDonald, T.C.; Newton, C.; Peebles, E.; Robinson, K.L. A synthesis of deepwater horizon impacts on coastal and nearshore living marine resources. Front. Mar. Sci. 2021, 7, 594862.
- Owusu, B.A.; Lim, A.; Intawong, C.; Rheanpumikankit, S.; Suksri, S.; Ingviya, T. Haematological, renal, and hepatic function changes among Rayong oil spill clean-up workers: A longitudinal study. Int. Arch. Occup. Environ. Health 2022, 95, 1481–1489.
- Ingviya, T.; Intawong, C.; Abubaker, S.; Strickland, P.T. Exposure assessment of rayong oil spill cleanup workers. Expo. Health 2020, 12, 617–628.
- Padikkal, S.; Sumam, K.S.; Sajikumar, N. Sustainability indicators of water sharing compacts. Environ. Dev. Sustain. 2018, 20, 2027–2042.
- Zhang, W.F.; Liu, N.; Cao, Y.Z.; Lin, X.; Liu, Y.N.; Feng, L. Superwetting porous materials for wastewater treatment: From immiscible oil/water mixture to emulsion separation. Adv. Mater. Interfaces 2017, 4, 1700029.
- Guha, I.F.; Varanasi, K.K. Separating nanoscale emulsions: Progress and challenges to date. Curr. Opin. Colloid Interface Sci. 2018, 36, 110–117.
- Putatunda, S.; Bhattacharya, S.; Sen, D.; Bhattacharjee, C. A review on the application of different treatment processes for emulsified oily wastewater. Int. J. Environ. Sci. Technol. 2019, 16, 2525–2536.
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. Oily wastewater treatment: Overview of conventional and modern methods, challenges, and future opportunities. Water 2021, 13, 980.
- Etkin, D.S.; Nedwed, T.J. Effectiveness of mechanical recovery for large offshore oil spills. Mar. Pollut. Bull. 2021, 163, 111848.
- Zhu, Y.Z.; Wang, D.; Jiang, L.; Jin, J. Recent progress in developing advanced membranes for emulsified oil/water separation. NPG Asia Mater. 2014, 6, e101.
- Zhang, R.; Sun, Y.H.; Guo, Z.G.; Liu, W.M. Janus membranes with asymmetric wettability applied in oil/water emulsion separations. Adv. Sustain. Syst. 2021, 5, 2000253.
- Cai, Y.H.; Shi, S.Q.; Fang, Z.; Li, J.Z. Design, development, and outlook of superwettability membranes in oil/water emulsions separation. Adv. Mater. Interfaces 2021, 8, 2100799.
- Kitao, T.; Zhang, Y.Y.; Kitagawa, S.; Wang, B.; Uemura, T. Hybridization of MOFs and polymers. Chem. Soc. Rev. 2017, 46, 3108–3133.
- Wang, C.H.; Liu, X.L.; Demir, N.K.; Chen, J.P.; Li, K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134.
- Ryu, U.; Jee, S.; Rao, P.C.; Shin, J.; Ko, C.; Yoon, M.; Park, K.S.; Choi, K.M. Recent advances in process engineering and upcoming applications of metal-organic frameworks. Coord. Chem. Rev. 2021, 426, 213544.
- Venkatesan, N.; Yuvaraj, P.; Fathima, N.N. Fabrication of non-fluorinated superhydrophobic and flame retardant porous material for efficient oil/water separation. Mater. Chem. Phys. 2022, 286, 126190.
- Borazjani, A.R.; Akhlaghi, B.; Abbasi, M.; Osfouri, S. Investigation of petroleum products dehydration using natural zeolite and activated carbon. Pet. Sci. Technol. 2023, 1–20.
- Chen, X.P.; Li, Y.M.; Yang, Y.S.; Zhang, D.; Guan, Y.H.; Bao, M.T.; Wang, Z.N. A super-hydrophobic and antibiofouling membrane constructed from carbon sphere-welded MnO2 nanowires for ultra-fast separation of emulsion. J. Membr. Sci. 2022, 653, 120514.
- Rego, R.M.; Kuriya, G.; Kurkuri, M.D.; Kigga, M. MOF based engineered materials in water remediation: Recent trends. J. Hazard. Mater. 2021, 403, 123605.
- Mukherjee, S.; Sharma, S.; Ghosh, S.K. Hydrophobic metal-organic frameworks: Potential toward emerging applications. APL Mater. 2019, 7, 050701.
- Bhuyan, A.; Ahmaruzzaman, M. Metal-organic frameworks: A new generation potential material for aqueous environmental remediation. Inorg. Chem. Commun. 2022, 140, 109436.
- Beydaghdari, M.; Saboor, F.H.; Babapoor, A.; Karve, V.V.; Asgari, M. Recent Advances in MOF-Based Adsorbents for Dye Removal from the Aquatic Environment. Energies 2022, 15, 34.
- Zheng, M.Y.; Chen, J.Y.; Zhang, L.; Cheng, Y.; Lu, C.Y.; Liu, Y.F.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J.Q. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022, 31, 103514.
- Dong, X.Y.; Li, Y.Y.; Li, D.Q.C.; Liao, D.H.; Qin, T.R.; Prakash, O.; Kumar, A.; Liu, J.Q. A new 3D 8-connected Cd(II) MOF as a potent photocatalyst for oxytetracycline antibiotic degradation. CrystEngComm 2022, 24, 6933–6943.
- Li, L.T.; Zou, J.F.; Han, Y.T.; Liao, Z.H.; Lu, P.F.; Nezamzadeh-Ejhieh, A.; Liu, J.Q.; Peng, Y.Q. Recent advances in Al(iii)/In(iii)-based MOFs for the detection of pollutants. N. J. Chem. 2022, 46, 19577–19592.
- Ke, F.; Pan, A.; Liu, J.Q.; Liu, X.X.; Yuan, T.; Zhang, C.Y.; Fu, G.N.; Peng, C.Y.; Zhu, J.F.; Wan, X.C. Hierarchical camellia-like metal-organic frameworks via a bimetal competitive coordination combined with alkaline-assisted strategy for boosting selective fluoride removal from brick tea. J. Colloid Interface Sci. 2023, 642, 61–68.
- Ge, B.; Yang, H.; Xu, X.C.; Ren, G.N.; Zhao, X.C.; Pu, X.P.; Li, W.Z. Facile synthesis of superhydrophobic ZIF-8/bismuth oxybromide photocatalyst aerogel for oil/water separation and hazardous pollutant degradation. Appl. Nanosci. 2020, 10, 1409–1419.
- Ahmad, N.; Samavati, A.; Nordin, N.; Jaafar, J.; Ismail, A.F.; Malek, N. Enhanced performance and antibacterial properties of amine-functionalized ZIF-8-decorated GO for ultrafiltration membrane. Sep. Purif. Technol. 2020, 239, 116554.
- Miao, W.N.; Tian, Y.; Jiang, L. Bioinspired superspreading surface: From essential mechanism to application. Acc. Chem. Res. 2022, 55, 1467–1479.
- Zhao, W.J. Bio-inspired superwettable materials: An interview with Lei Jiang. Natl. Sci. Rev. 2017, 4, 781–784.
- Gore, P.M.; Naebe, M.; Wang, X.G.; Kandasubramanian, B. Nano-fluoro dispersion functionalized superhydrophobic degummed & waste silk fabric for sustained recovery of petroleum oils & organic solvents from wastewater. J. Hazard. Mater. 2022, 426, 127822.
- Wang, Y.; Zhao, W.N.; Han, M.; Guan, L.; Han, L.; Hemraj, A.; Tam, K.C. Sustainable superhydrophobic surface with tunable nanoscale hydrophilicity for water harvesting applications. Angew. Chem. Int. Ed. 2022, 61, e202115238.
- Zheng, L.Z.; Li, H.Q.; Lai, X.J.; Huang, W.; Lin, Z.Y.; Zeng, X.R. Superwettable Janus nylon membrane for multifunctional emulsion separation. J. Membr. Sci. 2022, 642, 119995.
- Yang, J.; Li, H.N.; Chen, Z.X.; He, A.; Zhong, Q.Z.; Xu, Z.K. Janus membranes with controllable asymmetric configurations for highly efficient separation of oil-in-water emulsions. J. Mater. Chem. A 2019, 7, 7907–7917.
- Li, L.; Xu, Z.Z.; Sun, W.; Chen, J.; Dai, C.L.; Yan, B.; Zeng, H.B. Bio-inspired membrane with adaptable wettability for smart oil/water separation. J. Membr. Sci. 2020, 598, 117661.
- Song, P.; Lu, Q.Y. Porous clusters of metal-organic framework coated stainless steel mesh for highly efficient oil/water separation. Sep. Purif. Technol. 2020, 238, 116454.
- Gao, X.; Ma, Q.; Jin, Z.W.; Nian, P.; Wang, Z. Switchable superlyophobic zeolitic imidazolate framework-8 film-coated stainless-steel meshes for selective oil-water emulsion separation with high flux. N. J. Chem. 2020, 44, 13534–13541.
- Yang, X.H.; Li, S.P.; Yao, Y.Q.; Zhao, J.; Zhu, Z.G.; Chai, C.P. Preparation and characterization of polypropylene non-woven fabric/ZIF-8 composite film for efficient oil/water separation. Polym. Test 2021, 100, 107263.
- Zhou, P.Z.; Cheng, J.; Yan, Y.Y.; Xu, S.P.; Zhou, C.L. Ultrafast preparation of hydrophobic ZIF-67/copper mesh via electrodeposition and hydrophobization for oil/water separation and dyes adsorption. Sep. Purif. Technol. 2021, 272, 118871.
- Gao, J.; Wei, W.; Yin, Y.; Liu, M.H.; Zheng, C.B.; Zhang, Y.F.; Deng, P.Y. Continuous ultrathin UiO-66-NH2 coatings on a polymeric substrate synthesized by a layer-by-layer method: A kind of promising membrane for oil-water separation. Nanoscale 2020, 12, 6658–6663.
- Zhu, X.M.; Yu, Z.X.; Zeng, H.J.; Feng, X.F.; Liu, Y.C.; Cao, K.Y.; Li, X.Y.; Long, R.X. Using a simple method to prepare UiO-66-NH2/chitosan composite membranes for oil-water separation. J. Appl. Polym. Sci. 2021, 138, e50765.
- Li, H.Y.; Mu, P.; Li, J.; Wang, Q.T. Inverse desert beetle-like ZIF-8/PAN composite nanofibrous membrane for highly efficient separation of oil-in-water emulsions. J. Mater. Chem. A 2021, 9, 4167–4175.
