Radiation therapy (RT) in the management of pelvic cancers remains a clinical challenge to urologists given the sequelae of urethral stricture disease secondary to fibrosis and vascular insults. The management of post-radiation urethral stricture consists of conservative, endoscopic, and primary reconstructive options. Endoscopic approaches remain an option, but with limited long-term success. Despite concerns with graft take, reconstructive options such as urethroplasties in this population with buccal grafts have shown long-term success rates ranging from 70 to 100%. Robotic reconstruction is augmenting previous options with faster recovery times. Radiation-induced stricture disease is challenging with multiple interventions available, but with successful outcomes demonstrated in various cohorts including urethroplasties with buccal grafts and robotic reconstruction.
- :radiation injury
- urethral stricture
- reconstructive urology
- wound healing
1. Introduction
2. Prevalence
Pelvic RT administered for rectal cancer can impact the bladder and urethra due to the short distance between these organs. Urinary adverse events (AE) are to be expected; however, there is a dearth of studies following potential urologic sequela [6]. Studies that tracked urinary AE revealed that about 4% of patients experienced severe AE [7]. When examining patients diagnosed with prostate cancer who solely received EBRT, a study performed by David et al. revealed a 28.4% 10-year cumulative incidence rate of hospital admissions due to urinary AE [8]. The absence of studies presents a potential future area of exploration and collaboration among diverse disciplines and specialties. Muise et al. conducted a review of a cohort of 67,527 patients diagnosed with prostate cancer; 72.62% received RT, 24.86% underwent a radical prostatectomy (RP), 4.94% underwent a RP followed by RT, and 0.46% received RT followed by RP. Within five years of completing treatment, 8.44% of those treated with RT developed urethral strictures, compared to the 5.35% diagnosed with strictures after RP [9]. When both modalities were used, the rates of urethral stricture were higher, with 11.22% of patients experiencing stenosis after salvage RT and 19.34% after salvage RP [9].3. Stricture Etiology/Physiology
When the urethral lumen is subjected to repetitive damage, an atypical narrowing is formed due to these damages causing structural alterations to the supporting spongiosum and connective tissue underlying the urethral lumen [17,18][10][11]. As a result of these alterations, urine can extravasate into the neighboring spongiosum and trigger fibrotic changes. This process leads to the formation of a plaque which is classified as a stricture if it is circumferential [19][12]. The fibrosis present in urethral strictures demonstrates unique properties when compared to fibrosis in other areas of the body, such as a protracted healing process and involvement of much of the periurethral tissue [20][13]. In a study by Hofer et al., it was found that the parallels between urethral healing and dermal wound healing consisted of acute inflammatory, proliferative, and remodeling phases. These phases exhibited a 50% longer duration with cellular infiltration and cytokine changes that went beyond the injury site and affected a significant portion of periurethral tissue [20][13]. Important distinctions between urethral remodeling and dermal remodeling include differences in the length of the proliferative phase, which peaks at day 5 in dermal remodeling, while in urethral remodeling, students have demonstrated evidence of remodeling on day 10 [20][13]. Evidence from animal studies suggests strictures result from an increase in collagen and a decrease in smooth muscle content. This leads to compact, fibrotic, and poorly compliant tissue, although it is important to note that these findings have not been established in human studies [21][14]. Human studies have instead shown a decrease in the ratio of Type I collagen to Type III when comparing normal urethral spongiosum to urethral strictures [22][15]. Typical urethral spongiosum is composed of 75% Type I collagen and 25% Type III, compared to 16% Type I collagen and 84% Type III in the spongiosum of urethral strictures [22][15].4. Radiation-Induced Changes in Urologic Tissue
RT causes unavoidable radiation injury to normal tissues, resulting in acute and late effects on parenchyma, stroma, and vascular structures. Effects that manifest within hours of exposure are characterized as acute changes and can consist of an increase in vascular permeability, lymphocyte adhesion and infiltration, and endothelial cell edema [26][16]. Effects manifesting months to years later are characterized as late changes and result from the reduction in stem cells or progenitor cells. Late effects include organ dysfunction, fibrosis, and necrosis [26][16]. Following RT, the urothelium exhibits parenchymal and epithelial changes such as cellular atypia, neoplasia, dysplasia, metaplasia, necrosis, and atrophy. One of the most notable and consistent delayed effects of RT is atrophy [27][17]. Delayed necrosis present in the acute phase is usually secondary to ischemia, leading to fissures in the epithelial lining. Fibrosis, the presence of fibrinous exudate, and atypical fibroblasts are stromal lesions that are commonly visualized in the lower urinary tract. Radiation-induced fibrosis and vascular insufficiency present as delayed effects. The extent of these sequelae is dependent on the surrounding tissues that are impacted and, in the case of blood vessels, is proportional to the size of the vessels impacted. Damage to small capillaries typically results in obliteration, as they are most radiosensitive leading to endothelial swelling and increased permeability, while damage to medium-sized vessels results in fibrinoid necrosis and thrombosis [28][18]. Damage to large vessels is rare. Radiation-induced endothelial apoptosis plays a prominent role in the series of acute vascular changes. Late-stage radiation changes produce vascular effects involving thickening of the basement membrane, scarring of surrounding tissues alongside the development of telangiectasias, and a decline in clonogenic capacity, which presumably contributes to the late-stage radiation response to typical parenchyma [29][19].5. Pathways of Radiation-Induced Endothelial Cell Death
The behavior of endothelial cells when exposed to radiation has been extensively studied and understood. The macroscopic changes observed in acute radiation toxicity occur when irradiated endothelial cells undergo structural changes and produce a range of growth factors, chemoattractants, and biomarkers [26][16]. The prior literature has investigated different pathways involved in radiation-induced stricture formation; however, they are predominantly influenced by damage to the membrane and are mediated by ceramide production and acid sphingomyelinases (ASMases). Most studies exploring this pathway utilized single doses of radiation ranging from 10 to 20 Gy [6,26][6][16]. Ceramide activates the ceramide-activated protein kinase (CAPK) and the ceramide-activated phosphatase, leading to apoptosis through the activation of the MAPK8 pathway, the mitochondrial pathway, and the death receptor pathway [26,31,32][16][20][21]. The inhibition of protein kinase C (PKC) by ceramide-activated phosphatase is a critical step, given that PKC participates in antiapoptotic signaling and can impede sphingomyelin hydrolysis, thus obstructing the release of ceramide from cellular membranes [33,34,35][22][23][24]. Caspase 9 functions as an alternative activation pathway of caspases for apoptosis through mitochondrial proteins. This process is initiated by ceramides, as well. BCL-2-associated protein x (BAX) and BCL-2 antagonist of cell death protein (BAD) are proapoptotic proteins that promote apoptosis when CAPK is induced by ceramide [37][25]. When BAD binds to antiapoptotic proteins BCL2 and BCL2L1, cytochrome C is released and then caspase 9 is activated, which stops the suppression of apoptosis which is controlled by BAX [38,39][26][27]. Ceramide can be released through cell death or TNF receptor activation. This release leads to a direct apoptotic pathway using different adapter protein complexes including the TNFαR-associated death domain and the Fas-associated death domain [26][16]. The aforementioned domains start the induction of cytoplasmic promoters of cell death such as procaspase 8, which cleaves and activates effector caspases. Ceramide can also be released from radiation-induced DNA damage since the ceramide synthase can be activated by DNA double-strand breaks. This pathway would need de novo protein synthesis, which would result in slower kinetics over a longer period of time as a proapoptotic mechanism compared to the sudden release of ceramide once ASMase is activated [26][16]. The balance between pro- and anti-apoptotic signaling cascades and radiation dose determines the amount of endothelial cell apoptosis. For example, ionizing radiation does not lead to endothelial apoptosis directly from the mechanisms listed above, but it also activates anti-apoptotic pathways.6. Cellular and Extracellular Components of Fibrosis
Fibrotic tissue changes are the result of the decreased decomposition and increased production of extracellular matrix (ECM) proteins, especially collagen, which can be caused by radiation exposure. The premature terminal differentiation of potentially mitotic progenitor fibroblasts into irreversible postmitotic fibrocytes can be activated by ionizing radiation [45,46,47][28][29][30]. Growth factors, tissue-specific collagen, cytokines, and matrix molecules are produced by these differentiated fibrocytes that are the main component of the fibroblast system [46,47,48][29][30][31]. This accumulation of postmitotic fibrocytes may explain why radiation results in an increased synthesis and extracellular deposition of collagen, which is remarkable for post-radiation fibrotic tissue. Cytokines are important in the mechanisms of radiation-induced injury. TGF-β1 is a cytokine responsible for the proliferation and differentiation of fibroblasts into postmitotic fibrocytes, which secrete collagens and other extracellular matrix proteins. TGF-β1 also controls extracellular matrix homeostasis, which is responsible for the increased production and decreased degradation of extracellular collagen molecules [26,27,28][16][17][18]. The activation of TGF-β1 and plasmin activator inhibitor 1 by radiation exposure plays a significant role in the process of fibrotic tissue changes. IL-17 is another cytokine that plays an important role in mediating the response of cells to radiation-related damage such as neutrophil recruitment [49,50][32][33]. Bessout et al. were able to show that IL-17 creation was increased in CD4 T cells in mice that had undergone colorectal irradiation [51][34]. Other cytokines that appear after irradiation are cytokines IL-1β and TGF-β [51][34]. They are involved in Th17 differentiation, a CD4 T cell which secretes IL-17 [51,52][34][35]. Excessive accumulation of ECM proteins such as collagen is the most commonly observed component of tissue fibrosis after radiation exposure, mainly through increased synthesis and decreased degradation. The reduced degradation of extracellular collagen that is newly synthesized and deposited can be attributed to the gene expression and production of tissue inhibitors of matrix metalloproteinases (MMP), which is mediated by TGF-β1 [55][36]. Another mechanism for fibrinolysis and ECM degradation regulation is the plasmin activator (PA) system. Plasmin can break down ECM through proteolytic activity and induction of latent MMPs [56][37]. The PA system is controlled by a group of PA inhibitors, and plasmin activator inhibitor (PAI-1) is the most significant. TGF-β1, TNF-α, and IL-1 are signaling molecules that can stimulate PAI-1 secretion.7. Radiation-Induced Histologic Changes
Radiation causes a variety of histologic alterations including vascular modifications, fibrotic changes, cellular depletions, and inflammatory responses, which manifests progressively as the tissue gets further from the site of radiation exposure (Figure 1).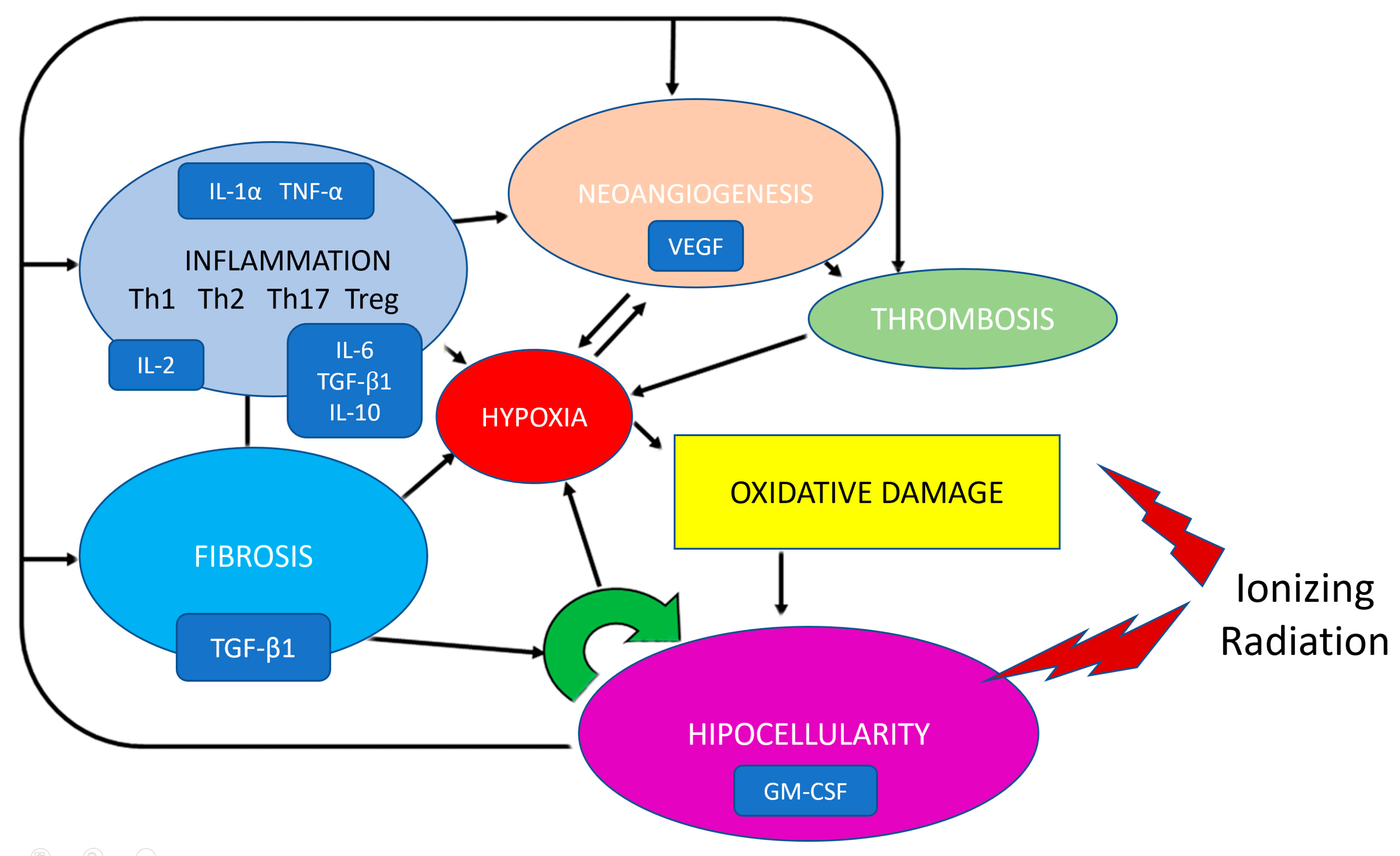
8. Pathogenesis of Radiation Strictures
8. Pathogenesis of Radiation Strictures
9. Surgical Pitfalls and Options for Treating Radiation Strictures
In order to create a treatment plan for a patient with a suspected post-RT stricture, a complete workup is necessary including a physical exam, history of prior urological instrumentation, treatments, and incontinence, as well as diagnostic testing such as urodynamic studies and lab tests. Direct visualization of the stricture and assessment of adjacent tissue should be performed, with retrograde urethrogram (RUG) combined with voiding cystourethrogram (VCUG) used if the retrograde scope cannot pass through the stricture. Additional cross-sectional imaging modalities including CT scan and/or MRI may be required to assess associated pathology (i.e., presence and extent of calcified/necrotic prostatic cavity, or urethrosymphyseal or recto-urethral fistula). Treatment options for post-RT urethral stricture are conservative management (clean intermittent catheterization or chronic catheter placement), endoscopic management (dilation, direct visualization internal urethrotomy [DVIU]), and primary reconstructive options, with joint decision making to be performed between physician and patient based on oncologic and performance status, stricture location/length/number, bladder status, and current level of continence [78,79,80].10. Conservative Management
11. Endoscopic Management
Dilation and DVIU are endoscopic procedures that can be used in initial interventions for stricture disease; however, these measures have limited long-term success in patients with radiation-induced urethral strictures. A study by Merrick et al. revealed that among 29 patients who had undergone endoscopic treatment of their strictures, 31% needed multiple procedures to achieve patency, and 3 of the patients required suprapubic tube placement after developing recurrent strictures [14][45]. Similar findings from other publications revealed that nearly half of patients treated endoscopically needed subsequent interventions for stricture treatment. Notably, one study highlighted the significant risk of de novo incontinence associated with endoscopic approaches [13][44]. The pursuit of a more durable result following endoscopic management has led to the adjunctive use of injectable substances which may inhibit fibrosis and thus prevent recurrent stenosis. The injection of steroids and mitomycin C following DVIU have demonstrated patency rates of 83% and 90%, respectively [85,86,87][57][58][59]. Although these numbers must be considered in their proper context, as multiple procedures are often needed to achieve them, a sub-analysis of radiated patients shows significantly worse success rates, and mitomycin can have severe adverse events resulting in the need for cystectomy [87,88,89][59][60][61].12. Reconstructive Techniques—Excision and Primary Anastomosis (EPA)
EPA for radiation-induced urethral strictures is typically performed via perineal access and tends to be more complicated compared to EPA performed on non-radiated urethral strictures for two main reasons: (1) the significant radiation-induced fibrosis which makes urethral mobilization difficult and (2) radiated fields have poor vascularity resulting in poor wound healing. The success rate of EPA, defined as not requiring additional procedures, for radiation-induced urethral stricture is reported to be 65–95% [90,91,92,93,94][62][63][64][65][66]. A multi-institutional retrospective study by Voelzke et al. identified the following factors, age, stricture length, and EBRT + BT, as associated with stricture recurrence after EPA [95][67]. One major limitation of the study is the heterogeneity in auxiliary surgical maneuvers (use of gracilis flaps, inferior pubectomy, crural separation) employed to complete the reconstruction, thus the effect of this excisional technique alone on the outcomes is difficult to ascertain.13. Reconstructive Techniques—Buccal Mucosa Graft Urethroplasty
In the past, augmentation techniques were not performed on radiation patients due to concerns regarding the viability of the graft in such hostile environments. Recent studies suggest that augmented urethroplasties using buccal mucosa grafts (BMG), the most versatile grafts currently used in urologic practice, are a viable repair technique for post-radiation strictures and have outcomes similar to non-radiated patients in the short term and medium term [67][38]. Buccal mucosa grafts (ventral and dorsal onlays) have been used in several suggested approaches of augmentation urethroplasty which provide patients with outstanding outcomes with regard to postoperative patency rates; they have been documented to be from 71 to 75% and even 100% in one small series [83,92,96][64][68][69]. A study by Ahyai et al. on outcomes following ventral onlay BMG urethroplasty reported an overall success rate was 71% at a mean follow up of 26.5 months, and rates of de novo incontinence and erectile dysfunction were 10.5% and 6.3%, respectively [97][70]. Blakely et al. documented findings on three patients with membranous urethral strictures who had undergone dorsal onlay BMG urethroplasty after RT. The results showed that all patients were able to maintain patency with no instances of de novo incontinence during the follow up at 8 months [95][67]. A subsequent multicenter retrospective review by Policastro et al. of 79 patients with posterior urethral stenosis secondary to radiation therapy who underwent dorsal onlay buccal mucosal urethroplasty with a 3 cm mean stricture length demonstrated an 82.3% stricture-free rate at a mean 21 months of follow up, 8% de novo stress urinary incontinence, and 91% patient satisfaction [98][71].14. Robotic Techniques
Robotic surgery has grown as a possible alternative to endoscopic management and open surgery in the treatment of radiation-induced urethral strictures. Open surgery for radiation-induced urethral strictures can lead to longer recovery times, additional interventions, and wound complications due to the hampered healing process present in irradiated tissue [101,102][72][73]. A review of open post-radiation urologic reconstructive procedures found a morbidity rate up to 54% [100,101][72][74]. In comparison, robotic surgery has been linked with reduced early postoperative morbidities for other major urologic procedures. This approach may translate to post-radiation reconstruction, potentially improving outcomes and decreasing morbidities [101,103,104][72][75][76]. Traditionally, open perineal surgery was used for the reconstruction of the posterior urethra, but this technique grew to be more challenging after RT since the natural planes of the tissue are eliminated and fibrosis is present [93,101][65][72]. In these circumstances, robotic surgery can improve visualization and improve dexterity in a tight working space around the bladder neck, allowing for the accurate placement of proximal sutures [101,105,106][72][77][78]. In patients undergoing a repair of vesicourethral anastomotic stenosis, robotic reconstruction of posterior urethral stenosis demonstrated a 100% patency rate. However, in two out of the seven patients, artificial urinary sphincter placement was needed [107,108][79][80]. A true assessment of the robotic approach for the repair of post-radiation stenosis is limited due to the exclusion of patients with a history of radiation in most of the current literature.References
- CDC. US Cancer Statistics 2019. Available online: cdc.gov/USCS (accessed on 1 February 2023).
- Tang, C.; Hoffman, K.E.; Allen, P.K.; Gabel, M.; Schreiber, D.; Choi, S.; Chapin, B.F.; Nguyen, Q.; Davis, J.W.; Corn, P.; et al. Contemporary prostate cancer treatment choices in multidisciplinary clinics referenced to national trends. Cancer 2019, 126, 506–514.
- Del Giudice, F.; Huang, J.; Li, S.; Sorensen, S.; Enemchukwu, E.; Maggi, M.; Salciccia, S.; Ferro, M.; Crocetto, F.; Pandolfo, S.D.; et al. Contemporary trends in the surgical management of urinary incontinence after radical prostatectomy in the United States. Prostate Cancer Prostatic Dis. 2022, 26, 367–373.
- Boorjian, S.A.; Eastham, J.A.; Graefen, M.; Guillonneau, B.; Karnes, R.J.; Moul, J.W.; Schaeffer, E.M.; Stief, C.; Zorn, K.C. A Critical Analysis of the Long-Term Impact of Radical Prostatectomy on Cancer Control and Function Outcomes. Eur. Urol. 2012, 61, 664–675.
- Elliott, S.P.; Meng, M.V.; Elkin, E.P.; McAninch, J.W.; Duchane, J.; Carroll, P.R.; CaPSURE Investigators. Incidence of urethral stricture after primary treatment for prostate cancer: Data from CaPSURE. J. Urol. 2007, 178, 529–534.
- Sterling, J.; Policastro, C.; Nikolavsky, D. Pathophysiology of radiation-induced urethral strictures and therapeutic strategies optimizing outcomes of surgical repair. In Scientific Advances in Reconstructive Urology and Tissue Engineering; Academic Press: Cambridge, MA, USA, 2022; pp. 51–80.
- Liberman, D.; Mehus, B.; Elliott, S.P. Urinary adverse effects of pelvic radiotherapy. Transl. Androl. Urol. 2014, 3, 186–195.
- David, R.V.; Kahokehr, A.A.; Lee, J.; Watson, D.I.; Leung, J.; O’callaghan, M.E. Incidence of genitourinary complications following radiation therapy for localised prostate cancer. World J. Urol. 2022, 40, 2411–2422.
- Muise, A.; Pan, M.M.; Rose, B.; Buckley, J.C. Functional outcomes after prostate cancer treatment: A comparison between single and multiple modalities. Urol. Oncol. Semin. Orig. Investig. 2023, 41, 104.e1–104.e9.
- Cavalcanti, A.G.; Yucel, S.; Deng, D.Y.; McANINCH, J.W.; Baskin, L.S. The Distribution of Neuronal and Inducible Nitric Oxide Synthase in Urethral Stricture Formation. J. Urol. 2004, 171, 1943–1947.
- Simsek, A.; Aldamanhori, R.; Chapple, C.R.; MacNeil, S. Overcoming scarring in the urethra: Challenges for tissue engineering. Asian J. Urol. 2018, 5, 69–77.
- Mundy, A.R.; Andrich, D.E. Urethral strictures. BJU Int. 2011, 107, 6–26.
- Hofer, M.D.; Cheng, E.Y.; Bury, M.I.; Park, E.; Xu, W.; Hong, S.J.; Kaplan, W.E.; Sharma, A.K. Analysis of Primary Urethral Wound Healing in the Rat. Urology 2014, 84, 246.e1–246.e7.
- Singh, M.; Blandy, J. The Pathology of Urethral Stricture. J. Urol. 1976, 115, 673–676.
- Baskin, L.S.; Constantinescu, S.C.; Howard, P.S.; McAninch, J.W.; Ewalt, D.H.; Duckett, J.W.; Snyder, H.M.; Macarak, E.J. Biochemical Characterization and Quantitation of the Collagenous Components of Urethral Stricture Tissue. J. Urol. 1993, 150, 642–647.
- Rodemann, H.P.; Blaese, M.A. Responses of Normal Cells to Ionizing Radiation. Semin. Radiat. Oncol. 2007, 17, 81–88.
- Fajardo, L.F. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005, 44, 13–22.
- Fajardo, L.F. Is the pathology of radiation injury different in small vs large blood vessels? Cardiovasc. Radiat. Med. 1999, 1, 108–110.
- Peña, L.A.; Fuks, Z.; Kolesnick, R.N. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: Protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000, 60, 321–327.
- Reyes, J.G.; Robayna, I.G.; Delgado, P.S.; González, I.H.; Aguiar, J.Q.; Rosas, F.E.; Fanjul, L.F.; de Galarreta, C.M.R. c-Jun Is a Downstream Target for Ceramide-activated Protein Phosphatase in A431 Cells. J. Biol. Chem. 1996, 271, 21375–21380.
- Dressler, K.A.; Mathias, S.; Kolesnick, R.N. Tumor Necrosis Factor-α Activates the Sphingomyelin Signal Transduction Pathway in a Cell-Free System. Science 1992, 255, 1715–1718.
- Müller, G.; Ayoub, M.; Storz, P.; Rennecke, J.; Fabbro, D.; Pfizenmaier, K. PKC zeta is a molecular switch in signal transduction of TNF-alpha, bifunctionally regulated by ceramide and arachidonic acid. EMBO J. 1995, 14, 1961–1969.
- Haimovitz-Friedman, A.; Kan, C.C.; Ehleiter, D.; Persaud, R.S.; McLoughlin, M.; Fuks, Z.; Kolesnick, R.N. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J. Exp. Med. 1994, 180, 525–535.
- Haimovitz-Friedman, A.; Balaban, N.; McLoughlin, M.; Ehleiter, D.; Michaeli, J.; Vlodavsky, I.; Fuks, Z. Protein kinase C mediates basic fibroblast growth factor protection of endothelial cells against radiation-induced apoptosis. Cancer Res. 1994, 54, 2591–2597.
- Basu, S.; Bayoumy, S.; Zhang, Y.; Lozano, J.; Kolesnick, R. BAD Enables Ceramide to Signal Apoptosis via Ras and Raf-1. J. Biol. Chem. 1998, 273, 30419–30426.
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.-I.; Jones, D.P.; Wang, X. Prevention of Apoptosis by Bcl-2: Release of Cytochrome c from Mitochondria Blocked. Science 1997, 275, 1129–1132.
- Belka, C.; Budach, W. Anti-apoptotic Bcl-2 proteins: Structure, function and relevance for radiation biology. Int. J. Radiat. Biol. 2002, 78, 643–658.
- Bayreuther, K.; Francz, P.; Rodemann, H. Fibroblasts in normal and pathological terminal differentiation, aging, apoptosis and transformation. Arch. Gerontol. Geriatr. 1992, 15, 47–74.
- Rodemann, H.P.; Binder, A.; Burger, A.; Güven, N.; Löffler, H.; Bamberg, M. The underlying cellular mechanism of fibrosis. Kidney Int. Suppl. 1996, 54, S32–S36.
- Burger, A.; Löffler, H.; Bamberg, M.; Rodemann, H.P. Molecular and cellular basis of radiation fibrosis. Int. J. Radiat. Biol. 1998, 73, 401–408.
- Rodemann, H.; Bamberg, M. Cellular basis of radiation-induced fibrosis. Radiother. Oncol. 1995, 35, 83–90.
- Paun, A.; Kunwar, A.; Haston, C.K. Acute adaptive immune response correlates with late radiation-induced pulmonary fibrosis in mice. Radiat. Oncol. 2015, 10, 45.
- Takigawa, N.; Segawa, Y.; Saeki, T.; Kataoka, M.; Ida, M.; Kishino, D.; Fujiwara, K.; Ohsumi, S.; Eguchi, K.; Takashima, S. Bronchiolitis obliterans organizing pneumonia syndrome in breast-conserving therapy for early breast cancer: Radiation-induced lung toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 751–755.
- Bessout, R.; Demarquay, C.; Moussa, L.; René, A.; Doix, B.; Benderitter, M.; Sémont, A.; Mathieu, N. TH17 predominant T-cell responses in radiation-induced bowel disease are modulated by treatment with adipose-derived mesenchymal stromal cells. J. Pathol. 2015, 237, 435–446.
- Garrido-Mesa, N.; Algieri, F.; Rodriguez Nogales, A.; Galvez, J. Functional plasticity of Th17 cells: Implications in gastrointestinal tract function. Int. Rev. Immunol. 2013, 32, 493–510.
- Martin, M.; Lefaix, J.; Delanian, S. TGF-beta1 and radiation fibrosis: A master switch and a specific therapeutic target? Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 277–290.
- Mayer, M. Biochemical and biological aspects of the plasminogen activation system. Clin. Biochem. 1990, 23, 197–211.
- Hughes, M.; Caza, T.; Li, G.; Daugherty, M.; Blakley, S.; Nikolavsky, D. Histologic characterization of the post-radiation urethral stenosis in men treated for prostate cancer. World J. Urol. 2019, 38, 2269–2277.
- Hanin, L.; Zaider, M. A mechanistic description of radiation-induced damage to normal tissue and its healing kinetics. Phys. Med. Biol. 2013, 58, 825–839.
- Martin, J.M.; Richardson, M.; Siva, S.; Cardoso, M.; Handmer, M.; Sidhom, M. Mechanisms, mitigation, and management of urinary toxicity from prostate radiotherapy. Lancet Oncol. 2022, 23, e534–e543.
- Rodda, S.; Tyldesley, S.; Morris, W.J.; Keyes, M.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; et al. ASCENDE-RT: An Analysis of Treatment-Related Morbidity for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost with a Dose-Escalated External Beam Boost for High- and Intermediate-Risk Prostate Cancer. Int. J. Radiat. Oncol. 2017, 98, 286–295.
- Awad, M.A.; Gaither, T.W.; Osterberg, E.C.; Murphy, G.P.; Baradaran, N.; Breyer, B.N. Prostate cancer radiation and urethral strictures: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2018, 21, 168–174.
- Hindson, B.R.; Millar, J.L.; Matheson, B. Urethral strictures following high-dose-rate brachytherapy for prostate cancer: Analysis of risk factors. Brachytherapy 2012, 12, 50–55.
- Sullivan, L.; Williams, S.G.; Tai, K.H.; Foroudi, F.; Cleeve, L.; Duchesne, G. Urethral stricture following high dose rate brachytherapy for prostate cancer. Radiother. Oncol. 2009, 91, 232–236.
- Merrick, G.S.; Butler, W.M.; Wallner, K.E.; Galbreath, R.W.; Anderson, R.L.; Allen, Z.A.; Adamovich, E. Risk Factors for the Development of Prostate Brachytherapy Related Urethral Strictures. J. Urol. 2006, 175, 1376–1381.
- Sowerby, R.J.; Gani, J.; Yim, H.; Radomski, S.B.; Catton, C. Long-term complications in men who have early or late radio-therapy after radical prostatectomy. Can. Urol. Assoc. J. 2014, 8, 253–258.
- Herschorn, S.; Elliott, S.; Coburn, M.; Wessells, H.; Zinman, L. SIU/ICUD Consultation on Urethral Strictures: Posterior Urethral Stenosis After Treatment of Prostate Cancer. Urology 2014, 83, S59–S70.
- Merrick, G.S.; Butler, W.M.; Wallner, K.E.; Galbreath, R.W.; Lief, J.H. Long-term urinary quality of life after permanent prostate brachytherapy. Int. J. Radiat. Oncol. 2003, 56, 454–461.
- Allen, Z.A.; Merrick, G.S.; Butler, W.M.; Wallner, K.E.; Kurko, B.; Anderson, R.L.; Murray, B.C.; Galbreath, R.W. Detailed urethral dosimetry in the evaluation of prostate brachytherapy-related urinary morbidity. Int. J. Radiat. Oncol. 2005, 62, 981–987.
- Zaffuto, E.; Gandaglia, G.; Fossati, N.; Dell’Oglio, P.; Moschini, M.; Cucchiara, V.; Suardi, N.; Mirone, V.; Bandini, M.; Shariat, S.F.; et al. Early Postoperative radiotherapy is assocciated with worse functional outcomes in patients with prostate cancer. J. Urol. 2017, 197, 669–675.
- Tibbs, M.K. Wound healing following radiation therapy: A review. Radiother. Oncol. 1997, 42, 99–106.
- Barry, J.M. Visual urethrotomy in the management of the obliterated membranous urethra. Urol. Clin. N. Am. 1989, 16, 319–324.
- Stein, D.M.; Santucci, R.A. Pro: Endoscopic realignment for pelvic fracture urethral injuries. Transl. Androl. Urol. 2015, 4, 72–78.
- Yasuda, K.; Yamanishi, T.; Isaka, S.; Okano, T.; Masai, M.; Shimazaki, J. Endoscopic Re-Establishment of Membranous Urethral Disruption. J. Urol. 1991, 145, 977–979.
- Rozanski, A.T.; Moynihan, M.J.; Zhang, L.T.; Muise, A.C.; Holst, D.D.; Copacino, S.A.; Zinman, L.N.; Buckley, J.C.; Vanni, A.J. The Efficacy and Safety of a Conservative Management Approach to Radiation-Induced Male Urethral Strictures in Elderly Patients With Comorbidities. Société Int. d’Urologie J. 2022, 3, 14–20.
- Ravier, E.; Fassi-Fehri, H.; Crouzet, S.; Gelet, A.; Abid, N.; Martin, X. Complications after artificial urinary sphincter implantation in patients with or without prior radiotherapy. BJU Int. 2014, 115, 300–307.
- Eltahawy, E.; Gur, U.; Virasoro, R.; Schlossberg, S.M.; Jordan, G.H. Management of recurrent anastomotic stenosis following radical prostatectomy using holmium laser and steroid injection. BJU Int. 2008, 102, 796–798.
- Kravchick, S.; Lobik, L.; Peled, R.; Cytron, S. Transrectal Ultrasonography-Guided Injection of Long-Acting Steroids in the Treatment of Recurrent/Resistant Anastomotic Stenosis After Radical Prostatectomy. J. Endourol. 2013, 27, 875–879.
- Redshaw, J.D.; Broghammer, J.A.; Smith, T.G., 3rd; Voelzke, B.B.; Erickson, B.A.; McClung, C.D.; Elliott, S.P.; Alsikafi, N.F.; Presson, A.P.; Aberger, M.E.; et al. Intralesional injection of mitomycin-C at transurethral incision of bladder neck contracture may offer limited benefit: TURNS Study Group. J. Urol. 2015, 193, 587–592.
- Rozanski, A.T.; Zhang, L.T.; Holst, D.D.; Copacino, S.A.; Vanni, A.J.; Buckley, J.C. The Effect of Radiation Therapy on the Efficacy of Internal Urethrotomy With Intralesional Mitomycin C for Recurrent Vesicourethral Anastomotic Stenoses and Bladder Neck Contractures: A Multi-Institutional Experience. Urology 2020, 147, 294–298.
- Vanni, A.J.; Zinman, L.N.; Buckley, J.C. Radial Urethrotomy and Intralesional Mitomycin C for the Management of Recurrent Bladder Neck Contractures. J. Urol. 2011, 186, 156–160.
- Meeks, J.J.; Brandes, S.B.; Morey, A.F.; Thom, M.; Mehdiratta, N.; Valadez, C.; Granieri, M.A.; Gonzalez, C.M. Urethroplasty for Radiotherapy Induced Bulbomembranous Strictures: A Multi-Institutional Experience. J. Urol. 2011, 185, 1761–1765.
- Hofer, M.D.; Zhao, L.C.; Morey, A.F.; Scott, J.F.; Chang, A.J.; Brandes, S.B.; Gonzalez, C.M. Outcomes after Urethroplasty for Radiotherapy Induced Bulbomembranous Urethral Stricture Disease. J. Urol. 2014, 191, 1307–1312.
- Rourke, K.; Kinnaird, A.; Zorn, J. Observations and outcomes of urethroplasty for bulbomembranous stenosis after radiation therapy for prostate cancer. World J. Urol. 2015, 34, 377–382.
- Fuchs, J.S.; Hofer, M.D.; Sheth, K.R.; Cordon, B.H.; Scott, J.M.; Morey, A.F. Improving Outcomes of Bulbomembranous Urethroplasty for Radiation-induced Urethral Strictures in Post-Urolume Era. Urology 2016, 99, 240–245.
- Glass, A.S.; McAninch, J.W.; Zaid, U.B.; Cinman, N.M.; Breyer, B.N. Urethroplasty After Radiation Therapy for Prostate Cancer. Urology 2012, 79, 1402–1406.
- Voelzke, B.B.; Leddy, L.S.; Myers, J.B.; Breyer, B.N.; Alsikafi, N.F.; Broghammer, J.A.; Elliott, S.P.; Vanni, A.J.; Erickson, B.A.; Buckley, J.C.; et al. Multi-institutional outcomes and associations after excision and primary anastomosis for radiotherapy-associated bulbomembranous urethral stenoses following prostate cancer treatment. Urology 2021, 152, 117–122.
- Blakely, S.; Caza, T.; Landas, S.; Nikolavsky, D. Dorsal Onlay Urethroplasty for Membranous Urethral Strictures: Urinary and Erectile Functional Outcomes. J. Urol. 2015, 195, 1501–1507.
- Chung, P.H.; Esposito, P.; Wessells, H.; Voelzke, B.B. Incidence of Stress Urinary Incontinence After Posterior Urethroplasty for Radiation-induced Urethral Strictures. Urology 2018, 114, 188–192.
- Ahyai, S.A.; Schmid, M.; Kuhl, M.; Kluth, L.A.; Soave, A.; Riechardt, S.; Chun, F.K.-H.; Engel, O.; Fisch, M.; Dahlem, R. Outcomes of Ventral Onlay Buccal Mucosa Graft Urethroplasty in Patients after Radiotherapy. J. Urol. 2015, 194, 441–446.
- Policastro, C.G.; Simhan, J.; Martins, F.E.; Lumen, N.; Venkatesan, K.; Angulo, J.C.; Gupta, S.; Rusilko, P.; Pérez, E.A.R.; Redger, K.; et al. A multi-institutional critical assessment of dorsal onlay urethroplasty for post-radiation urethral stenosis. World J. Urol. 2020, 39, 2669–2675.
- Elbakry, A.A.; Pan, M.M.; Buckley, J.C. Frontiers in post-radiation urologic reconstruction; robotic surgery and near-infrared fluorescence imaging: A Narrative Review. AME Med. J. 2022, 7, 7.
- Toia, B.; Seth, J.; Ecclestone, H.; Pakzad, M.; Hamid, R.; Greenwell, T.; Ockrim, J. Outcomes of reconstructive urinary tract surgery after pelvic radiotherapy. Scand. J. Urol. 2019, 53, 156–160.
- Vetterlein, M.W.; Kluth, L.A.; Zumstein, V.; Meyer, C.P.; Ludwig, T.A.; Soave, A.; Riechardt, S.; Engel, O.; Dahlem, R.; Fisch, M.; et al. Buccal mucosal graft urethroplasty for radiation-induced urethral strictures: An evaluation using the extended Urethral Stricture Surgery Patient-Reported Outcome Measure (USS PROM). World J. Urol. 2020, 38, 2863–2872.
- Flamiatos, J.F.; Chen, Y.; Lambert, W.E.; Martinez Acevedo, A.; Becker, T.M.; Bash, J.C.; Amling, C.L. Open versus robot-assisted radical cystectomy: 30-day perioperative comparison and predictors for cost-to patient, complication, and read-mission. J. Robot. Surg. 2019, 13, 129–140.
- Khalil, M.I.; Tourchi, A.; Langford, B.T.; Bhandari, N.R.; Payakachat, N.; Davis, R.; Safaan, A.; Raheem, O.A.; Kamel, M.H. Early Postoperative Morbidity of Robotic Versus Open Radical Cystectomy in Obese Patients. J. Endourol. 2020, 34, 461–468.
- Kim, S.; Buckley, J.C. Robotic Lower Urinary Tract Reconstruction. Urol. Clin. N. Am. 2020, 48, 103–112.
- Unterberg, S.H.; Patel, S.H.; Fuller, T.W.; Buckley, J.C. Robotic assisted proximal perineal urethroplasty: Improving visualization and ergonomics. Urology 2019, 125, 230–233.
- Bearrick, E.N.; Findlay, B.L.; Maciejko, L.A.; Hebert, K.J.; Anderson, K.T.; Viers, B.R. Robotic urethral reconstruction out-comes in men with posterior urethral stenosis. Urology 2022, 161, 118–124.
- Kirshenbaum, E.J.; Zhao, L.C.; Myers, J.B.; Elliott, S.P.; Vanni, A.J.; Baradaran, N.; Erickson, B.A.; Buckley, J.C.; Voelzke, B.B.; Granieri, M.A.; et al. Patency and Incontinence Rates After Robotic Bladder Neck Reconstruction for Vesicourethral Anastomotic Stenosis and Recalcitrant Bladder Neck Contractures: The Trauma and Urologic Reconstructive Network of Surgeons Experience. Urology 2018, 118, 227–233.
