Nanocelluloses (NCs), with their remarkable characteristics, have proven to be one of the most promising “green” materials of our times and have received special attention from researchers in nanomaterials. A diversity of new functional materials with a wide range of biomedical applications has been designed based on the most desirable properties of NCs, such as biocompatibility, biodegradability, and their special physicochemical properties.
- nanocellulose
- nanocrystalline cellulose
- nanofibrillated cellulose
- bacterial cellulose
- hydrogels
- nanogels
- nanocomposites
- drug delivery
- wound healing
- tissue engineering
1. Introduction
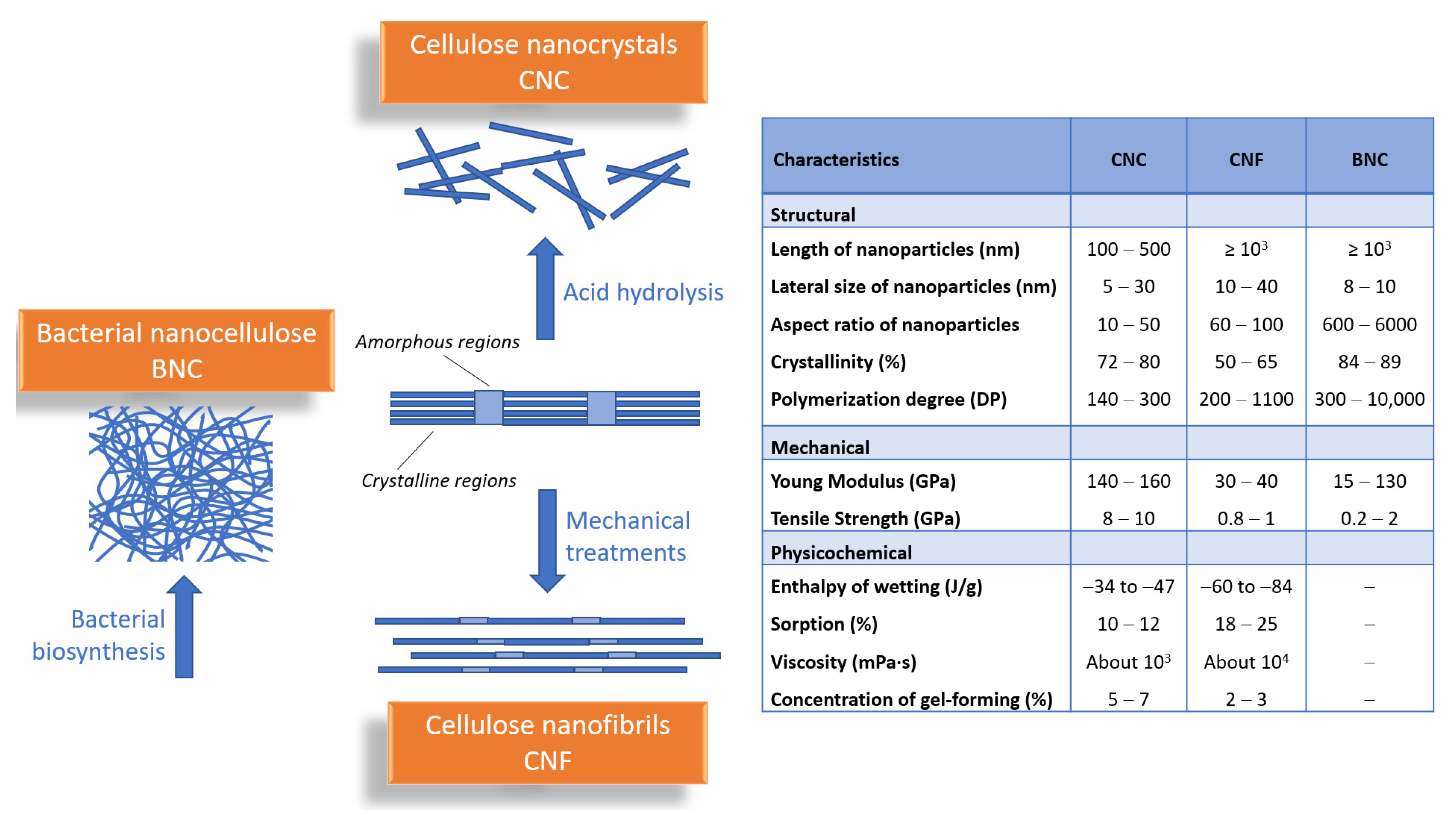
2. Advanced Functional Materials Based on Nanocellulose—General Characteristics
2.1. Hydrogels
With the progress of nanotechnology, hydrogels have received much more attention due to their particular and excellent characteristics. Hydrogels were the first biomaterials to be conceived for use in humans. They have moved forward to now mimic basic physiological processes and are essentials as bioactive implants in the sense of “in vivo” scaffolds. The use of hydrogels as biomaterials is strongly related to their properties. Hydrogels are three-dimensional network colloidal gels from hydrophilic polymer crosslinked able by swelling to absorb and retain large volumes of water in an aqueous environment [18][19][20][21][22][23]. In the swollen state, they have a soft and rubbery structure that mimics the behavior of extracellular matrix (ECM) in biological tissues [24]. Furthermore, hydrogels are conformable to different kinds of surfaces on which they are placed. These properties, in combination with their mucoadhesive nature, elasticity, swelling, and deswelling characteristics in response to environmental stimuli, make hydrogels potential candidates for biomedical applications [18][25]. Thus, hydrogels have found applications to produce different types of materials such as contact lenses [26], blood-contacting hydrogels [27], wound-healing bioadhesives [28], artificial kidney membranes [29], artificial skin [30], vocal cord replacement [31][32], and artificial tendons [33]. Depending on the size of the obtained particles, hydrogels may be classified as macro-, micro-, or nanogels. When they have particle sizes bigger than 100 m, they are usually called macrogels, while gels with particle sizes up to the micrometer range are called microgels. Finally, if these gels are smaller than 100 nm, they are usually considered nanogels [34][35].2.2. Nanogels
Nanogels, also called “hydrogel nanoparticles”, “nanoscalar polymer networks”, “gel nanoparticles”, or “nanoscale hydrogels”, are combine the properties of gels with those of colloids [18]. Generally, they have a spherical shape and size between 20 and 200 nm [36]. Hydrogels, at the nanometer scale, have a great potential in the field of biomedical applications, e.g., as drug-delivery systems, as they combine the characteristics of hydrogels with the advantages of nanoparticles [36][37]. Reducing the size of hydrogel particles in the nano range is reflected in increasing the solubility of hydrophobic drugs, improving the accumulation of drugs in tumors, but also by reducing cytotoxic side effects and increasing the stability of therapeutic agents against enzymatic and chemical degradation [34]. The nanogels also possess some desirable properties, such as high drug-loading capacities, chemical stability, and mechanical properties to avoid the disassembly or fracture during transport, and sensitive response behavior to ensure rapid drug release in response to the relevant stimuli [38]. In addition to their excellent applicability in the drug delivery field, nanogels have also found applications in other biomedical fields, such as chemotherapy [39][40], diagnosis of diseases [41], vaccines delivery [42], biocatalysis [43], and generation of bioactive scaffolds in regenerative medicine [34]. They have also been studied for use in diabetes treatments [44] and gene and protein delivery [41][45]. Nanohydrogels prepared from natural sources have drawn huge attention due to their vast applications in pharmacy, medicine, tissue engineering, cancer therapy, and drug delivery [46]. The use of nanocellulosic materials in obtaining hydrogels from renewable materials has been a much-desired goal that has been achieved for many hydrogel types [47][48][49]. Several smart hydrogels such as injectable hydrogels [33][50][51], shape memory [52][53], supramolecular hydrogels [54][55], double-membrane hydrogels [56], temperature-sensitive hydrogels [57], and many other hydrogels types based on nanocellulose with potential for biomedical applications have been developed. However, on their own, nanocellulose materials do not gel [58]. Different ways are used to perform, for example, for the gelation of CNC suspensions: by simply increasing the concentration of suspension due to a decrease in the electrostatic double-layer distance [59]; by modifying the solvent conditions through ionic strength increase [60]; by addition of polymers [58]; by sonication [61]; and by hydrothermal treatment at elevated temperature [47]. Nanocrystalline cellulose, with their high rigidity and relatively low anisotropy, are well-suited to act as templates for aligned structures (e.g., artificial muscle-like materials) while providing toughness and flexibility. With collagen, for instance, this afforded networks with mechanical properties similar to tendon and ligaments and excellent biocompatibility [62]. Nanofibrillated cellulose (CNFs) is the type of nanocellulose most likely to form hydrogels due to the length of the nanofibrils. CNFs form gels with much higher elasticity than those resulting from CNCs. CNF suspensions exhibit gelation (G′ > G″, G′ ∝ ω0, and G″ ∝ ω0, where, G′ is the storage modulus, G″ is the loss modulus, ω is the frequency) even down to a concentration near to 0.1 wt.%, i.e., the critical gelation concentration, above which the nanofibrils form interconnected networks [63]. CNFs will afford such structures at concentration ranges of 0.05–6 wt.% [62]. The simplest case is offered by pristine CNFs, which spontaneously form hydrogels, probably promoted by their length and interacting entanglements [64].2.3. Nanocomposites
Currently, research is also progressing in the field of nanocomposite hydrogels, including functionalized nanomaterials [18]. In general, in order to improve or modify certain properties, polymeric matrices of nanocomposites are reinforced with nanoparticles/nanofillers [65]. In particular, hydrogels are reinforced with nanoscale materials to obtain nanocomposites with high mechanical strength characteristics or are combined with nanoparticles that confer antibacterial or magnetic properties [34].2.3.1. Nanocellulose Materials as “Reinforcing Agents” into Polymer Matrices
Over the past decade, composite materials have attracted a great deal of interest, and particular attention has been focused on the use of nanocellulose as an alternative to inorganic reinforcing agents in polymer matrices for the production of fully “green” composites [66][67][68]. Nanocellulose, owing to its exceptionally high mechanical properties (high specific strength and modulus), high surface area, high aspect ratio, and low environmental impact, has greater advantages as reinforcing filler in comparison to glass fibers, silica, carbon black, and other expensive nanosized fillers [65]. Thus, composite materials with natural fillers have not only met the environmental appeal but also contributed to developing low-density materials with improved properties [69]. Hydrogels entirely made of biopolymers and reinforced with nanocellulose can be classified as “green” nanocomposite materials because of their renewable and biodegradable design [70]. The design of cellulose-based biocomposites is a pathway with many alternatives due to the wide variety of cellulose fibers with specific geometries, the diversity of polymers and manufacturing processes, the multitude of types of reinforcements, and the possibilities of orientation and arrangement of fibers [17]. Nanocrystalline cellulose has been investigated as reinforcing agents for a variety of polymeric systems due to their large aspect ratio, high specific strength and modulus, low density, high surface area, and unique optical properties [51][62][65][71][72]. CNCs have been used as reinforcing agents in a wide range of polymer matrices, from the most common to the most unusual, such as: poly(vinyl alcohol), poly(oxyethylene), polyethylene glycol, poly(N-isopropylacrylamide), starch, natural rubber, or polyurethane [67][73][74][75][76]. Nanofibrillated cellulose (NFC) has excellent properties for mechanical reinforcement due to its special morphology that combines the advantages of the length of the fibers in the micrometers range with those of their width in the nanometers range [69]. The use of CNF networks as reinforcing elements together with a suitable matrix polymer is an efficient reinforcement solution for high-quality, specialized applications of bio-based composites. The combination of nanofiber flexibility, aspect ratio, and strength is the main advantage of CNFs in various applications [77]. For instance, comparing the reinforcement capacity of CNC and NFC (at the same addition) using poly(ethylene oxide) (PEO) as the polymer matrix, Xu and coworkers [75] reported that the nanocomposites reinforced with NFC demonstrated higher-strength and elastic modulus than nanocomposites with CNC. However, CNC-based nanocomposites presented a higher strain of failure. The higher strength values of NFC-based nanocomposites are the effect of their high aspect ratio of cellulose nanofibrils that favors more entanglement and network percolation. By comparison, bacterial nanocellulose (BNC), having the highest purity of all nanocellulose materials, doubled by high crystallinity and excellent biological affinity, is the ideal reinforcing component for biopolymer composites [78].2.3.2. Nanocellulose Materials as “Matrices” for Different Reinforcing Agents
The inclusion of biocompatible and/or bioactive compounds as components of the composite is the proper way to overcome certain limitations of nanocellulose materials, improving their biocompatibility, antimicrobial activity, or water-holding capacity [79]. Generally, nanocellulose can be reinforced with different polymers with specific properties, obtaining a material with different characteristics from those of starting materials [65]. Nanocellulose can also be used as a substrate for the incorporation of inorganic nanoparticles, such as carbon nanotubes, graphene, and graphene oxide to obtain hydrogels with antibacterial, antiviral, antifungal, magnetic, electrical, and mechanical properties. The high specific surface area, the presence of reducing functional groups, and the ability to form aqueous suspensions are the main arguments for the use of nanocelluloses as a support for metal/metal oxide nanoparticles [80]. The process of composite formation is performed through physicochemical interactions or by mechanical capture of nanoparticles in the structural matrix of nanocellulose [81]. Nanocellulose-based compounds have found applications in various biomedical areas, from dressings to drug administration and even as a basis for scaffolding in regenerative medicine [82]. Silver particles have been used as potential agents with a broad antibacterial activity and low presumed toxicity to coat cellulosic materials for biomedical applications. The composite was prepared by immersing BNC in a silver ammonium solution and showed to be effective as dressing in wound-healing applications by decreasing inflammation and promoting wound-healing [30]. The silver nanoparticles were incorporated in crystalline nanocellulose by microwave-assisted synthesis, and the composites proved to have high antibacterial properties against E. coli (Gram-negative bacteria) and S. aureus (Gram-positive bacteria) [83]. Barua and coworkers [84] prepare copper-copper oxide nanoparticles (Cu–CuO) NP-coated CNFs through a green reductive technique, which exhibited promising antimicrobial activity against Gram-positive and Gram-negative bacteria and fungal species.3. Nanocellulose-Based Materials in Pharmaceutical/Medical Applications
The nanocellulose materials, used as independent functional material or as reinforcement units in composite materials, have received tremendous attention in a wide variety of applications, including foods, packaging, cosmetics, biomedical implants, optics, water filtration, hygienic applications, and so forth [3][14][17]. However, especially in biomedical fields, they appear to have significant advantages due to their intrinsic biodegradability and biocompatibility [14]. However, other interesting features should also be considered, such as mechanical properties, low risk of cytotoxicity, its three-dimensional (3D) nanofibrous network, and last but not least, its natural source [62][85][86].3.1. Nanocellulose-Based Materials in Drug-Delivery Systems (DDS)
An ideal drug carrier should be nontoxic, non-immunogenic, biocompatible, and biodegradable; enhance drug solubility and stability and have high drug-loading capacity; and be capable of reaching correct concentrations at a proper rate determined by an optimal [36]. Other equally important criteria to be met are related to its size and surface characteristics because these two parameters control the residence time in the bloodstream and the target site. More exactly, the size needs to be sufficiently large enough to prevent rapid penetration into fenestrated blood vessels, yet sufficiently small to avoid phagocytosis. The surface nature also decides the duration and destination of the drug carrier in the circulatory system. For instance, a hydrophilic surface will most likely make the carrier avoid phagocytosis by macrophages, and this hydrophilicity can be accomplished either by covering the surface with a hydrophilic polymer (i.e., PEG) or by using block copolymers with hydrophilic and hydrophobic areas [62]. Being a natural nanosized material, nanocellulose features meet the necessary criteria mentioned above regarding its function as a vehicle for DDS: its horizontal measurements extend from 5 to 20 nm, and the longitudinal measurement ranges from 10 nm to a few microns; each of its monomers bears three hydroxyl groups with the ability to form hydrogen bonds, which plays a major role in the surface hydrophilicity. Of course, the nanocellulose unique properties should not be overlooked because they make this material play an important role among drug-delivery vectors, such as high crystallinity, biocompatibility, biodegradability, high surface area, unique mechanical and rheological properties, liquid absorption capacity, and porosity [36][85][87][88]. The three types of nanocellulose are quite similar to each other, yet there are distinct differences that set them apart. These differences make each type of nanocellulose better suited for a certain drug-delivery system compared to the others [87]. The researchers summarize the recent reports on nanocellulose-based drug-delivery systems in Table 1.|
NCs Type |
Drug Delivery System |
Drug |
Drug-Release Conditions |
Drug Release |
|---|
|
Material |
Cellulose Source |
Material Toxicological Experiment | Mechanism |
Ref. |
|||||
|---|---|---|---|---|---|---|---|---|---|
Cells | Lines |
Cellulose Source |
Toxicological Experiment Toxicological Results |
Cells Lines |
Toxicological Results Results and Possible Application |
Results and Ref. |
|||
Possible | Application |
|
Material |
Cellulose Source |
Toxicological Experiment |
Cells Lines |
Toxicological Results | Ref. |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Results and | Possible | Application |
Ref. |
|||||||||||
|
CNC |
||||||||||||||
|
CNC | cCNC/SA double-membrane hydrogels |
CH, EGF |
PBS, pH 7.4, 37 °C; t90% = 3 days (CH); t90% = 4 to 8 days (EGF). |
Swelling/erosion |
||||||||||
|
BNC | [ | ] | ||||||||||||
CNF |
TEMPO-oxidized CNC/CSos |
PrHy, IMI |
||||||||||||
|
CNC | Breast cancer |
Cotton (Whatman 1 filter paper) PBS, pH 7.4, room temp.; t40% [50] |
||||||||||||
MTS assay; | = 12 min (PrHy); t 80% = 2 h (IMI). |
- |
ATP assay. |
BEAS 2B hMDMs [90] |
||||||||||
Cytotoxicity at 100 mg/mL; | ||||||||||||||
|
BNC scaffolds |
G. xylinus |
CCk-8 assay |
HUVECs, SMCs, |
No micronuclei induction after exposure to 2.5–100 mg/mL; No induction of proinflammatory cytokines in hMDMs. |
Toxicity impact on lungs or bone marrow |
[135] |
Fibroblasts |
CNC-HDQ complex |
HDQ |
Double crosslinking 3D-printed CNF hydrogels |
Skin TE |
|||
BC tubes have no toxic or side effects on vessel-related cells cultured on their surface; | the surface of BC tubes was beneficial for cell attachment, proliferation, and ingrowth. |
CNC CNC- carboxyl groups dH [ |
Softwood cellulose pulp2O, room temp., in the dark; t40% = 1 h; t80% = 4 h. |
111] |
||||||||||
MTS assay |
- |
CaCO-2, HeLa, MDCK, J774 [91] |
||||||||||||
Vascular | TE | [166] |
CNC not exhibit any significant cytotoxicity; can exert stress on cells if they possess a high charge density; Charge-dependent decrease in mitochondrial activity (charge contents > 3.9 mmol/g). |
Drug delivery |
[136] |
CS/CNC nanocomposite hydrogels |
CNF/PVA bilayer scaffold C |
|||||||
|
c-CNCs |
Skin TE |
SGF, pH 1.2, 37 |
t-CNCs [ |
Cotton Tunicate from °C; 115120 min: 65% (0.5% CS/CNC); 50% (2.5% CS/CNC). |
] Ritger–Peppas model; n = 0.61–0.66; Non-Fickian diffusion. |
[92] |
||||||||
Stuela clava |
LDH assay |
A549 MDM MDDC |
The aspect ratio in combination with CNCs dose influences the uptake by the 3D co-culture system of the human epithelial airway barrier system. |
Toxicity impact on lungs |
[137] |
QC/cCNC/β-GP nanocomposite hydrogels |
CNF/Gel/ApA DOX |
Bone TE PBS, pH 7.4, 37 °C; t90% = 4 days (0% cCNC); t90% = 7 days (1% cCNC); t90% = 17 days (2.5% cCNC). |
Swelling/erosion |
[93] |
||||
[ | ||||||||||||||
|
CNC | ] |
|||||||||||||
Wood pulp |
TB assay |
A549 |
CNC were nontoxic to A549 cells; CNC induced a robust inflammatory response; CNC particles induced a more robust inflammatory response compared to NCF. |
Comparable toxicity of CNC with CNF |
[138] |
Gel/CNC nanocomposite hydrogels |
TPh |
SGF, pH 1.2, 37 °C; 24 h: 90% (5% CNC); 85% (10% CNC); 60% (25% CNC). |
CNC |
- |
[94] |
|||
|
CNCgel CNCdry |
CNC/PVA nanocomposites |
Wood pulp Skin TE |
LDH assay [117] |
|||||||||||
MH-S | Low conc. (1.5 and 5 μg/cm2) induce no cytotoxicity; A high dose of CNCdry induced a decrease in cell viability; CNC exposure further altered the secretion of cytokines. |
Toxicity impact on lungs |
[139] |
m-CNC/Alg hydrogels |
Gel/HA/CNC hydrogels |
Skin wound repair |
[118] |
|||||||
|
CNC |
Ibu |
PBS, pH 7.4, 37 °C; t = 0–30 min; 45%–60% burst release; t = 30–330 min; sustained release. |
Wheat bran Fickian diffusion |
MTT assay |
Caco-2 [95] |
|||||||||
Dose-dependent decrease in cell viability, but only with significant results above 1000 μg/mL; | The cell viability decreased significantly upon contact with CNC90 (88.09%) at 2000 μg/mL, although CNC30 (92.81%) and CNC60 (93.11%) did not significantly decrease the cell viability. |
Biocompatible nanocomposites |
[140] |
CNF |
Col/CNC/GMs PDA/TEMPO-CNF composite hydrogels |
TCH |
PBS; “On-off” drug release under NIR irradiation; 120 min: 60% (pH 5.0); 30% (pH 7.4); 15 h: 70% (pH 5.0); 55% (pH 7.4). |
Blood vessel Korsmeyer–Peppas model; Non-Fickian diffusion. |
||||||
] |
CNF/HPMC nanocomposites | |||||||||||||
|
PEG-grafted CNC nanocomposites |
Bone TE |
[120] |
||||||||||||
|
CNC/PVA hybrid hydrogels |
Soft TE |
[121] |
||||||||||||
|
CNC/PAAm composite hydrogels |
TE |
[122] |
||||||||||||
|
KT |
a-CNC/Gel hydrogels composite |
Breast cancer |
[123] |
|||||||||||
|
TEMPO-CNC reinforced PVA hydrogels |
Corneal implant |
[124] |
||||||||||||
|
BNC |
BNC/Fibrin composites |
New blood vessel |
[78] |
|||||||||||
|
BNC-Gel/HAp nanocomposites |
Bone TE |
[125] |
||||||||||||
|
Alg/BNC/Col composite |
TE |
[126] |
||||||||||||
|
3D BNC/PMS scaffolds |
TE; soft tissues regeneration |
[127] |
||||||||||||
|
DBC/Col-p |
TE; tissues regeneration |
[128] |
||||||||||||
|
BNC/PA/Gel/HAp |
Bone repair |
[129] |
||||||||||||
|
(BNC-Col)-Ap/OGP peptides |
Bone TE |
[130] |
||||||||||||
|
BNC-CNTs composites |
Bone regeneration |
|||||||||||||
|
K-CNC R-CNC |
Rubberwood fiber Kenaf-bast fiber |
MTT assay |
RAW 264.7 HaCaT |
Cytotoxicity of K-CNC and R-CNC is not significant up to 700 μg/mL; K-CNC and R-CNC induced the formation of ROS in RAW264.7 macrophages. |
Biocompatible nanocomposites |
[141] |
||||||||
|
CNC CNC-FL CNC-HM |
Cellulose pulp |
MTT assay |
ATCC PCS201012, A375 |
No cytotoxicity in direct and indirect contact assays. |
Drug delivery |
[142] |
||||||||
|
CNC in nanocomposites |
||||||||||||||
|
Collagen/CNCs/ GMs scaffolds |
MCC |
MTT assay |
HUVECs |
No cytotoxicity; Excellent biocompatibility. |
Vascular TE |
[143] |
||||||||
|
CNC CNC-AEM CNC-AEMA |
Softwood pulp |
ATP assay |
J774 A.1 PBMNC |
One cationic CNC induced secretion of proinflammatory cytokine IL-1b associated with increase mitochondrial-derived ROS and extracellular ATP levels. |
Drug and DNA delivery systems |
[144] |
||||||||
|
PLA/CNCg-PEG nanocomposites |
Southern pine |
Live/dead assays |
hMSCs |
Suitable biocompatibility; Nontoxic effect on hMSCs proliferation. |
Bone TE |
[145] |
||||||||
|
TEMPO-CNC reinforced PVA hydrogels |
MCC |
AB assay |
HCE-2cells |
Nontoxicity; Excellent biocompatibility; The HCE-2 cells viability above the 70%. |
Ophthalmic applications |
[146] |
||||||||
|
CNF |
Bleached dissolving pulp Norway spruce (Picea bies) |
MTT assay [3H]-thymidine uptake assay |
L929; Thymocytes PBMNCs |
CNFs were not cytotoxic; CNC has non-inflammatory and on-immunogenic properties. |
Implantable biomaterials TE |
[151] |
||||||||
|
CNF |
Pinus radiata pulp |
LDH assay MTT assay |
HEK NHDF |
No toxic effect for keratinocytes and fibroblasts; Non-immunotoxic. |
||||||||||
|
Octenidine-loaded BNC |
K. xylinus |
ATP assay |
HaCaT | Wound dressings |
Pure BNC has no influence on HaCaT viability; OCT/BNC extracts exhibited time and concentration-dependent toxicity; cell-damaging effects were observed at extract conc >10% and longer incubation times (24 and 48 h). |
Active wound dressing [152] |
||||||||
[ | ] |
CNF CNC |
Wood pulp |
TB assay |
||||||||||
|
BNC |
Sugar cane molasses | A549 |
CNF caused a significant decrease in cell viability, at 72 h; Decrease in GSH levels after exposure to CNF. |
CNC toxicity |
LDH activity |
HepG2/C3A |
BC is not cytotoxic (conc. < 170 μg/mL); BNC has a protective effect against CP-induced myelotoxicity and enotoxicity. |
Biomaterial TE [138] |
||||||
[ | ] |
U-NFC A-NFC C-NFC |
||||||||||||
|
Vaccarin- loaded BNC |
Never-dried bleached sulfite softwood dissolving pulp |
G. xylinusAB assay LDH assays |
HDF |
Toxicity impacts on dermal, lung, and macrophage cells |
MTT assay MRC-5 THP-1 |
[153] |
||||||||
L929 | No cytotoxicity for treated NFC; HDF and MRC-5 cells: the metabolic activity of the treated cells was comparable to that of the negative control; THP-1 cells: a higher metabolic activity of the NFC-treated; U-CNF has an inflammatory response, which was suppressed when surface charges were introduced on the CNFs. |
BNC-Vac has lower toxicity and better biocompatibility than BNC; RGR for both BNC and BNC-Vac was above 74%. |
Wound dressing |
[169] |
CNF |
Bleached Eucalyptus Globulus kraft pulp |
MTT assay |
A549 THP-1 |
||||||
|
Gentamycin-loaded BNC | Cytotoxic effect at the highest dose tested; | Genotoxic effects in A549 cells in the co-cultures; No oxidative DNA damages. |
TE |
K. xylinus |
NR assay |
U2-OS |
No cytotoxicity on osteoblast culture after 24 h; gentamycin released from G-BNC after 8 h (400 mg/L) and 16 h (600 mg/L) is enough to eliminate S. aureus and P. aeruginosa biofilms. [ |
Bone regeneration TE154] |
||||||
[ | ] |
CNF |
||||||||||||
|
Curcumin- loaded BNC |
Curauá fibers (Ananas erectifolius L. B. Smith) |
K. xylinus Cytotoxicity assays ISO 10993-5 |
MTS assay Vero |
HNDF CNF shows no cytotoxicity and suitable biocompatibility; The morphology and basic functions of the cells are not affected by the direct contact with the tested materials. |
The cytotoxic effect on the cells depended on the conc. of curcumin; at 0.5 mg/mL C, a strong cytotoxicity for BNC-C and BNC-DC180; BNC-DC300 suitable cytotoxicity, even at higher extract conc. Scaffold TE |
[ |
Wound dressing 155] |
PBS, pH 7.4; 8 h: 95% (5% CNF), 62% (0.5% CNF), 56% (0.75% CNF), 37% (1% CNF). | ||||||
[ | ] |
CNF |
||||||||||||
|
BNC-GTMAC |
Softwood bleached kraft fiber |
BNC-GHDE LDH |
G. xylinus assay |
AB assay Caco-2, HT-29MTX Raji B |
HaCaT Minimal or no cytotoxicity in a cellular model of the intestinal epithelium (for CNC-25 at 0.75% and 1.5% w/w, as well as for CNF-50 at 0.75% w/w). |
No cytotoxicity; Suitable wound closure rates in the presence of the samples, with complete coverage of the scratched area after 5 days. Biocompatible material |
Non-Fickian diffusion; n = 0.52–0.61. |
[97] |
||||||
|
CNF/Alg hydrogels |
MH |
SGF, pH 1.2; SIF, pH 7.4, 37 °C; T40% = 90 min (CNF/Alg-50/50, SGF) t80% = 145 min (CNF/Alg-50/50, SIF). |
Fickian diffusion mechanism |
[98] |
||||||||||
|
BNC |
BNC-SA hybrid hydrogels |
Ibu |
PBS, pH 1.5, 7.0 and 11.8; 37 °C; 24 h: 90% (pH 11.8); 80% (pH 7.0); and 60% (pH 1.5); PBS, pH 7.4; 0.15 V, 0.3 V, 0.5 V; 24 h: 95% (0.5 V); 85% (0.3 V and 0.15 V); 80% (0 V). |
Korsmeyer–Peppas model; Non-Fickian diffusion; pH; n = 0.498–0.772; E-field; n = 0.700–0.491. |
[99] |
|||||||||
|
TCH-loaded BNC composites |
TCH |
HEPES buffers, pH 7, 37 °C; 3 h: ~100% (free TCH); 90% (0.5% TCH); 60% (0.3% TCH); 20% (0.1% TCH); 10% (0.05% TCH); |
- |
[100] |
||||||||||
|
OCT-loaded BNC |
OCT |
PBS, pH 7.4, 32 °C; 8 h: 82.7% ± 2.6% in first; 24 h 91.8% ± 2.0% after |
Ritger–Peppas model; n = 0.51–0.55; Non-Fickian diffusion. |
[101] |
||||||||||
|
PI-loaded BNC |
PI |
PI buffer, 32 °C; t84% = 48 h. |
Ritger–Peppas model; n = 0.608–0.612 |
[102] |
||||||||||
|
PHMB-loaded BNC |
PHMB |
PHMB buffer, 32 °C; t87% = 48 h. |
Ritger–Peppas model; n = 0.863–0.871 |
[102] |
||||||||||
Abbreviations: cCNC—Cationic cellulose nanocrystals; SA—Sodium alginate; CH—Ceftazidime hydrate; EGF—Epidermal growth factor human; PBS—Phosphate-buffered saline; CSos—Chitosan oligosaccharide; PrHy—Procaine hydrochloride; IMI—Imipramine hydrochloride; HDQ—Hydroquinone; C—Curcumin; CS—Chitosan; QC—Quaternized cellulose; β-GP—β-glycerophosphate; DOX—Doxorubicin; Gel—Gelatin; TPh—Theophylline; m-CNC—Magnetic cellulose nanocrystals; NIR—Near-infrared spectroscopy; PDA—Polydopamine; HPMC—Hydroxypropylmethyl cellulose; KT—Ketorolac tromethamine; Alg—Alginate; MH—Metformin hydrochloride; SGF—Simulated gastric fluid; SIF—Simulated intestinal fluid; HEPES—(4-(2-hydroxyethyl)-1-piperazine- ethanesulfonic acid); TCH—Tetracycline hydrochloride; AM—Acrylamide; Ibu—Ibupofren; OCT—Octenidine; PI—Povidone-iodine; PHMB—Polihexanide; dH2O—Distilled water.
3.2. Nanocellulose-Based Materials in Wound-Healing Applications
3.3. Nanocellulose-Based Materials in Tissue Engineering Applications
Tissue engineering is another area of exciting potential applications for nanocellulose materials [16]. These applications include skin, bone, and cartilage tissue, engineering of blood vessels and soft tissues, repairing congenital heart defects, or ophthalmologic applications [103]. An ideal scaffold for tissue engineering is a material with high porosity, interconnected pores with adequate size, the presence of functional groups for cell adherence, and a high surface-to-volume ratio. Furthermore, tissue engineering scaffolds should possess suitable mechanical properties and should be nontoxic for cells. All these physicochemical properties are essential for cells growth and proliferation, vascularization, and nutrient transfer [109][110]. Cellulose-based materials have been shown to have great biomedical potential in the construction of ECM-mimicking scaffolds owing to their intrinsic characteristics, such as biocompatibility, lack of cytotoxicity, tunable 3D architecture and porous microstructures, and desired mechanical properties, although in vivo degradability still remains under discussion due to lack of the relevant enzyme in the human body [48][62][111][112]. In particular, the use of cellulose nanoparticles to improve the performance of other polymers used as scaffolds is another topic of interest for the researchers. Nanocellulose is considered one of the most promising nano-reinforcement materials of composites due to its nanoscale size, high aspect ratio, and excellent mechanical strength. Compared to other nanofillers, the incorporation of nanocellulose from natural sources could greatly improve the mechanical property of the polymer matrix, but without the sacrifice of its biocompatibility and biodegradability. Last but not least, the presence of surface hydroxyl groups in nanocellulose not only makes the chemical modification easy but also enhances the hydrophilicity of the materials, which is beneficial to the growth and proliferation of cells [113][114]. Nanocellulose-based materials applications in tissue engineering are vast and varied, and Table 2 presents some examples, from the multitude of applications in this field, for each type of nanocellulosic material.|
NCs Type |
TE Systems |
Applications |
References |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
CNF |
CNF/CS nanocomposites |
Artificial skin |
[30] |
||||||||||||||||
|
CNF-based thixotropic gels | |||||||||||||||||||
[ | |||||||||||||||||||
Wound dressing | |||||||||||||||||||
] | |||||||||||||||||||
[ | ] |
CNF |
Banana peel bran |
||||||||||||||||
|
BNC in nanocomposites | MTT assay |
Caco-2 |
CNF conc. < 500 mg/mL are not cytotoxic to Caco-2 cells; Viability of Caco-2 decreased with increasing CNF conc. |
Biocompatible material |
[157] |
||||||||||||||
|
U-NFC A-NFC C-NFC P-NFC S-NFC |
Never-dried bleached sulfite softwood dissolving pulp |
Resazurin Assay |
Caco-2 |
None of the NFCs inducing cytotoxic effects in the intestinal cells; | |||||||||||||||
|
BNC/ALG bilayer composites | The differences in physics-chemical properties of the studied NFCs were not reflected in the Caco-2 response in terms of metabolic activity and cell membrane integrity. | Drug release in gastrointestinal tract (GIT) |
G. xylinus |
ISO10993-5:2009 |
hNCs hMNC |
The composites were found to be noncytotoxic, with a cell viability of 98% and a uniform distribution of cells on the entire porous layer. |
Neocartilage TE [158] |
||||||||||||
PVA/CNC | |||||||||||||||||||
nanocomposites |
Sugarcane bagasse |
MTT assay |
L929 | ||||||||||||||||
[ | ] |
U-NFC C-NFC H-NFC P-NFC S-NFC |
Never-dried bleached sulfite softwood dissolving pulp |
MTS Assay |
BEAS-2B |
||||||||||||||
|
BNC-COL-Ap composites |
G. xylinus |
MTT assay | No cytotoxicity for the highest tested dose (500 μg/mL) for any of the NFCs; |
None of the NFCs induced genotoxic effects; |
Osteoblastic cells All samples were able to increase intracellular formation of ROS. |
The composites did not exhibit cytotoxicity effects. In vitro toxicity of NFCs |
Bone regeneration TE [159] |
||||||||||||
[ | ] |
c-CNF cys-CNF |
Never-dried bleached sulfite softwood dissolving pulp |
PB assay |
hDF |
cys-CNF did not induce toxic effects on hDF when tested at a concentration up to 0.5 mg/mL, nor did the starting material c-NF cys-CNF presented a dual action in vitro: inhibition of metalloproteinase and radical scavenging activity. |
|||||||||||||
|
ALG/BCN/COL composite | Wound dressing |
A. xylinum |
CCk-8 assay | [ |
MC3T3-E1 hAMS |
MC3T3-E1 and hams cells were viable and proliferate well, after 2 and 5 days of incubation—suitable cytocompatibility. |
TE 160] |
||||||||||||
[ | ] |
CNF in nanocomposites |
|||||||||||||||||
|
BC-PHEMA composites |
A. xylinum |
AB assay |
rMSCs |
BC-PHEMA composites are nontoxic and biocompatible; did not influence the morphology and proliferation of the rMSCs. |
Wound dressing |
[176] |
CNF L-CNF CNC L-CNC |
||||||||||||
|
BC/COL composites | Dissolving pulp |
AB assay |
G. xylinus A549 |
Live/ Dead assay THP-1 |
Cytotoxic and inflammatory responses were dependent on type, size, and hydrophobicity Low or inexistent toxicity of all CNMs in A549 cells Dose-dependent cytotoxic and inflammatory responses in THP-1 cells. |
TE |
UCBMSCs |
No cytotoxicity; Provide advanced microenvironment for UCB-MSCs viability and in vitro proliferation; Significantly elevated proteins and calcium deposition. [161] |
|||||||||||
Bone regeneration | TE |
[177] |
Noncytotoxic effect; Strong attachment and proliferation of human fibroblast skin cells on the scaffold. |
CNF /GEL/ApA TE scaffolds |
Bleached birch pulp |
MTT assay |
MSCs |
||||||||||||
|
GEL/BNC nanocomposite | CNFs and CNF-COOHs have no cytotoxicity; | CNF-COOH-ApA cells expressed a low level of stress, visible through lower cell density and the cell inclusions. |
Bone TE |
A. xylinum |
MTT assay [ |
HEK293 |
BNG showed negligible cytotoxicity. ] |
||||||||||||
Wound dressing | [ | ] | |||||||||||||||||
[ | ] |
GA-HA-CNC hydrogels |
NFC hydrogels crosslinked with Ca MCC |
CCK-8 assay |
2+ |
Bleached sulfite softwood pulp NIH-3T3 |
AB assay Cell viability, at 1, 4, and 7 days, higher than 70% limit; No foreign body response. |
hDF |
|||||||||||
|
BNC-GEL nanocomposite | Cell viability about 78% indicates no toxic effects. | No inflammatory response of blood-derived mononuclear cells was observed in relation to the cytokines secretion. |
Skin TE |
Wound healing |
- |
MTS assay |
MRC-5 |
The samples have no cytotoxicity, and the cells retained their morphology in direct contact with the membrane, The cells attaching to the GEL porous site, while not attaching to the GEL thin-coated BC side. [148] |
|||||||||||
Bone regeneration | [ | ] | |||||||||||||||||
TE | [ | ] |
CS/Gel/NCC/CP nanocomposites |
TEMPO-CNF hydrogel Soft wood cellulose fibers |
MTT assay |
Bleached birch kraft pulp |
MTT assay Fibroblast cell |
hDF |
Nontoxicity effect and great hDF cells viability. |
||||||||||
|
Chitosan-BNC |
K. xylinus |
MTT assay | Lack of cytotoxicity after 3 days of increasing the cells’ viability. |
Wound healing |
[149] |
||||||||||||||
Wound healing |
GM07492 |
No cytotoxicity for the BC group and BC-Chi-Cip group; Ciprofloxacin-loaded BC-Chi samples exhibited a significant but slight decrease in the metabolic activity of cells (moderate cytotoxicity). |
[164] |
||||||||||||||||
Wound dressing | [ |
CNC/PVA |
MCC |
[131] |
|||||||||||||||
] | AB assay |
HCE-2 |
Nontoxic and cytocompatible profile of the CNC-PVA hydrogel; Suitable biocompatibility toward HCE-2. |
Ophthalmic applications |
[150] |
BNC |
Ear cartilage TE |
[132] |
|||||||||||
|
BNC/Alg bilayer composite |
Neocartilage formation |
[133] |
|||||||||||||||||
|
BNC/CS composites |
Cartilage tissue regeneration |
[134] |
|||||||||||||||||
Abbreviations: CS—Chitosan; PVA—Poly(vinyl) alcohol; Gel—Gelatin; ApA—Phosphonate; HA—Hyaluronic acid; Col—Collagen; GMs—Gelatin microspheres; PEG—Poly(ethylene glycol); PAAm—Polyacrylamide; a-CNC—Anionic CNC; HAp—Hydroxyapatite; Alg—Alginate; PMS—Paraffin microspheres; DBC—Dialdehyde bacterial cellulose; Col-p—Collagen peptide; PA—Procyanidin; Ap—Carbonate apatite; OGP—Osteogenic growth peptide; CNTs—Carbon nanotubes.
4. Toxicological Evaluation and Potential Limitations of NCs-Based Materials
4.1. Toxicological Evaluation of NC-Based Materials
Abbreviations: BEAS 2B—Human bronchial epithelial cells; hMDMs—Monocyte-derived macrophages; Caco-2—Human colon carcinoma cells; HeLa—Human cervix; MDCK—Dog kidney; J774—Mouse macrophages; A549—Epithelial cells; MDM—Human blood monocyte-derived macrophages; MDDC—Dendritic cells; MH-S—Murine alveolar macrophages; RAW 264.7—Macrophages; ATCC PCS201012—Primary human fibroblasts; A375—Malignant melanoma cells; J774A.1—Mouse monocyte/macrophage; PBMNC—Peripheral blood mononuclear cells; hMSCs—Human mesenchymal stem cells; L929—Mouse fibroblast; NIH-3T3—Fibroblast; HCE-2—Human corneal epithelial cells; LDH assay—Lactate dehydrogenase cytotoxicity assay; MTT assay—(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay; TB assay—Trypan blue exclusion staining cell viability assay; MTS assay—CellTiter-Glo luminescent cell viability assay; MCC—Microcrystalline cellulose.
NFC/QCRs nanocomposites | ||||||
Brown algae | ||||||
MTT assay | ||||||
|
GO/n-HAp/BNC/b-glucan biocomposite |
- |
NR assay L929 |
Cells viability is higher than 80% (for 5 to 1000 μg/mL CNFs/QCRs), indicating that there is no cytotoxicity. |
Wound healing |
[165] |
|
MC3T3-E1 | ||||||
All samples had suitable potential for cell adhesion and proliferation with very low cytotoxicity | ||||||
The order of the cell viability: BgC-1.4 (93%) > BgC-1.3 (79.8%) > BgC-1.2 (71.4%) > BgC-1.1 (68.9%). | ||||||
Bone regeneration | ||||||
TE | ||||||
[ | ] | |||||
Abbreviations: HUVECs—Human umbilical vein endothelial cells; SMCs—Smooth muscle cells; HaCaT—Human keratinocytes cells; HepG2/C3A—Human C3A hepatoma cells; L929—Mouse skin fibroblast cells; U2-OS—Osteoblast cell; HNDF—Human neonatal dermal fibroblasts; MC3T3-E1—Mouse osteoblastic cells; rMSCs—Mouse mesenchymal stem cells; UCBMSCs—Human umbilical cord blood-derived mesenchymal stem cells; MRC-5—Normal lung tissues cells; GM07492—Human fibroblast cells; CCk-8 assay—Cholecystokinin-octopeptide proliferation assay; ATP assay—Adenosine triphosphate assay; LDH assay—Lactate dehydrogenase assay; NR assay—Neutral red assay; MTS assay—CellTiter 96® Aqueous Non-Radioactive Cell Proliferation assay; AB assay—Alamar blue assay; G. xylinus—Gluconacetobacter xylinus; K. xylinus—Komagataeibacter xylinus; A. xylinum—Acetobacter xylinum; BNC- GTMAC—BNC functionalized with glycidyl trime-thylammonium chloride (GTMAC); BNC-GHDE—BNC functionalized with glycidyl hexadecyl ether (GHDE).
4.2. In Vivo Degradability of Nanocellulose-Based Materials
Besides biocompatibility and nontoxicity, biodegradability is another requirement for materials with applications in pharmaceutical and biomedical fields [182]. Generally, biodegradability is the property of a material to decompose into simple molecules under the action of biological elements (e.g., enzymes) [183]. Biodegradation is controlled by the material properties (e.g., molecular weight, crystallinity), and thus the biodegradation time can vary from months to years depending on its amorphous/crystalline or hydrophilic/hydrophobic behaviors [184]. For some biomedical applications (e.g., artificial heart valves or menisci), nonbiodegradable materials may be acceptable, whereas, for other applications (e.g., tissue regeneration), the bioresorbable materials that enable tissue regeneration at rates appropriate to their degradation are preferable, biodegradation being an essential property in this regard [185]. NCs are considered to be generally biodegradable because it derives from its natural sources—cellulose. However, there are some distinct differences between cellulose and industrially-obtained NC in terms of their size, morphology, surface characteristics, or physicochemical properties, so it cannot be entirely assumed that nanocellulose has the same biodegradability as cellulose in its native state [186]. Indeed, the studies performed to evaluate the in vivo degradability of nanocellulose showed that its biodegradation still remains a challenge given that NC itself does not undergo complete degradation in the human body due to the lack of specific enzymes [187]. The biodegradation of nanocellulose is usually performed by cellulolytic microorganisms, which produce enzymes (endoglucanases, cellobiohydrolases, and β-glucosidases) that act synergistically, leading to depolymerization of cellulose. In typical enzymatic hydrolysis, endoglucanases and cellobiohydrolases are responsible for the degradation of cellulose to cellobiosis, followed by its hydrolysis to free glucose molecules [188]. Given the facts mentioned above, some efforts have been made to find ways to improve the cellulose fibers’ biodegradation, making them resorbable by the organism [182]. For instance, as demonstrated in an earlier study [189], the incorporation of cellulase enzymes in bacterial cellulose and especially the conjunction of these enzymes with β-glucosidase led to a loss of integrity of almost 80% of tested materials after 2 days. A more recent study showed that the incorporation of the proteinase K, a kind of serine proteases, into scaffolds based on poly(butylene succinate) (PBS)/poly (lactic acid) (PLA) reinforced with NFCs, accelerated the degradation of the NFC-based scaffolds, acting mainly on the C=O bond of PLA. It showed promising prospects in improving the biodegradability of nanocellulose-based scaffolds by incorporating degradable polymers or related enzymes [190]. The introduction of N-acetyl-glucosamine residues (GlcNAc) into bacterial cellulose (BC) molecule during its synthesis by metabolically engineered Gluconacetobacter xylinus is another attempt that renders the cellulosic biopolymers susceptible to in vivo degradation. It was noticed that the modified-BC with high content of GlcNAc became more susceptible to biodegradation (e.g., to lysozyme and cellulase) compared with BC produced by the control strain. The susceptibility of modified-BC to in vivo degradation was evaluated by its subcutaneous implantation in the female BALB/c mice backs. Visual inspection of implant sites, after 10- and 20-days post-implantation, respectively, indicated little to no degradation of the cellulose produced by the control strain, while the modified-BC was almost entirely degraded after 10 days and completely undetectable after 20 days [191][192]. The oxidation process, using various chemicals, including metaperiodate, hypochlorite, dichromate, nitrogen dioxide, or TEMPO (2,2,6,6-tetramethylpiperidn-1-yl)oxyl) [193][194][195][196][197], makes cellulose materials more vulnerable to hydrolysis and therefore potentially degradable by the human body. The only impediment of this process is that it can significantly alter the structure of cellulose, disturbing the unique, medically desirable properties of cellulose, such as its high tensile strength and conformability. However, this “disadvantage” becomes an “advantage” in soft tissue applications where the material often needs to readily conform to the various contours of the body and to have adequate strength for tissue support and allow easy handling [196]. 2,3-dialdehyde celluloses (DAC), obtained via periodate oxidation, have been considered degradable, although only at a slow rate [198]. In tissue engineering applications, cellulose acetate (acetyl cellulose) and ethyl cellulose have hardly been considered as scaffold materials since they are known to degrade very slowly or are practically non-resorbable in vivo. By oxidation with periodate, which has a regioselective action at the C2 and C3 atoms in the glucopyranose units, a structure with two aldehyde groups is formed that is less stable at physiological conditions. Thus, oxidized acetyl cellulose sponges, implanted subcutaneously into Wistar rats in vivo, showed a partial degradation of about 47% after 60 weeks, while for ethyl cellulose the proportion was only 18%) [199]. If the chemical oxidation by is coupled with γ-irradiation, as observed with bacterial cellulose used in soft tissue repair [196], resorbable and fully conformable materials are obtained. In vivo, these oxidized materials showed degradation as early as 2 weeks and continued to be slowly degraded until 26 weeks. A potential mechanism of oxidized-BNC resorption was considered, consisting of two steps: initial rapid resorption via hydrolysis of oxidized domains resulting in degradation of about 70%–80%, followed by the formation of short oligosaccharides that are further cleared by phagocytosis. In this last stage, the action of macrophages and giant cells breaks down the short glucan chains to the point where they can be consumed by the cells [196]. Oxidized bacterial cellulose (OBC) coupled with two degradable polymers, such as chitosan (CS) and collagen (COL), leads to nanocomposites (OBC/COL/CS) with excellent biodegradation. In vivo degradation performance of nanocomposites was evaluated by subcutaneous implantation using male Sprague–Dawley rats and was compared with oxidized regenerated plant cellulose (ORC), which is among the most widely used absorbable topical hemostatic agents for internal bleeding control. ORC has relatively poor biodegradability in vivo. Its complete absorption requires as long as 8 weeks [200]. After implantation for 7 days, sample residues stained purple-brown can be seen on the majority of sections. However, after 30 days, neither residual fragments of samples remaining in the subcutaneous tissue nor an evident pathological response of the implanted sites were observed in nanocomposites groups with oxidized-BC (OBC, OBC/CS, and OBC/COL/CS). In contrast, in the ORC group, even though the fiber diameter decreased markedly compared with that observed after the first 7 days, residual fragments were still found after 30 days [201]. Unlike oxidized-BC, unmodified BC shows poor degradation when is used, for instance, as porous scaffolds for bone tissue engineering, alone or in combination with gelatin via different crosslinking techniques and coating hydroxyapatite. At six weeks after implantation in the nude mice model, the results of the histological analysis showed clearly visible fibers in all scaffolds, suggesting poor degradation of the bacterial cellulose that unfortunately partly inhibited the new bone formation [129]. Similar to bacterial cellulose, the biodegradability of nanocrystalline cellulose (CNCs) and nanofiber cellulose (CNFs), respectively, also evaluated in various studies, is improved by modification (e.g., TEMPO-oxidation) or in combination with other biodegradable polymers. For instance, cellulose nanocrystals combined with biodegradable collagen and gelatin microspheres containing basic fibroblast growth factor (bFGF) were evaluated as a biodegradable platform for long-term release and consequent angiogenic boosting. The scaffolds were implanted into the subcutaneous tissue of the rat backs, and their biodegradability was assessed at 3, 7, and 10 days. With prolonged times, the gross views revealed that scaffolds size was reduced following implantation. On the 3rd day, all of the implanted scaffolds maintained their shape. On the 7th day, the scaffolds based on collagen/CNCs appeared to be smaller in size than other groups. Following that, on the 10th day, no residue of these scaffolds was observed, which may have been caused by degradation in vivo [119]. On the other hand, injectable hydrogels based on TEMPO-oxidized cellulose nanofiber (TOCNFs), methyl cellulose, carboxymethyl cellulose, and polyethylene glycol, with different concentrations (0.2% and 1% w/v) of TOCNFs were tested regarding their degradation in vivo. The biodegradability was evaluated using male rats by direct dorsal administration of 0.5 mL of injectable hydrogels and observation after two weeks by opening the injection site. Degradation studies revealed that nanocellulose fibers do not undergo complete degradation after 14 days. Moreover, the TOCNFs concentration in the hydrogel was inversely proportional to hydrolytic degradation rate: a higher concentration of TOCNFs in the hydrogel determines a lower degradation capacity, as evidenced by the much smaller size of the hydrogels with 0.2% TOCNFs after 2 weeks [202]. Last but not least, cellulose fibers derived from Styela clava tunics (SCT-CF) are also considered slowly degradable compared with wood pulp cellulose fibers (WP-CF). The biodegradability was evaluated by film subcutaneous implantation into Sprague–Dawley (SD) rats for various lengths of time. The weight loss was greater in cellulose films from Styela clava (almost 24% of their initial weight) than in films prepared from wood pulp cellulose (less than 10%). It was considered that the higher susceptibility of SCT-CF to degradation would be due to its content of about 98% α-cellulose and very low concentrations of ββ-cellulose and, additionally, a lower crystallinity index of SCT-CF (10.71%) unlike WP-CF (33.78%) [203]. Thereby, it can be concluded that, in terms of biodegradation, the cellulose fibers may be considered nonbiodegradable in vivo or, at best, slowly degradable, but their susceptibility to the action of biological agents can be improved, to a greater or lesser degree, by various methods, i.e., by chemical modification, by association with different well-known degradable polymers or by incorporating of related enzymes. Moreover, biodegradability is a complex characteristic that not only depends on cellulose fiber nature and obtaining methodology but also on its crystallinity form, degree of polymerization, morphology, chemical derivatization, swelling, etc.References
- Mokhena, T.C.; John, M.J. Cellulose nanomaterials: New generation materials for solving global issues. Cellulose 2020, 27, 1149–1194.
- Pachuau, L. Application of Nanocellulose for Controlled Drug Delivery. In Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications, 1st ed.; Jawaid, M., Mohammad, F., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Chapter 1; pp. 1–19.
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466.
- Raghav, N.; Sharma, M.R.; Kennedy, J.F. Nanocellulose: A mini-review on types and use in drug delivery systems. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031.
- Kamel, S.; Khattab, T.A. Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors 2020, 10, 67.
- Naderi, A. Nanofibrillated cellulose: Properties reinvestigated. Cellulose 2017, 24, 1933–1945.
- Maiuolo, L.; Algieri, V.; Olivito, F.; Tallarida, M.A.; Costanzo, P.; Jiritano, A.; De Nino, A. Chronicle of Nanocelluloses (NCs) for Catalytic Applications: Key Advances. Catalysts 2021, 11, 96.
- Mirtaghavi, A.; Luo, J.; Muthuraj, R. Recent Advances in Porous 3D Cellulose Aerogels for Tissue Engineering Applications: A Review. J. Compos. Sci. 2020, 4, 152.
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748.
- Ioelovich, M. Characterization of Various Kinds of Nanocellulose. In Handbook of Nanocellulose and Cellulose Nanocom-Posites, 1st ed.; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Chapter 2; pp. 51–100.
- Khalil, H.A.; Adnan, A.; Yahya, E.; Olaiya, N.; Safrida, S.; Hossain, S.; Balakrishnan, V.; Gopakumar, D.; Abdullah, C.; Oyekanmi, A.; et al. A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymers 2020, 12, 1759.
- Hamad, W.Y. Cellulose Nanocrystals and Nanofibrils in Advanced Applications. In Handbook of Nanocellulose and Cellulose Nanocomposites, 1st ed.; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Chapter 24; pp. 799–832.
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial Nanocellulose—A Biobased Polymer for Active and Intelligent Food Packaging Applications: Recent Advances and Developments. Polymers 2020, 12, 2209.
- Xue, Y.; Mou, Z.; Xiao, H. Nanocellulose as a sustainable biomass material: Structure, properties, present status and future prospects in biomedical applications. Nanoscale 2017, 9, 14758–14781.
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270.
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719–41737.
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Koloor, S.S.R.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231.
- Jacob, S.; Nair, A.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357.
- Nair, S.K.; Basu, S.; Sen, B.; Lin, M.-H.; Kumar, A.N.; Yuan, Y.; Cullen, P.J.; Sarkar, D. Colloidal Gels with Tunable Mechanomorphology Regulate Endothelial Morphogenesis. Sci. Rep. 2019, 9, 1–17.
- Ciolacu, D.; Cazacu, G. New Green Hydrogels Based on Lignin. J. Nanosci. Nanotechnol. 2018, 18, 2811–2822.
- Del Valle, L.J.; Díaz, A.; Puiggalí, J. Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives. Gels 2017, 3, 27.
- Ciolacu, D.; Rudaz, C.; Vasilescu, M.; Budtova, T. Physically and chemically cross-linked cellulose cryogels: Structure, properties and application for controlled release. Carbohydr. Polym. 2016, 151, 392–400.
- Ooi, S.Y.; Ahmad, I.; Amin, M.C.I.M. Effect of Cellulose Nanocrystals Content and pH on Swelling Behaviour of Gelatin Based Hydrogel. Sains Malays. 2015, 44, 793–799.
- Shojaeiarani, J.; Bajwa, D.; Shirzadifar, A. A review on cellulose nanocrystals as promising biocompounds for the synthesis of nanocomposite hydrogels. Carbohydr. Polym. 2019, 216, 247–259.
- Pandey, M.; Mohamad, N.; Amin, M.C.I.M. Bacterial Cellulose/Acrylamide pH-Sensitive Smart Hydrogel: Development, Characterization, and Toxicity Studies in ICR Mice Model. Mol. Pharm. 2014, 11, 3596–3608.
- Tummala, G.K.; Felde, N.; Gustafsson, S.; Bubholz, A.; Schröder, S.; Mihranyan, A. Light scattering in poly(vinyl alcohol) hydrogels reinforced with nanocellulose for ophthalmic use. Opt. Mater. Express 2017, 7, 2824.
- Basu, A.; Lindh, J.; Ålander, E.; Strømme, M.; Ferraz, N. On the use of ion-crosslinked nanocellulose hydrogels for wound healing solutions: Physicochemical properties and application-oriented biocompatibility studies. Carbohydr. Polym. 2017, 174, 299–308.
- Li, J.; Yu, F.; Chen, G.; Liu, J.; Li, X.-L.; Cheng, B.; Mo, X.-M.; Chen, C.; Pan, J.-F. Moist-Retaining, Self-Recoverable, Bioadhesive, and Transparent in Situ Forming Hydrogels to Accelerate Wound Healing. ACS Appl. Mater. Interfaces 2019, 12, 2023–2038.
- Jansen, K.; Schuurmans, C.C.; Jansen, J.; Masereeuw, R.; Vermonden, T. Hydrogel-Based Cell Therapies for Kidney Regeneration: Current Trends in Biofabrication and In Vivo Repair. Curr. Pharm. Des. 2017, 23, 3845–3857.
- Wu, T.; Farnood, R.; O’Kelly, K.; Chen, B. Mechanical behavior of transparent nanofibrillar cellulose–chitosan nanocomposite films in dry and wet conditions. J. Mech. Behav. Biomed. Mater. 2014, 32, 279–286.
- Latifi, N.; Asgari, M.; Vali, H.; Mongeau, L. A tissue-mimetic nano-fibrillar hybrid injectable hydrogel for potential soft tissue engineering applications. Sci. Rep. 2018, 8, 1–18.
- Gaston, J.; Thibeault, S.L. Hyaluronic acid hydrogels for vocal fold wound healing. Biomatter 2013, 3, e23799.
- Liu, R.; Zhang, S.; Chen, X. Injectable hydrogels for tendon and ligament tissue engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1333–1348.
- De Lima, C.S.A.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; Camacho-Cruz, L.A.; Bucio, E.; Kadlubowski, S.S. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics 2020, 12, 970.
- Rusu, D.; Ciolacu, D.; Simionescu, B.C. Cellulose-Based Hydrogels in Tissue Engineering Applications. Cellul. Chem. Technol. 2019, 53, 907–923.
- Moscovici, M.; Hlevca, C.; Casarica, A.; Pavaloiu, R.D. Nanocellulose and Nanogels as Modern Drug Delivery Systems. In Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications, 1st ed.; Jawaid, M., Mo-hammad, F., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Chapter 9; pp. 209–269.
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649.
- Miao, L.; Zhang, M.; Tu, Y.; Lin, S.; Hu, J. Stimuli-Responsive Cellulose-Based Hydrogels. In Cellulose-Based Super-Absorbent Hydrogels, Polymers and Polymeric Composites: A Reference Series, 1st ed.; Mondal, M.I.H., Ed.; Springer: Cham, Switzerland, 2019; Chapter 9; pp. 269–308.
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Design and engineering of nanogels for cancer treatment. Drug Discov. Today 2011, 16, 457–463.
- Xu, F.; Zhu, J.; Lin, L.; Zhang, C.; Sun, W.; Fan, Y.; Yin, F.; Van Hest, J.C.M.; Wang, H.; Du, L.; et al. Multifunctional PVCL nanogels with redox-responsiveness enable enhanced MR imaging and ultrasound-promoted tumor chemotherapy. Theranostics 2020, 10, 4349–4358.
- Sivaram, A.J.; Rajitha, P.; Maya, S.; Jayakumar, R.; Sabitha, M. Nanogels for delivery, imaging and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 509–533.
- Ferreira, S.A.R.M.; Gama, F.M.; Vilanova, M. Polymeric nanogels as vaccine delivery systems. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 159–173.
- Yan, M.; Ge, J.; Liu, Z.; Ouyang, P. Encapsulation of Single Enzyme in Nanogel with Enhanced Biocatalytic Activity and Stability. J. Am. Chem. Soc. 2006, 128, 11008–11009.
- Chou, H.-S.; Larsson, M.; Hsiao, M.-H.; Chen, Y.-C.; Röding, M.; Nydén, M.; Liu, D.-M. Injectable insulin-lysozyme-loaded nanogels with enzymatically-controlled degradation and release for basal insulin treatment: In vitro characterization and in vivo observation. J. Control. Release 2016, 224, 33–42.
- Kandil, R.; Merkel, O.M. Recent progress of polymeric nanogels for gene delivery. Curr. Opin. Colloid Interface Sci. 2019, 39, 11–23.
- Akram, M.; Hussain, R. Nanohydrogels: History, development, and applications in drug delivery. In Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications, 1st ed.; Jawaid, M., Mohammad, F., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Chapter 11; pp. 297–330.
- Lewis, L.; Derakhshandeh, M.; Hatzikiriakos, S.G.; Hamad, W.Y.; MacLachlan, M. Hydrothermal Gelation of Aqueous Cellulose Nanocrystal Suspensions. Biomacromolecules 2016, 17, 2747–2754.
- Krontiras, P.; Gatenholm, P.; AHägg, D. Adipogenic differentiation of stem cells in three-dimensional porous bacterial nanocellulose scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 103, 195–203.
- Picheth, G.F.; Pirich, C.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106.
- Sanandiya, N.D.; Vasudevan, J.; Das, R.; Lim, C.T.; Fernandez, J.G. Stimuli-responsive injectable cellulose thixogel for cell encapsulation. Int. J. Biol. Macromol. 2019, 130, 1009–1017.
- De France, K.; Chan, K.J.W.; Cranston, E.D.; Hoare, T. Enhanced Mechanical Properties in Cellulose Nanocrystal–Poly(oligoethylene glycol methacrylate) Injectable Nanocomposite Hydrogels through Control of Physical and Chemical Cross-Linking. Biomacromolecules 2016, 17, 649–660.
- Lu, Y.; Han, J.; Ding, Q.; Yue, Y.; Xia, C.; Ge, S.; Van Le, Q.; Dou, X.; Sonne, C.; Lam, S.S. TEMPO-oxidized cellulose nanofibers/polyacrylamide hybrid hydrogel with intrinsic self-recovery and shape memory properties. Cellulose 2021, 28, 1469–1488.
- Song, K.; Zhu, W.; Li, X.; Yu, Z. A novel mechanical robust, self-healing and shape memory hydrogel based on PVA reinforced by cellulose nanocrystal. Mater. Lett. 2020, 260, 126884–126887.
- Liu, S.; Jin, M.; Chen, Y.; Gao, H.; Shi, X.; Cheng, W.; Ren, L.; Wang, Y. High internal phase emulsions stabilised by supramolecular cellulose nanocrystals and their application as cell-adhesive macroporous hydrogel monoliths. J. Mater. Chem. B 2017, 5, 2671–2678.
- Masruchin, N.; Park, B.-D.; Causin, V. Influence of sonication treatment on supramolecular cellulose microfibril-based hydrogels induced by ionic interaction. J. Ind. Eng. Chem. 2015, 29, 265–272.
- Hua, J.; Liu, C.; Ng, P.F.; Fei, B. Bacterial cellulose reinforced double-network hydrogels for shape memory strand. Carbohydr. Polym. 2021, 259, 117737.
- McKee, J.R.; Hietala, S.; Seitsonen, J.; Laine, J.; Kontturi, E.; Ikkala, O. Thermoresponsive Nanocellulose Hydrogels with Tunable Mechanical Properties. ACS Macro. Lett. 2014, 3, 266–270.
- Talantikite, M.; Beury, N.; Moreau, C.; Cathala, B.; Leray, N. Arabinoxylan/Cellulose Nanocrystal Hydrogels with Tunable Mechanical Properties. Langmuir 2019, 35, 13427–13434.
- Sabet, S.S. Shear Rheology of Cellulose Nanocrystal (CNC) Aqueous Suspensions. Ph.D. Thesis, University of British Columbia Library, Vancouver, BC, Canada, 2013.
- Shafiei-Sabet, S.; Hamad, W.Y.; Hatzikiriakos, S.G. Ionic strength effects on the microstructure and shear rheology of cellulose nanocrystal suspensions. Cellulose 2014, 21, 3347–3359.
- Heath, L.; Thielemans, W. Cellulose nanowhisker aerogels. Green Chem. 2010, 12, 1448–1453.
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625.
- Heise, K.; Kontturi, E.; Allahverdiyeva, Y.; Tammelin, T.; Linder, M.B.; Ikkala, O. Nanocellulose: Recent Fundamental Advances and Emerging Biological and Biomimicking Applications. Adv. Mater. 2021, 33, e2004349.
- Kontturi, E.; Laaksonen, P.; Linder, M.; Nonappa, N.; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced Materials through Assembly of Nanocelluloses. Adv. Mater. 2018, 30, e1703779.
- Saba, N.; Jawaid, M. Recent advances in nanocellulose-based polymer nanocomposites. In Woodhead Publishing Series in Composites Science and Engineering, Cellulose-Reinforced Nanofibre Composites, 1st ed.; Jawaid, M., Boufi, S., Abdul Khalil, H.P.S., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Chapter 4; pp. 89–112.
- Dufresne, A. Cellulose nanomaterial reinforced polymer nanocomposites. Curr. Opin. Colloid Interface Sci. 2017, 29, 1–8.
- Prusty, K.; Swain, S.K. Cellulose-based nanohydrogels for tissue engineering applications. In Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications, 1st ed.; Jawaid, M., Mohammad, F., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Chapter 4; pp. 67–90.
- Gonzalez, J.S.; Ludueña, L.N.; Ponce, A.; Alvarez, V.A. Poly(vinyl alcohol)/cellulose nanowhiskers nanocomposite hydrogels for potential wound dressings. Mater. Sci. Eng. C 2014, 34, 54–61.
- Ferreira, F.V.; Pinheiro, I.F.; De Souza, S.F.; Mei, L.H.I.; Lona, L.M.F. Polymer Composites Reinforced with Natural Fibers and Nanocellulose in the Automotive Industry: A Short Review. J. Compos. Sci. 2019, 3, 51.
- Nascimento, D.M.D.; Nunes, Y.L.; Figueirêdo, M.C.B.; Azeredo, H.; Aouada, F.; Feitosa, J.P.A.; Rosa, M.F.; Dufresne, A. Nanocellulose nanocomposite hydrogels: Technological and environmental issues. Green Chem. 2018, 20, 2428–2448.
- Halib, N.; Perrone, F.; Cemazar, M.; Dapas, B.; Farra, R.; Abrami, M.; Chiarappa, G.; Forte, G.; Zanconati, F.; Pozzato, G.; et al. Potential Applications of Nanocellulose-Containing Materials in the Biomedical Field. Materials 2017, 10, 977.
- Chen, Y.; Xu, W.; Liu, W.; Zeng, G. Responsiveness, swelling, and mechanical properties of PNIPA nanocomposite hydrogels reinforced by nanocellulose. J. Mater. Res. 2015, 30, 1797–1807.
- Abitbol, T.; Johnstone, T.; Quinn, T.M.; Gray, D.G. Reinforcement with cellulose nanocrystals of poly(vinyl alcohol) hydrogels prepared by cyclic freezing and thawing. Soft Matter 2011, 7, 2373–2379.
- Han, J.; Lei, T.; Wu, Q. High-water-content mouldable polyvinyl alcohol-borax hydrogels reinforced by well-dispersed cellulose nanoparticles: Dynamic rheological properties and hydrogel formation mechanism. Carbohydr. Polym. 2014, 102, 306–316.
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose Nanocrystals vs. Cellulose Nanofibrils: A Comparative Study on Their Microstructures and Effects as Polymer Reinforcing Agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009.
- Yang, J.; Han, C.-R.; Duan, J.-F.; Xu, F.; Sun, R.-C. Mechanical and Viscoelastic Properties of Cellulose Nanocrystals Reinforced Poly(ethylene glycol) Nanocomposite Hydrogels. ACS Appl. Mater. Interfaces 2013, 5, 3199–3207.
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969.
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2014, 21, 1–30.
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571.
- Kaushik, M.; Moores, A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637.
- Hu, Z.; Cranston, E.D.; Ng, R.; Pelton, R. Tuning Cellulose Nanocrystal Gelation with Polysaccharides and Surfactants. Langmuir 2014, 30, 2684–2692.
- Chinga-Carrasco, G. Potential and Limitations of Nanocelluloses as Components in Biocomposite Inks for Three-Dimensional Bioprinting and for Biomedical Devices. Biomacromolecules 2018, 19, 701–711.
- Li, S.-M.; Jia, N.; Ma, M.-G.; Zhang, Z.; Liu, Q.-H.; Sun, R.-C. Cellulose—Silver nanocomposites: Microwave-assisted synthesis, characterization, their thermal stability, and antimicrobial property. Carbohydr. Polym. 2011, 86, 441–447.
- Barua, S.; Das, G.; Aidew, L.; Buragohain, A.K.; Karak, N. Copper-copper oxide coated nanofibrillar cellulose: A promising biomaterial. RSC Adv. 2013, 3, 14997–15004.
- Kupnik, K.; Primožič, M.; Kokol, V.; Leitgeb, M. Nanocellulose in Drug Delivery and Antimicrobially Active Materials. Polymers 2020, 12, 2825.
- Mertaniemi, H.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Gandía, C.; Mäkitie, A.; Partanen, J.; Ikkala, O.; Yliperttula, M. Human stem cell decorated nanocellulose threads for biomedical applications. Biomaterials 2016, 82, 208–220.
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88.
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396.
- Lin, N.; Gèze, A.; Wouessidjewe, D.; Huang, J.; Dufresne, A. Biocompatible Double-Membrane Hydrogels from Cationic Cellulose Nanocrystals and Anionic Alginate as Complexing Drugs Codelivery. ACS Appl. Mater. Interfaces 2016, 8, 6880–6889.
- Akhlaghi, S.P. Surface Modification and Characterization of Cellulose Nanocrystals for Biomedical Applications. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2014.
- Taheri, A.; Mohammadi, M. The Use of Cellulose Nanocrystals for Potential Application in Topical Delivery of Hydroquinone. Chem. Biol. Drug Des. 2014, 86, 102–106.
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H. Enhancement of Curcumin Bioavailability Using Nanocellulose Reinforced Chitosan Hydrogel. Polymers 2017, 9, 64.
- You, J.; Cao, J.; Zhao, Y.; Zhang, L.; Zhou, J.; Chen, Y. Improved Mechanical Properties and Sustained Release Behavior of Cationic Cellulose Nanocrystals Reinforeced Cationic Cellulose Injectable Hydrogels. Biomacromolecules 2016, 17, 2839–2848.
- Ooi, S.Y.; Ahmad, I.; Amin, M.C.I.M. Cellulose nanocrystals extracted from rice husks as a reinforcing material in gelatin hydrogels for use in controlled drug delivery systems. Ind. Crop. Prod. 2016, 93, 227–234.
- Supramaniam, J.; Adnan, R.; Kaus, N.H.M.; Bushra, R. Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system. Int. J. Biol. Macromol. 2018, 118, 640–648.
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36.
- Orasugh, J.T.; Saha, N.R.; Rana, D.; Sarkar, G.; Mollick, M.R.; Chattoapadhyay, A.; Mitra, B.C.; Mondal, D.; Ghosh, S.K.; Chattopadhyay, D. Jute cellulose nano-fibrils/hydroxypropylmethylcellulose nanocomposite: A novel material with potential for application in packaging and transdermal drug delivery system. Ind. Crop. Prod. 2018, 112, 633–643.
- Guo, T.; Pei, Y.; Tang, K.; He, X.; Huang, J.; Wang, F. Mechanical and drug release properties of alginate beads reinforced with cellulose. J. Appl. Polym. Sci. 2017, 134, 44495–44503.
- Shi, X.; Zheng, Y.; Wang, G.; Lin, Q.; Fan, J. pH- and electro-response characteristics of bacterial cellulose nanofiber/sodium alginate hybrid hydrogels for dual controlled drug delivery. RSC Adv. 2014, 4, 47056–47065.
- Shao, W.; Liu, H.; Wang, S.; Wu, J.; Huang, M.; Min, H.; Liu, X. Controlled release and antibacterial activity of tetracycline hydrochloride-loaded bacterial cellulose composite membranes. Carbohydr. Polym. 2016, 145, 114–120.
- Moritz, S.; Wiegand, C.; Wesarg, F.; Hessler, N.; Müller, F.A.; Kralisch, D.; Hipler, U.-C.; Fischer, D. Active wound dressings based on bacterial nanocellulose as drug delivery system for octenidine. Int. J. Pharm. 2014, 471, 45–55.
- Wiegand, C.; Moritz, S.; Hessler, N.; Kralisch, D.; Wesarg, F.; Müller, F.A.; Fischer, D.; Hipler, U.-C. Antimicrobial functionalization of bacterial nanocellulose by loading with polihexanide and povidone-iodine. J. Mater. Sci. Mater. Med. 2015, 26, 1–14.
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164.
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-García, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Mäkitie, A.; Luukko, K.; et al. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release 2016, 244, 292–301.
- Cattelaens, J.; Turco, L.; Berclaz, L.M.; Huelsse, B.; Hitzl, W.; Vollkommer, T.; Bodenschatz, K.J. The Impact of a Nanocellulose-Based Wound Dressing in the Management of Thermal Injuries in Children: Results of a Retrospective Evaluation. Life 2020, 10, 212.
- Qiu, Y.; Qiu, L.; Cui, J.; Wei, Q. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater. Sci. Eng. C 2016, 59, 303–309.
- Mohamad, N.; Amin, M.C.I.M.; Pandey, M.; Ahmad, N.; Rajab, N.F. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohydr. Polym. 2014, 114, 312–320.
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-Responsive Poly(N-Isopropylacrylamide)-Cellulose Nanocrystals Hybrid Hydrogels for Wound Dressing. Polymers 2017, 9, 119.
- Sanyang, M.L.; Saba, N.; Jawaid, M.; Mohammad, F.; Salit, M.S. Bacterial nanocellulose applications for tissue engineering. In Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications, 1st ed.; Jawaid, M., Mohammad, F., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Chapter 3; pp. 47–66.
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963.
- Xu, C.; Molino, B.Z.; Wang, X.; Cheng, F.; Xu, W.; Molino, P.; Bacher, M.; Su, D.; Rosenau, T.; Willför, S.; et al. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J. Mater. Chem. B 2018, 6, 7066–7075.
- Murizan, N.I.S.; Mustafa, N.S.; Ngadiman, N.H.A.; Yusof, N.M.; Idris, A. Review on Nanocrystalline Cellulose in Bone Tissue Engineering Applications. Polymers 2020, 12, 2818.
- Huang, A.; Peng, X.; Geng, L.; Zhang, L.; Huang, K.; Chen, B.; Gu, Z.; Kuang, T. Electrospun poly (butylene succinate)/cellulose nanocrystals bio-nanocomposite scaffolds for tissue engineering: Preparation, characterization and in vitro evaluation. Polym. Test. 2018, 71, 101–109.
- Subhedar, A.; Bhadauria, S.; Ahankari, S.; Kargarzadeh, H. Nanocellulose in biomedical and biosensing applications: A review. Int. J. Biol. Macromol. 2021, 166, 587–600.
- Ghafari, R.; Jonoobi, M.; Amirabad, L.M.; Oksman, K.; Taheri, A.R. Fabrication and characterization of novel bilayer scaffold from nanocellulose based aerogel for skin tissue engineering applications. Int. J. Biol. Macromol. 2019, 136, 796–803.
- Gorgieva, S.; Girandon, L.; Kokol, V. Mineralization potential of cellulose-nanofibrils reinforced gelatine scaffolds for promoted calcium deposition by mesenchymal stem cells. Mater. Sci. Eng. C 2017, 73, 478–489.
- Lam, N.T.; Chollakup, R.; Smitthipong, W.; Nimchua, T.; Sukyai, P. Utilizing cellulose from sugarcane bagasse mixed with poly(vinyl alcohol) for tissue engineering scaffold fabrication. Ind. Crop. Prod. 2017, 100, 183–197.
- Yin, F.; Lin, L.; Zhan, S. Preparation and properties of cellulose nanocrystals, gelatin, hyaluronic acid composite hydrogel as wound dressing. J. Biomater. Sci. Polym. Ed. 2019, 30, 190–201.
- Li, W.; Lan, Y.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y. In vitro and in vivo evaluation of a novel collagen/cellulose nanocrystals scaffold for achieving the sustained release of basic fibroblast growth factor. J. Biomater. Appl. 2015, 29, 882–893.
- Zhang, C.; Salick, M.R.; Cordie, T.M.; Ellingham, T.; Dan, Y.; Turng, L.-S. Incorporation of poly(ethylene glycol) grafted cellulose nanocrystals in poly(lactic acid) electrospun nanocomposite fibers as potential scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 49, 463–471.
- Tummala, G.K. Hydrogels of Poly(Vinyl Alcohol) and Nanocellulose for Ophthalmic Applications. Synthesis, Characterization, Biocompatibility and Drug Delivery Studies. Ph.D. Thesis, UPPSALA University, Uppsala, Sweden, 2018.
- Yang, J.; Han, C. Mechanically Viscoelastic Properties of Cellulose Nanocrystals Skeleton Reinforced Hierarchical Composite Hydrogels. ACS Appl. Mater. Interfaces 2016, 8, 25621–25630.
- Prince, E.; Alizadehgiashi, M.; Campbell, M.; Khuu, N.; Albulescu, A.; De France, K.; Ratkov, D.; Li, Y.; Hoare, T.; Kumacheva, E. Patterning of Structurally Anisotropic Composite Hydrogel Sheets. Biomacromolecules 2018, 19, 1276–1284.
- Tummala, G.K.; Rojas, R.; Mihranyan, A. Poly(vinyl alcohol) Hydrogels Reinforced with Nanocellulose for Ophthalmic Applications: General Characteristics and Optical Properties. J. Phys. Chem. B 2016, 120, 13094–13101.
- Martínez Ávila, H.; Schwarz, S.; Feldmann, E.M.; Mantas, A.; von Bomhard, A.; Gatenholm, P.; Rotter, N. Biocompatibility evaluation of densified bacterial nanocellulose hydrogel as an implant material for auricular cartilage regeneration. Appl. Microbiol. Biotechnol. 2014, 98, 7423–7435.
- Yan, H.; Huang, D.; Chen, X.; Liu, H.; Feng, Y.; Zhao, Z.; Dai, Z.; Zhang, X.; Lin, Q. A novel and homogeneous scaffold material: Preparation and evaluation of alginate/bacterial cellulose nanocrystals/collagen composite hydrogel for tissue engineering. Polym. Bull. 2017, 75, 985–1000.
- Osorio, M.; Fernández-Morales, P.; Gañán, P.; Zuluaga, R.; Kerguelen, H.; Ortiz, I.; Castro, C. Development of novel three-dimensional scaffolds based on bacterial nanocellulose for tissue engineering and regenerative medicine: Effect of processing methods, pore size, and surface area. J. Biomed. Mater. Res. Part A 2019, 107, 348–359.
- Zheng, Y.; Wen, X.; Wu, J.; Wang, L.-N.; Yuan, Z.; Peng, J.; Meng, H. Immobilization of collagen peptide on dialdehyde bacterial cellulose nanofibers via covalent bonds for tissue engineering and regeneration. Int. J. Nanomed. 2015, 10, 4623–4637.
- Huang, Y.; Wang, J.; Yang, F.; Shao, Y.; Zhang, X.; Dai, K. Modification and evaluation of micro-nano structured porous bacterial cellulose scaffold for bone tissue engineering. Mater. Sci. Eng. C 2017, 75, 1034–1041.
- Saska, S.; Teixeira, L.N.; Raucci, L.M.S.D.C.; Scarel-Caminaga, R.M.; Franchi, L.P.; dos Santos, R.A.; Santagneli, S.H.; Capela, M.V.; de Oliveira, P.T.; Takahashi, C.S.; et al. Nanocellulose-collagen-apatite composite associated with osteogenic growth peptide for bone regeneration. Int. J. Biol. Macromol. 2017, 103, 467–476.
- Park, S.; Park, J.; Jo, I.; Cho, S.-P.; Sung, D.; Ryu, S.; Park, M.; Min, K.-A.; Kim, J.; Hong, S.; et al. In situ hybridization of carbon nanotubes with bacterial cellulose for three-dimensional hybrid bioscaffolds. Biomaterials 2015, 58, 93–102.
- Nimeskern, L.; Ávila, H.M.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21.
- Martínez Ávila, H.; Feldmann, E.M.; Pleumeekers, M.M.; Nimeskern, L.; Kuo, W.; de Jong, W.C.; Schwarz, S.; Müller, R.; Hendriks, J.; Rotter, N.; et al. Novel bilayer bacterial nanocellulose scaffold supports neocartilage formation in vitro and in vivo. Biomaterials 2015, 44, 122–133.
- Li, G.; Nandgaonkar, A.G.; Habibi, Y.; Krause, W.E.; Wei, Q.; Lucia, L.A. An environmentally benign approach to achieving vectorial alignment and high microporosity in bacterial cellulose/chitosan scaffolds. RSC Adv. 2017, 7, 13678–13688.
- Catalán, J.; Ilves, M.; Järventaus, H.; Hannukainen, K.-S.; Kontturi, E.; Vanhala, E.; Alenius, H.; Savolainen, K.M.; Norppa, H. Genotoxic and immunotoxic effects of cellulose nanocrystals in vitro. Environ. Mol. Mutagen. 2015, 56, 171–182.
- Hosseinidoust, Z.; Sim, G.; Alam, N.; Tufenkji, N.; Van De Ven, T.G.M. Cellulose nanocrystals with tunable surface charge for nanomedicine. Nanoscale 2015, 7, 16647–16657.
- Endes, C.; Mueller, S.; Kinnear, C.; Vanhecke, D.; Foster, E.J.; Petri-Fink, A.; Weder, C.; Clift, M.J.D.; Rothen-Rutishauser, B. Fate of Cellulose Nanocrystal Aerosols Deposited on the Lung Cell Surface In Vitro. Biomacromolecules 2015, 16, 1267–1275.
- Menas, A.L.; Yanamala, N.; Farcas, M.; Russo, M.; Friend, S.; Fournier, P.M.; Star, A.; Iavicoli, I.; Shurin, G.V.; Vogel, U.; et al. Fibrillar vs. crystalline nanocellulose pulmonary epithelial cell responses: Cytotoxicity or inflammation? Chemosphere 2017, 171, 671–680.
- Erdem, J.S.; Alswady-Hoff, M.; Ervik, T.K.; Skare, Ø.; Ellingsen, D.G.; Zienolddiny, S. Cellulose nanocrystals modulate alveolar macrophage phenotype and phagocytic function. Biomaterials 2019, 203, 31–42.
- Xiao, Y.; Liu, Y.; Wang, X.; Li, M.; Lei, H.; Xu, H. Cellulose nanocrystals prepared from wheat bran: Characterization and cytotoxicity assessment. Int. J. Biol. Macromol. 2019, 140, 225–233.
- Tuerxun, D.; Pulingam, T.; Nordin, N.I.; Chen, Y.W.; Bin Kamaldin, J.; Julkapli, N.B.M.; Lee, H.V.; Leo, B.F.; Bin Johan, M.R. Synthesis, characterization and cytotoxicity studies of nanocrystalline cellulose from the production waste of rubber-wood and kenaf-bast fibers. Eur. Polym. J. 2019, 116, 352–360.
- Meschini, S.; Pellegrini, E.; Maestri, C.A.; Condello, M.; Bettotti, P.; Condello, G.; Scarpa, M. In vitro toxicity assessment of hydrogel patches obtained by cation-induced cross-linking of rod-like cellulose nanocrystals. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 687–697.
- Li, W.; Lan, Y.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y. In vitro and in vivo evaluation of a novel collagen/cellulose nanocrystals scaffold for achieving the sustained release of basic fibroblast growth factor. J. Biomater. Appl. 2015, 29, 882–893.
- Sunasee, R.; Araoye, E.; Pyram, D.; Hemraz, U.D.; Boluk, Y.; Ckless, K. Cellulose nanocrystal cationic derivative induces NLRP3 inflammasome-dependent IL-1β secretion associated with mitochondrial ROS production. Biochem. Biophys. Rep. 2015, 4, 1–9.
- Zhang, C.; Salick, M.R.; Cordie, T.M.; Ellingham, T.; Dan, Y.; Turng, L.-S. Incorporation of poly(ethylene glycol) grafted cellulose nanocrystals in poly(lactic acid) electrospun nanocomposite fibers as potential scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 49, 463–471.
- Tummala, G.K.; Rojas, R.; Mihranyan, A. Poly(vinyl alcohol) Hydrogels Reinforced with Nanocellulose for Ophthalmic Applications: General Characteristics and Optical Properties. J. Phys. Chem. B 2016, 120, 13094–13101.
- Lam, N.T.; Chollakup, R.; Smitthipong, W.; Nimchua, T.; Sukyai, P. Utilizing cellulose from sugarcane bagasse mixed with poly(vinyl alcohol) for tissue engineering scaffold fabrication. Ind. Crop. Prod. 2017, 100, 183–197.
- Yin, F.; Lin, L.; Zhan, S. Preparation and properties of cellulose nanocrystals, gelatin, hyaluronic acid composite hydrogel as wound dressing. J. Biomater. Sci. Polym. Ed. 2019, 30, 190–201.
- Akhavan-Kharazian, N.; Izadi-Vasafi, H. Preparation and characterization of chitosan/gelatin/nanocrystalline cellulose/calcium peroxide films for potential wound dressing applications. Int. J. Biol. Macromol. 2019, 133, 881–891.
- Tummala, G.K.; Lopes, V.R.; Mihranyan, A.; Ferraz, N. Biocompatibility of Nanocellulose-Reinforced PVA Hydrogel with Human Corneal Epithelial Cells for Ophthalmic Applications. J. Funct. Biomater. 2019, 10, 35.
- Colic, M.; Mihajlović, D.; Mathew, A.P.; Naseri, N.; Kokol, V. Cytocompatibility and immunomodulatory properties of wood based nanofibrillated cellulose. Cellulose 2015, 22, 763–778.
- Nordli, H.R.; Chinga-Carrasco, G.; Rokstad, A.M.; Pukstad, B. Producing ultrapure wood cellulose nanofibrils and evaluating the cytotoxicity using human skin cells. Carbohydr. Polym. 2016, 150, 65–73.
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: Surface chemistry aspect. Part. Fibre Toxicol. 2017, 14, 1–13.
- Ventura, C.; Lourenço, A.F.; Sousa-Uva, A.; Ferreira, P.J.; Silva, M.J. Evaluating the genotoxicity of cellulose nanofibrils in a co-culture of human lung epithelial cells and monocyte-derived macrophages. Toxicol. Lett. 2018, 291, 173–183.
- Souza, S.F.; Mariano, M.; Reis, D.; Lombello, C.B.; Ferreira, M.; Sain, M. Cell interactions and cytotoxic studies of cellulose nanofibers from Curauá natural fibers. Carbohydr. Polym. 2018, 201, 87–95.
- DeLoid, G.M.; Cao, X.; Molina, R.M.; Silva, D.I.; Bhattacharya, K.; Ng, K.W.; Loo, S.C.J.; Brain, J.D.; Demokritou, P. Toxicological effects of ingested nanocellulose in in vitro intestinal epithelium and in vivo rat models. Environ. Sci. Nano 2019, 6, 2105–2115.
- Tibolla, H.; Pelissari, F.; Martins, J.; Lanzoni, E.M.; Vicente, A.; Menegalli, F.; Cunha, R. Banana starch nanocomposite with cellulose nanofibers isolated from banana peel by enzymatic treatment: In vitro cytotoxicity assessment. Carbohydr. Polym. 2019, 207, 169–179.
- Lopes, V.R.; Strømme, M.; Ferraz, N. In Vitro Biological Impact of Nanocellulose Fibers on Human Gut Bacteria and Gastrointestinal Cells. Nanomaterials 2020, 10, 1159.
- Aimonen, K.; Suhonen, S.; Hartikainen, M.; Lopes, V.; Norppa, H.; Ferraz, N.; Catalán, J. Role of Surface Chemistry in the In Vitro Lung Response to Nanofibrillated Cellulose. Nanomaterials 2021, 11, 389.
- Blasi-Romero, A.; Palo-Nieto, C.; Sandström, C.; Lindh, J.; Strømme, M.; Ferraz, N. In Vitro Investigation of Thiol-Functionalized Cellulose Nanofibrils as a Chronic Wound Environment Modulator. Polymers 2021, 13, 249.
- Yanamala, N.; Kisin, E.R.; Menas, A.L.; Farcas, M.; Khaliullin, T.O.; Vogel, U.; Shurin, G.V.; Schwegler-Berry, D.; Fournier, P.M.; Star, A.; et al. In Vitro Toxicity Evaluation of Lignin-(Un)coated Cellulose Based Nanomaterials on Human A549 and THP-1 Cells. Biomacromolecules 2016, 17, 3464–3473.
- Gorgieva, S.; Girandon, L.; Kokol, V. Mineralization potential of cellulose-nanofibrils reinforced gelatine scaffolds for promoted calcium deposition by mesenchymal stem cells. Mater. Sci. Eng. C 2017, 73, 478–489.
- Basu, A.; Lindh, J.; Ålander, E.; Strømme, M.; Ferraz, N. On the use of ion-crosslinked nanocellulose hydrogels for wound healing solutions: Physicochemical properties and application-oriented biocompatibility studies. Carbohydr. Polym. 2017, 174, 299–308.
- Xu, W.; Pranovich, A.; Uppstu, P.; Wang, X.; Kronlund, D.; Hemming, J.; Öblom, H.; Moritz, N.; Preis, M.; Sandler, N.; et al. Novel biorenewable composite of wood polysaccharide and polylactic acid for three dimensional printing. Carbohydr. Polym. 2018, 187, 51–58.
- Gao, H.; Zhong, Z.; Xia, H.; Hu, Q.; Ye, Q.; Wang, Y.; Chen, L.; Du, Y.; Shi, X.; Zhang, L. Construction of cellulose nanofibers/quaternized chitin/organic rectorite composites and their application as wound dressing materials. Biomater. Sci. 2019, 7, 2571–2581.
- Zang, S.; Zhang, R.; Chen, H.; Lu, Y.; Zhou, J.; Chang, X.; Qiu, G.; Wu, Z.; Yang, G. Investigation on artificial blood vessels prepared from bacterial cellulose. Mater. Sci. Eng. C 2015, 46, 111–117.
- Moritz, S.; Wiegand, C.; Wesarg, F.; Hessler, N.; Müller, F.A.; Kralisch, D.; Hipler, U.-C.; Fischer, D. Active wound dressings based on bacterial nanocellulose as drug delivery system for octenidine. Int. J. Pharm. 2014, 471, 45–55.
- Pinto, F.C.M.; De-Oliveira, A.C.A.; De-Carvalho, R.R.; Gomes-Carneiro, M.R.; Coelho, D.R.; Lima, S.V.C.; Paumgartten, F.J.R.; Aguiar, J.L.A. Acute toxicity, cytotoxicity, genotoxicity and antigenotoxic effects of a cellulosic exopolysaccharide obtained from sugarcane molasses. Carbohydr. Polym. 2016, 137, 556–560.
- Qiu, Y.; Qiu, L.; Cui, J.; Wei, Q. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater. Sci. Eng. C 2016, 59, 303–309.
- Junka, A.; Bartoszewicz, M.; Dziadas, M.; Szymczyk, P.; Dydak, K.; Żywicka, A.; Owczarek, A.; Bil-Lula, I.; Czajkowska, J.; Fijałkowski, K. Application of bacterial cellulose experimental dressings saturated with gentamycin for management of bone biofilm in vitro and ex vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 30–37.
- Zikmundova, M.; Vereshaka, M.; Kolarova, K.; Pajorova, J.; Svorcik, V.; Bacakova, L. Effects of Bacterial Nanocellulose Loaded with Curcumin and Its Degradation Products on Human Dermal Fibroblasts. Materials 2020, 13, 4759.
- Orlando, I.; Basnett, P.; Nigmatullin, R.; Wang, W.; Knowles, J.C.; Roy, I. Chemical Modification of Bacterial Cellulose for the Development of an Antibacterial Wound Dressing. Front. Bioeng. Biotechnol. 2020, 8, 1–19.
- Martínez Ávila, H.; Feldmann, E.M.; Pleumeekers, M.M.; Nimeskern, L.; Kuo, W.; de Jong, W.C.; Schwarz, S.; Müller, R.; Hendriks, J.; Rotter, N.; et al. Novel bilayer bacterial nanocellulose scaffold supports neocartilage formation in vitro and in vivo. Biomaterials 2015, 44, 122–133.
- Saska, S.; Teixeira, L.N.; Raucci, L.M.S.D.C.; Scarel-Caminaga, R.M.; Franchi, L.P.; dos Santos, R.A.; Santagneli, S.H.; Capela, M.V.; de Oliveira, P.T.; Takahashi, C.S.; et al. Nanocellulose-collagen-apatite composite associated with osteogenic growth peptide for bone regeneration. Int. J. Biol. Macromol. 2017, 103, 467–476.
- Yan, H.; Huang, D.; Chen, X.; Liu, H.; Feng, Y.; Zhao, Z.; Dai, Z.; Zhang, X.; Lin, Q. A novel and homogeneous scaffold material: Preparation and evaluation of alginate/bacterial cellulose nanocrystals/collagen composite hydrogel for tissue engineering. Polym. Bull. 2017, 75, 985–1000.
- Hobzova, R.; Hrib, J.; Sirc, J.; Karpushkin, E.; Michálek, J.; Janouskova, O.; Gatenholm, P. Embedding of Bacterial Cellulose Nanofibers within PHEMA Hydrogel Matrices: Tunable Stiffness Composites with Potential for Biomedical Applications. J. Nanomater. 2018, 2018, 5217095.
- Noh, Y.K.; Costa, A.D.S.D.; Park, Y.S.; Du, P.; Kim, I.-H.; Park, K. Fabrication of bacterial cellulose-collagen composite scaffolds and their osteogenic effect on human mesenchymal stem cells. Carbohydr. Polym. 2019, 219, 210–218.
- Ye, S.; Jiang, L.; Su, C.; Zhu, Z.; Wen, Y.; Shao, W. Development of gelatin/bacterial cellulose composite sponges as potential natural wound dressings. Int. J. Biol. Macromol. 2019, 133, 148–155.
- Gorgieva, S.; Hribernik, S. Microstructured and Degradable Bacterial Cellulose–Gelatin Composite Membranes: Mineralization Aspects and Biomedical Relevance. Nanomaterials 2019, 9, 303.
- Cacicedo, M.L.; Pacheco, G.; Islan, G.A.; Alvarez, V.A.; Barud, H.S.; Castro, G.R. Chitosan-bacterial cellulose patch of ciprofloxacin for wound dressing: Preparation and characterization studies. Int. J. Biol. Macromol. 2020, 147, 1136–1145.
- Khan, M.U.A.; Haider, S.; Haider, A.; Razak, S.I.A.; Kadir, M.R.A.; AShah, S.; Javed, A.; Shakir, I.; Al-Zahrani, A.A. Development of porous, antibacterial and biocompatible GO/n-HAp/bacterial cellulose/β-glucan biocomposite scaffold for bone tissue engineering. Arab. J. Chem. 2021, 14, 102924.
- Queirós, E.; Pinheiro, S.; Pereira, J.; Prada, J.; Pires, I.; Dourado, F.; Parpot, P.; Gama, M. Hemostatic Dressings Made of Oxidized Bacterial Nanocellulose Membranes. Polysaccharides 2021, 2, 6.
- Goswami, P.; O’Haire, T. Developments in the use of green (biodegradable), recycled and biopolymer materials in technical nonwovens. In Advances in Technical Nonwovens; Elsevier BV: Amsterdam, The Netherlands, 2016; Volume 181, pp. 97–114.
- Ghalia, M.A.; Dahman, Y. Advanced nanobiomaterials in tissue engineering: Synthesis, properties, and applications. In Nanobiomaterials in Soft Tissue Engineering, 1st ed.; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; Chapter 6; pp. 141–172.
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325.
- Singh, G.; Chandoha-Lee, C.; Zhang, W.; Renneckar, S.; Vikesland, P.J.; Pruden, A. Biodegradation of nanocrystalline cellulose by two environmentally-relevant consortia. Water Res. 2016, 104, 137–146.
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460.
- Athukoralalage, S.S.; Balu, R.; Dutta, N.K.; Choudhury, N.R. 3D Bioprinted Nanocellulose-Based Hydrogels for Tissue Engineering Applications: A Brief Review. Polymers 2019, 11, 898.
- Hu, Y.; Catchmark, J.M. In vitro biodegradability and mechanical properties of bioabsorbable bacterial cellulose incorporating cellulases. Acta Biomater. 2011, 7, 2835–2845.
- Luo, H.; Cha, R.; Li, J.; Hao, W.; Zhang, Y.; Zhou, F. Advances in tissue engineering of nanocellulose-based scaffolds: A review. Carbohydr. Polym. 2019, 224, 115144.
- Jacek, P.; Dourado, F.; Gama, M.; Bielecki, S. Molecular aspects of bacterial nanocellulose biosynthesis. Microb. Biotechnol. 2019, 12, 633–649.
- Yadav, V.; Paniliatis, B.J.; Shi, H.; Lee, K.; Cebe, P.; Kaplan, D.L. Novel In Vivo-Degradable Cellulose-Chitin Copolymer from Metabolically Engineered Gluconacetobacter xylinus. Appl. Environ. Microbiol. 2010, 76, 6257–6265.
- Levanič, J.; Šenk, V.P.; Nadrah, P.; Poljanšek, I.; Oven, P.; Haapala, A. Analyzing TEMPO-Oxidized Cellulose Fiber Morphology: New Insights into Optimization of the Oxidation Process and Nanocellulose Dispersion Quality. ACS Sustain. Chem. Eng. 2020, 8, 17752–17762.
- Isogai, A.; Hänninen, T.; Fujisawa, S.; Saito, T. Review: Catalytic oxidation of cellulose with nitroxyl radicals under aqueous conditions. Prog. Polym. Sci. 2018, 86, 122–148.
- Yang, H.; Chen, D.; Van De Ven, T.G.M. Preparation and characterization of sterically stabilized nanocrystalline cellulose obtained by periodate oxidation of cellulose fibers. Cellulose 2015, 22, 1743–1752.
- Czaja, W.; Kyryliouk, D.D.; De Paula, C.A.; Buechter, D.D. Oxidation of γ-irradiated microbial cellulose results in bioresorbable, highly conformable biomaterial. J. Appl. Polym. Sci. 2014, 131, 39995–40006.
- Bulut, Y.; Aksit, A. A comparative study on chemical treatment of jute fiber: Potassium dichromate, potassium permanganate and sodium perborate trihydrate. Cellulose 2013, 20, 3155–3164.
- Li, J.; Wan, Y.; Li, L.; Liang, H.; Wang, J. Preparation and characterization of 2,3-dialdehyde bacterial cellulose for potential biodegradable tissue engineering scaffolds. Mater. Sci. Eng. C 2009, 29, 1635–1642.
- Elçin, A.E. In VitroandIn VivoDegradation of Oxidized Acetyl- and Ethyl-Cellulose Sponges. Artif. Cells Blood Substit. Biotechnol. 2006, 34, 407–418.
- Potter, M.J.; Chauhan, A.; Rowe, D. Surgicel: An effective tool to avoid free flap pedicle kinking in the head and neck. ANZ J. Surg. 2013, 83, 95–96.
- Yuan, H.; Chen, L.; Hong, F.F. A Biodegradable Antibacterial Nanocomposite Based on Oxidized Bacterial Nanocellulose for Rapid Hemostasis and Wound Healing. ACS Appl. Mater. Interfaces 2019, 12, 3382–3392.
- Sultana, T.; Van Hai, H.; Abueva, C.; Kang, H.J.; Lee, S.-Y.; Lee, B.-T. TEMPO oxidized nano-cellulose containing thermo-responsive injectable hydrogel for post-surgical peritoneal tissue adhesion prevention. Mater. Sci. Eng. C 2019, 102, 12–21.
- Song, S.H.; Kim, J.E.; Lee, Y.J.; Kwak, M.H.; Sung, G.Y.; Kwon, S.H.; Son, H.J.; Lee, H.S.; Jung, Y.J.; Hwang, D.Y. Cellulose film regenerated from Styela clava tunics have biodegradability, toxicity and biocompatibility in the skin of SD rats. J. Mater. Sci. Mater. Electron. 2014, 25, 1519–1530.
