During evolution, the development of bone was critical for many species to thrive and function in the boundary conditions of Earth. Furthermore, bone also became a storehouse for calcium that could be mobilized for reproductive purposes in mammals and other species. The critical nature of bone for both function and reproductive needs during evolution in the context of the boundary conditions of Earth has led to complex regulatory mechanisms that require integration for optimization of this tissue across the lifespan. Three important regulatory variables include mechanical loading, sex hormones, and innervation/neuroregulation. The importance of mechanical loading has been the target of much research as bone appears to subscribe to the “use it or lose it” paradigm. Furthermore, because of the importance of post-menopausal osteoporosis in the risk for fractures and loss of function, this aspect of bone regulation has also focused research on sex differences in bone regulation.
- bone regulation
- mechanical loading
- sex hormones
- neural regulation
1. The Regulation of Bone by Mechanical Loading
2. Neural Regulation of Bone
2.1. Background
2.2. Loss of Neural Integrity on Bone
3. Possible Role of Neural Input in Fracture Healing and Altered Healing with Brain Trauma
4. Potential Neural Influences on Development of Post-Menopausal Osteoporosis (OP) and Age-Related OP
5. Space Flight, Bedrest, and Neural Regulation
5.1. Influence of Space Flight and Bedrest on Bone Regulation
As stated previously, bone as well as muscles and other MSK tissues appear to operate under the “use it or lose it” paradigm. One way to lose bone is via disuse, such as staying in bed and not engaging GRFs [1]. Disuse by removing oneself from GRFs, such as being immobilized/sedentary for the majority of the day for many days in a row can lead to the rapid loss of bone as an adult, occurring within days [205][180]. This occurs via uncoupling of bone maintenance with the excess of bone resorption, but the rate of bone loss is quite variable between individuals (reviewed in [6][181]), again possibly indicating that multiple steps are involved beyond loss of stimulation via GRF exposure. While prolonged bedrest can result from diseases or loss of mobility via aging and frailty, bed rest with six-degree head-down tilt is also used as a terrestrial surrogate for space flight (reviewed in [1,5][1][3]). For the latter, the participants are usually healthy younger males and females, although a current study is comprised of older individuals more reflective of astronaut ages or even older. This bedrest surrogate also allows for testing of potential countermeasures, such as exercise, short-arm centrifuges, or even drugs, such as bisphosphonates [206][182] and other anti-resorptive drugs that could be used in space (reviewed in [207,208][183][184]). One caveat of such bedrest studies is that as they are performed on Earth, the loss of bone still is occurring in the presence of 1 g gravity but without the GRF loading. In contrast, bone loss during space flight or living at low Earth orbit conditions on a vehicle, such as the International Space Station (ISS), occurs in microgravity and the absence of GRF loading. Similar to bone loss during bedrest, the rate of bone loss in astronauts is quite variable (discussed in [6][181]), indicating astronauts are heterogeneous with regard to their response to microgravity conditions. Furthermore, astronauts lose bone mostly in the lower extremities; the extremities are subjected to more GRF loading than the upper extremities. As evolution could not have anticipated space flight and exposure to microgravity, the finding of a heterogenous response to microgravity regarding bone loss implies that such variation arose in the systems controlling the rate of bone loss when they would be silent while on Earth, or potentially contributing to bone loss during aging. This is in contrast to the post-menopausal development of osteoporosis in a subset of females where bone loss is associated with loss of hormonal influence. Furthermore, as most astronauts have been males, but aged males with osteoporosis represent only 25% of patients with OP, there are some differences between astronauts losing bone in space and males on Earth developing OP on Earth. Thus, there are levels of complexity regarding the regulation of bone integrity that remain to be answered.5.2. Is There a Role for Neural Regulation Dysfunction during Bone Loss during Space Flight and Osteoporosis?
It is clear from the above discussion that bone is innervated, loss of innervation from SCI or stroke leads to loss of bone, bone is regulated differently in females and males, and bone loss can occur in response to a number of conditions, including space flight and the Earth analogue, prolonged bedrest. For many of these altered environments, the bone loss has been mainly attributed to the loss of mechanical loading, with counter measures focused on the use of exercise to overcome the “disuse atrophy”. However, a role for the innervation of bone in the loss of bone density with space flight and prolonged bedrest has not been explored in detail by the biomechanists, who have focused more on the direct effects of loading on bone cells rather than indirectly via the potential regulatory functions of mechanical loading on neural elements that in turn could influence bone cells. Furthermore, insights into the heterogeneity in bone loss associated with microgravity conditions has not been explained. Similarly, the focus of research on bone loss associated with menopause has been directed at the direct effects of hormones on bone cells in spite of the fact that only a subset of females develops clinically relevant osteoporosis after menopause. And similar to the heterogeneity of bone loss in microgravity, why females with osteoporosis also exhibit a variation in the rate and extent of bone loss has not been explained. Thus, in both of these situations, microgravity and post-menopausal osteoporosis, several aspects of the bone loss remain unaccounted for at the present time. With regard to hormonal regulation of bone, as discussed earlier, it is clear that bone cells can be affected by hormones, particularly in females. Most cells in the body express both nuclear and plasma membrane receptors for estrogen and progesterone, as well as androgens [209,210,211[185][186][187][188][189][190],212,213,214], with the non-genomic plasma membrane receptors mediating rapid responses. Hormones, such as estrogens, can influence a variety of neural activities [215,216,217[191][192][193][194][195],218,219], so it is not outside the realm of possibility that such hormones may influence the functioning of neural activity in bone. In a rat model, the influence of neuronal signals was diminished in estrogen-deficient females [220][196], potentially indicating a loss of neural regulatory mechanisms after menopause could contribute to bone loss. Depending on the extent of loss of the neural regulation (local synthesis versus dependence on systemic levels of hormones), there could be variation in the impact of the disruption of the neural influence. This variation could contribute to the development of osteoporosis and the rate of bone loss in the post-menopausal environment, but this will require more focused research to address this possibility. However, it should be pointed out that the regulation of bone is complex, particularly in females where the regulation must involve the integration of a variety of factors (mechanical loading, sex hormones, and innervation/nerves) that can vary across the lifespan. Thus, integration and establishment of which mechanisms have priority depends in part on whether one is pre-puberty, skeletally mature but cycling or pregnant/lactating, or in the post-menopausal state. The other variable that may play a role in the nature of the integration of factors regulating bone integrity is that of epigenetic modification [221][197], modifications that can occur at the life transition points or due to life experiences. Thus, epigenetic modifications, dependent in part on which cells are affected and where they are located, could alter the regulation of bone by any of the known variables (mechanical loading, neural input, hormonal factors) at different stages of life. However, this area will require additional research effort to better understand the local versus systemic impact of epigenetic alterations on bone regulation. Interestingly, most of the bone loss in microgravity by astronauts has been in males, so the complexities of deciphering the underlying neural regulatory mechanisms may be less than in females. However, as more female astronauts go into space for longer periods of time, the findings with the two sexes may be compared even though the numbers of each is still fairly small. As mentioned earlier, male astronauts lose bone in microgravity at variable rates, and exercise in microgravity conditions is only partially effective. This set of circumstances likely cannot be explained solely by a loss of mechanical loading as the only factor involved; although, it is possible that the exercises used in space do not adequately reflect loading on Earth. An additional potential explanation is that bone is regulated by a combination of factors, including neuroregulatory factors, and the conditions in space lead to loss of regulation by multiple factors and the exercise conditions in space do not replicate the conditions required to restore the neuro-component. Such a conclusion would also be supported by the effect of exercise on bone health in patients with spinal cord injuries, where such exercise is again only partially effective, indicating factors in addition to mechanical loading are required for a complete pattern for maintaining bone integrity. In this circumstance, it is clear that the neuro-component has been damaged and its contributions disrupted by the injury. Interestingly, the fact that bisphosphonates can alleviate bone loss both in space and after a SCI likely indicates that directly influencing bone–cell activity can block or interfere with the impact of loss of effectiveness of the regulatory systems. Bone regulation in post-puberty females is more complex than for males given that hormonal variation during the menstrual cycle, pregnancy, and lactation can also lead to modifications of bone integrity, modifications that are apparently mostly reversible. While bone cells express sex hormone receptors and thus could be directly influenced by hormones, it is also possible that some of the effects of sex hormones on bone cells is indirect via modulation of the regulatory activity of the neural elements. Whether these neural elements affected by sex hormones are those directing elements innervating bones or more central control elements in the brain or via the dorsal root ganglion would remain to be determined. A role for a central neural (i.e., brain)-localized mechanism may also be hypothesized to explain some of the variation in bone loss accompanying space flight and menopause. As only a subset of females develops clinically relevant post-menopausal osteoporosis and there is variation in the rate and extent of bone loss, variants of a central mechanism might better explain the systemic effects of osteoporosis rather than local effects in a variety of mechanical environments, local environments that may be subjected to individual epigenetic modifications [31,222][151][198]. Similarly, as space flight could not have been anticipated by evolution, the finding that male astronauts experience variable bone loss during space flight may mean that variation in a central regulatory mechanism (i.e., neural) may be silent during most of the lifespan while being maintained in a 1 g environment on Earth. However, in some males, there may be age-related loss of regulation at the neural level, leading to clinical osteoporosis, but at numbers much less than for post-menopausal females. Such heterogeneity between individual humans may reside in genetic variation that may not be evident if the individual remains on Earth. In this model of bone regulation with contributions from neural elements (likely central), mechanical loading, and sex hormones (mainly from females), the various regulatory systems must be integrated to allow for optimal functioning or functioning in a manner that allows for the regulation of bone within a window of physiological intactness throughout most of the lifespan. There may be some loss of integration with aging, and there must be some plasticity to achieve priority setting during development and then followed by growth and maturation. In this model of integration, some regulatory systems not only interact with bone cells directly but also interact with other regulatory systems to affect bone indirectly (i.e., sex hormones such as estrogen affecting neural regulation or ground reaction force the loading affecting both bone cells and the neural regulatory system). Some of these potential regulatory options are depicted in Figure 1.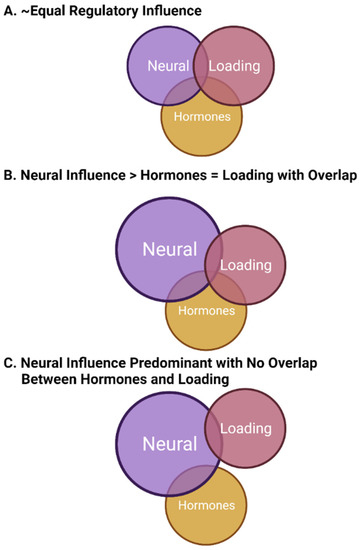
References
- Hart, D.A. Learning from human responses to deconditioning environment: Improved understanding of the “use it or lose it” principle. Front. Sports Act. Living 2021, 3, 685845.
- Hart, D.A.; Zernicke, R.F.; Shrive, N.G. Homo sapiens may incorporate daily acute cycles of “conditioning-deconditioning” to maintain musculoskeletal integrity: Need to integrate with biological clocks and circadian rhythm mediators. Int. J. Mol. Sci. 2022, 23, 9949.
- Hart, D.A.; Zernicke, R.F. Optimal human functioning requires exercise across the lifespan: Mobility in a 1 g environment is intrinsic to the integrity of multiple biological systems. Front. Physiol. 2020, 11, 156.
- Frost, H.M. Bone’s mechanostat: A 2003 update. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 275A, 1081–1101.
- Ferretti, J.L.; Cointry, G.R.; Capozza, R.F.; Frost, H.M. Bone mass, bone strength, muscle-bone interactions, osteopeias and osteoporosis. Mech. Aging Dev. 2003, 124, 269–279.
- Frost, H.M. A 2003 update of bone physiology and Wolff’s Law for clinicians. Angle Orthod. 2004, 74, 3–15.
- Choi, J.U.A.; Kijas, A.W.; Lauko, J.; Rowan, A.E. The mechanosensory role of osteocytes and implications for bone health and disease states. Front. Cell Dev. Biol. 2022, 9, 770143.
- Cao, W.; Helder, M.N.; Bravenboer, N.; Wu, G.; Jin, J.; Ten Bruggenkate, C.M.; Klein-Nulend, J.; Schulten, E.A.J.M. Is there a governing role of osteocytes in bone tissue regeneration? Curr. Osteopors. Rep. 2020, 18, 541–550.
- Wee, N.K.; Sims, N.A.; Morello, R. The osteocyte transcriptome: Discovering messages buried within bone. Curr. Osteoporos. Rep. 2021, 19, 604–615.
- Zeng, Y.; Riquelme, M.A.; Hua, R.; Zhang, J.; Acosta, F.M.; Gu, S.; Jiang, J.X. Mechanosensitive piezo1 calcium channel activates connexin 43 hemichannels through PI3K signaling pathway in bone. Cell Biosci. 2022, 12, 191.
- Savadipour, A.; Palmer, D.; Ely, E.V.; Collins, K.H.; Garcia-Castrorena, J.M.; Harissa, Z.; Kim, Y.S.; Oestreich, A.; Qu, F.; Rashidi, N.; et al. The role of PIEZO ion channels in the musculoskeletal system. Am. J. Physiol. Cell Physiol. 2023, 324, C728–C740.
- Wang, J.; Sun, Y.X.; Li, J. The role of mechanosensory Piezo1 in bone homeostasis and mechanobiology. Dev. Biol. 2023, 493, 80–88.
- Maycas, M.; Ardura, J.A.; de Castro, L.F.; Bravo, B.; Gortazar, A.R.; Esbit, P. Role of the parathyroid hormone type 1 receptor (PTH1R) as a mechanosensor in osteocyte survival. J. Bone Miner. Res. 2015, 30, 1231–1244.
- Maycas, M.; Esbrit, P.; Gortazar, A.R. Molecular mechanisms in bone mechanotransduction. Histol. Histopathol. 2017, 32, 751–760.
- Tirado-Cabrera, I.; Martin-Guerrero, E.; Heredero-Jimenez, S.; Ardura, J.A.; Gortazar, A.R. PTH1R translocation to primary cilia in mechanically-stimulated osteocytes prevents osteoblast formation via regulation of CXCL5 and IL-6 secretion. J. Cell Physiol. 2022, 237, 3927–3943.
- van Tol, A.F.; Schemenz, V.; Wagermaier, W.; Roschger, A.; Razi, H.; Vitienes, I.; Fratzl, P.; Willie, B.; Weinkamer, R. The mechanoresponse of bone is closely related to osteocyte lacunocanalicular network architecture. Proc. Natl. Acad. Sci. USA 2020, 117, 32251–32259.
- Yang, F.; Yu, W.; Huo, X.; Li, H.; Qi, Q.; Yang, X.; Shi, N.; Wu, X.; Chen, W. Effects of osteocyte shape on fluid flow and fluid shear stress of the loaded bone. Biomed. Res. Int. 2022, 2022, 3935803.
- Kameo, Y.; Ozasa, M.; Adachi, T. Computational framework for analyzing flow-induced strain on osteocyte as modulated by microenvironment. J. Mech. Behav. Biomed. Mater. 2022, 126, 105027.
- Wang, H.; Zheng, X.; Zhang, Y.; Huang, J.; Zhou, W.; Li, X.; Tian, H.; Wang, B.; Xing, D.; Fu, W.; et al. The endocrine role of bone: Novel functions of bone-derived cytokines. Biochem. Pharmacol. 2021, 183, 114308.
- Tasevski, V.; Sorbetti, J.M.; Chiu, S.S.; Shrive, N.G.; Hart, D.A. Influence of mechanical and biological signals on gene expression in human MG-63 cells: Evidence for a complex interplay between hydrostatic compression and vitamin D3 or TGF-beta1 on MMP-1 and MMP-3 mRNA levels. Biochem. Cell Biol. 2005, 83, 96–107.
- Bellido, T.; Delgado-Calle, J. Ex vivo organ cultures as models to study bone biology. JBMR Plus 2020, 4.
- Smyth, N.A.; Zachwieja, E.C.; Buller, L.T.; Miranda, A.D.; Steinlauf, S.D. Surgical approaches to the calcaneus and the sural nerve: There is no safe zone. Foot Ankle Surg. 2018, 24, 517–520.
- Feigl, G.C.; Schmid, M.; Zahn, P.K.; Gonzalez, C.A.A.; Litz, R.J. The posterior femoral cutaneous nerve contributes significantly to sensory innervation of the lower leg: An anatomical investigation. Br. J. Anaesth. 2020, 124, 308–313.
- Eguchi, Y.; Ohtori, S.; Yamashita, M.; Yamauchi, K.; Suzuki, M.; Orita, S.; Kamoda, H.; Arai, G.; Ishikawa, T.; Miyagi, M.; et al. Clinical applications of diffusion magnetic resonance imaging of the lumbar foraminal nerve root entrapment. Eur. Spine J. 2010, 19, 1874–1882.
- Bruns, T.M.; Wagenaar, J.B.; Bauman, M.J.; Gaunt, R.A.; Weber, D.J. Real-time control of hind limb functional electrical stimulation using feedback from dorsal root ganglia recordings. J. Neural Eng. 2013, 10, 026020.
- Savastano, L.E.; Laurito, S.R.; Fitt, M.R.; Rasmussen, J.A.; Polo, V.G.; Patterson, S.I. Sciatic nerve injury: A simple and subtle model for investigating many aspects of the nervous system damage and recovery. J. Neurosci. Methods. 2014, 227, 166–189.
- Bron, R.; Wood, R.J.; Brock, J.A.; Ivanusic, J.J. Piezo2 expression in corneal afferent neurons. J. Comp. Neurol. 2014, 522, 2967–2979.
- Nigg, B.; Enders, H. Barefoot running-some critical considerations. Footwear Sci. 2013, 5, 1–7.
- Madansingh, S.; Murphree, D.H.; Kaufman, K.R.; Fortune, E. Assessment of gait kinetics in post-menopausal women using tri-axial ankle accelerometers during barefoot walking. Gait Posture 2019, 69, 85–90.
- Brazill, J.M.; Beeve, A.T.; Craft, C.S.; Ivanusic, J.J.; Scheller, E.L. Nerves in bone: Evolving concepts in pain and anabolism. J. Bone Miner. Res. 2019, 34, 1393–1406.
- Dimitri, P.; Rosen, C. The central nervous system and bone metabolism: An evolving story. Calcif. Tissue Int. 2017, 100, 476–485.
- Xu, J.; Zhang, Z.; Zhao, J.; Meyers, C.A.; Lee, S.; Qin, Q.; James, A.W. Interaction between the nervous and skeletal systems. Front. Cell Dev. Biol. 2022, 10, 976736.
- Minoia, A.; Carbonare, L.D.; Schwamborn, J.C.; Bolognin, S.; Valenti, M.T. Bone tissue and the nervous system: What do they have in common? Cells 2022, 12, 51.
- Cooper, R.R. Nerves in cortical bone. Science 1968, 160, 127–128.
- Elefteriou, F. Impact of the autonomic nervous system on the skeleton. Physiol. Rev. 2018, 98, 1083–1112.
- Tomlinson, R.E.; Christiansen, B.A.; Giannone, A.A.; Genetos, D.C. The role of nerves in skeletal development, adaptation, and aging. Front. Endocrinol. 2020, 11, 646.
- Rajpar, I.; Tomlinson, R.E. Function of peripheral nerves in the development and healing of tendon and bone. Semin. Cell Dev. Biol. 2022, 123, 48–56.
- Abeynayake, N.; Arthur, A.; Gronthos, S. Crosstalk between skeletal and neural tissues is critical for skeletal health. Bone 2021, 142, 115645.
- Wan, Q.Q.; Qin, W.P.; Ma, Y.X.; Shen, M.J.; Li, J.; Zhang, Z.B.; Chen, J.H.; Tay, F.R.; Niu, L.N.; Jiao, K. Crosstalk between bone and nerves within bone. Adv. Sci. 2021, 8, 2003390.
- Liu, S.; Chen, T.; Wang, R.; Huang, H.; Fu, S.; Zhao, Y.; Wang, S.; Wan, L. Exploring the effect of the “quaternary regulation” theory of “peripheral nerve-angiogenesis-osteoclast-osteogenesis” on osteoporosis based on neuropeptides. Front. Endocrinol. 2022, 13, 908043.
- Bjurholm, A.; Kreicbergs, A.; Brodin, E.; Schulteberg, M. Substance P- and CGRP-immunoreactive nerves in bone. Peptides 1988, 9, 165–171.
- Hu, B.; Lv, X.; Wei, L.; Wang, Y.; Zheng, G.; Yang, C.; Zang, F.; Wang, J.; Li, J.; Wu, X.; et al. Sensory nerve maintains intervertebral disc extracellular matrix homeostasis via CGRP/CHSY1 axis. Adv. Sci. 2022, 9, e2202620.
- Xu, J.; Wang, J.; Chen, X.; Li, Y.; Mi, J.; Qin, L. The effects of calcitonin gene-related peptide on bone homeostasis and regeneration. Curr. Osteoporos. Rep. 2020, 18, 621–632.
- Jones, K.B.; Mollano, A.V.; Morcuende, J.A.; Cooper, R.R.; Saltzman, C.L. Bone and brain: A review of neural, hormonal, and musculoskeletal connections. Iowa Orthop. J. 2004, 24, 123–132.
- Wang, L.; Hou, S.; Sabsovich, I.; Guo, T.Z.; Wei, T.; Kingery, W.S. Mice lacking substance P have normal bone modeling but diminished bone formation, increased resorption, and accelerated osteopenia with aging. Bone 2021, 144, 115806.
- Li, F.X.; Xu, F.; Lin, X.; Wu, F.; Zhong, J.W.; Wang, Y.; Guo, B.; Zheng, M.H.; Chan, S.K.; Yuan, L.Q. The role of substance P in the regulation of bone and cartilage metabolic activity. Front. Endocrinol. 2020, 11, 77.
- Chen, Q.C.; Zhang, Y. The role of NPY in the regulation of bone metabolism. Front. Endocrinol. 2022, 13, 833485.
- Irie, K.; Hara-Irie, F.; Ozawa, H.; Yajima, T. Calcitonin gene-related peptide (CGRP)-containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc. Res. Tech. 2002, 58, 85–90.
- Wu, H.; Lin, X.Q.; Long, Y.; Wang, J. Calcitonin gene-related peptide is potential therapeutic target of osteoporosis. Heliyon 2022, 8, 12288.
- Wee, N.K.Y.; Novak, S.; Ghosh, D.; Root, S.H.; Dickerson, I.M.; Kalajzic, I. Inhibition of CGRP signaling impairs fracture healing in mice. J. Orthop. Res. 2023, 41, 1228–1239.
- Qiao, Y.; Wang, Y.; Zhou, Y.; Jiang, F.; Huang, T.; Chen, L.; Lan, J.; Yang, C.; Guo, Y.; Yan, S.; et al. The role of nervous system in adaptive response of bone to mechanical loading. J. Cell Physiol. 2019, 234, 7771–7780.
- Corr, A.; Smith, J.; Baldock, P. Neuronal control of bone remodeling. Toxicol. Pathol. 2017, 45, 894–903.
- Gajda, M.; Adriaensen, D.; Cichocki, T. Development of the innervation of long bones: Expression of the growth-associated protein. Folia Histochem. Cytobiol. 2000, 38, 103–110.
- Chartier, S.R.; Mitchell, S.A.T.; Majuta, L.A.; Mantyh, P.W. The changing sensory and sympathetic innervation of the young, adult, and aging mouse femur. Neuroscience 2018, 387, 178–190.
- Valdez, G. Effects of disease-afflicted and aging neurons on the musculoskeletal system. Bone 2019, 122, 31–37.
- Salo, P.T.; Tatton, W.G. Age-related loss of knee joint afferents in mice. J. Neurosci. Res. 1993, 35, 664–677.
- Salo, P.T.; Theriault, E. Number, distribution and neuropeptide content of rat knee joint afferents. J. Anat. 1997, 190 Pt 4, 515–522.
- Brighton, C.T.; Taddurin, G.T.; Goll, S.R.; Pollack, S.R. Treatment of denervation/disuse osteoporosis in the rat with a capacitively coupled electrical signal: Effects on bone formation and bone resorption. J. Orthop. Res. 1988, 6, 676–684.
- Brouwers, J.E.M.; Lambers, F.M.; van Riethergen, B.; Ito, K.; Huiskes, R. Comparison of bone loss induced by ovariectomy and neurectomy in rats analyzed by in vivo micro-CT. J. Orthop. Res. 2009, 27, 1521–1527.
- Ma, X.; Lv, J.; Sun, X.; Ma, J.; Xing, G.; Wang, Y.; Sun, L.; Wang, J.; Li, F.; Li, Y.; et al. Naringin ameliorates bone loss induced by sciatic neurectomy and increases semaphoring #A expression in denervated bone. Sci. Rep. 2015, 6, 24562.
- Li, Y.; Jie, L.; Tian, A.Y.; Zhong, S.; Tian, M.Y.; Zhong, Y.; Wang, Y.; Li, H.; Li, J.; Sun, X.; et al. Transforming growth factor beta is regulated by a glucocorticoid-dependent mechanism in denervation mouse bone. Sci. Rep. 2017, 7, 9925.
- Lin, C.C.; Chang, Y.T.; Lin, R.W.; Chang, C.W.; Wang, G.J.; Lai, K.A. Single pulsed electromagnetic field restores bone mass and microarchitecture in denervation/disuse osteopenic mice. Med. Eng. Phys. 2020, 80, 52–59.
- Bayram, P.; Karamese, S.A.; Ozdemir, B.; Salum, C.; Erol, H.S.; Karamese, M. Two flavonoids, baicalein and naringin, are effective as anti-inflammatory and anti-ocidant agents in a rat model of polymicrobial sepsis. Immunopharamacol. Immunotoxicol. 2023, 1–10.
- Brighton, C.T.; Katz, M.J.; Goll, S.R.; Nichols, C.E.; Pollack, S.R. Prevention and treatment of sciatic denervation disuse osteoporosis in the rat tibia with capacitively coupled electrical stimulation. Bone 1985, 6, 87–97.
- Tamaki, H.; Yotani, K.; Ogita, F.; Hayao, K.; Kirimto, H.; Onishi, H.; Kusuga, N.; Yamamoto, N. Low-frequency electrical stimulation of denervated skeletal muscle retards muscle and trabecular bone loss in aged rats. Int. J. Mol. Sci. 2019, 16, 822–830.
- Kawao, N.; Moritake, A.; Tatsumi, K.; Kaji, H. Roles of irisin in the linkage from muscle to bone during mechanical unloading in mice. Calcif. Tissue Int. 2018, 103, 24–34.
- Deng, J.; Cohen, D.J.; Redden, J.; McClure, M.J.; Boyan, B.D.; Schwartz, Z. Differential effects of nerectomy and botox-induced muscle paralysis on bone phenotype and titanium implant osseointegration. Bone 2021, 153, 116145.
- Qin, W.; Bauman, W.A.; Cardozo, C.P. Evolving concepts in neurogenic osteoporosis. Curr. Osteoporos. Rep. 2010, 8, 212–218.
- Morse, L.R.; Biering-Soerensen, F.; Carbone, L.D.; Cervinka, T.; Cirnigliaro, C.M.; Johnston, T.E.; Liu, N.; Troy, K.L.; Weaver, F.M.; Shuhart, C.; et al. Bone mineral density testing in spinal cord injury: 2019 ISCD official position. J. Clin. Densitom. 2019, 22, 554–566.
- Anderson, D.; Park, A.J. Prophylactic treatment of osteoporosis after SCI: Promising research, but not yet indicated. Spinal Cord Ser. Cases 2019, 5, 25.
- Invernizzi, M.; de Sire, A.; Reno, F.; Cisari, C.; Runza, L.; Baricich, A.; Carda, S.; Fusco, N. Spinal cord injury as a model of bone-muscle interactions: Therapeutic implications from in vitro and in vivo studies. Front. Endocrinol. 2020, 11, 204.
- Carda, S.; Cisari, C.; Invernizzi, M.; Bevilacqua, M. Osteoporosis after stroke: A review of the causes and potential treatments. Cerebrovas. Dis. 2009, 28, 191–200.
- Yang, F.Z.; Jehu, D.A.M.; Ouyang, H.; Lam, F.M.H.; Pang, M.Y.C. The impact of stroke on bone properties and muscl-bone relationship: A systematic review and meta-analysis. Osteoporo. Int. 2020, 31, 211–224.
- Sato, Y. Abnormal bone and calcium metabolism in patients after stroke. Arch. Phys. Med. Rehabil. 2000, 81, 117–121.
- Pluskiewicz, W. Skeletal consequences in patients after stroke. Endokrynol. Pol. 2011, 62, 48–50.
- Hsieh, C.Y.; Sung, S.F.; Huang, H.K. Drug treatment strategies for osteoporosis in stroke patients. Expert Opin. Pharmacother. 2020, 21, 811–821.
- Borschmann, K. Exercise protects bone after stroke, or does it? A narrative review of the evidence. Stroke Res. Treat. 2012, 2012, 103697.
- Borshmann, K.; Pang, M.Y.C.; Bernhardt, J.; Juliano-Burns, S. Strepping towards prevention of bone loss after stroke: A systematic review of the skeletal effects of physical activity after stroke. Int. J. Stroke 2012, 7, 330–335.
- Sallehuddin, H.; Ong, T.; Said, S.M.; Tarmizi, N.A.A.; Loh, S.P.; Lim, W.C.; Nedarajah, R.; Lim, H.T.; Zambri, N.H.M.; Ho, Y.Y.; et al. Non-pharmacological interventions for bone health after stroke: A systematic review. PLoS ONE 2022, 17, e0263935.
- Lichy, A.M.; Groah, S. Asymmetric lower-limb bone loss after spinal cord injury: Case report. J. Rehabil. Res. Dev. 2012, 49, 221–226.
- Bauman, W.A.; Cardozo, C.P. Osteoporosis in individuals with spinal cord injury. PMR 2015, 7, 188–201.
- Shams, R.; Drasites, K.P.; Zaman, V.; Matzelle, D.; Shields, D.C.; Sole, C.J.; Haque, A.; Banik, N.L. The pathophysiology of osteoporosis after spinal cord injury. Int. J. Mol. Sci. 2021, 22, 3057.
- Abdelrahman, S.; Ireland, A.; Winter, E.M.; Purcell, M.; Coupaud, S. Osteoporosis after spinal cord injury: Aetiology, effects and therapeutic approaches. J. Musculoskelet. Neuronal Interact. 2021, 21, 26–50.
- Antoniou, G.; Benetos, I.S.; Vlamis, J.; Pneumaticos, S.G. Bone mineral density post a spinal cord injury: A review of the current literature guidelines. Cureus 2022, 14, e23434.
- Bauman, W.A. Pharmacological approaches for bone health in persons with spinal cord injury. Curr. Opin. Pharmacol. 2021, 60, 346–359.
- Edwards, W.B.; Schnitzer, T.J.; Troy, K.L. Bone mineral and stiffness loss at the distal femur and proximal tibia in acute spinal cord injury. Osteoporos. Int. 2014, 25, 1005–1015.
- Lobos, S.; Cooke, A.; Simonett, G.; Ho, C.; Boyd, S.K.; Edwards, W.B. Trabecular bone score at the distal femur and proximal tibia in individuals with spinal cord injury. J. Clin. Densitom. 2019, 22, 249–256.
- Haider, I.T.; Simonian, N.; Saini, A.S.; Leung, F.M.; Edwards, W.B.; Schnitzer, T.J. Open-label clinical trial of alendronate after teriparatide therapy in people with spinal cord injury and low bone mineral density. Spinal Cord 2019, 57, 832–842.
- Weaver, F.M.; Le, B.; Ray, C.; Miskevics, S.; Gonzalez, B.; Carbone, L.D. Predicting osteoporosis medication receipt in veterans with a spinal cord injury: A retrospective cohort study. J. Spinal Cord Med. 2019, 42, 760–767.
- Sutor, T.W.; Kura, J.; Mattingly, A.J.; Otzel, D.M.; Yarrow, J.F. The effects of exercise and activity-based physical therapy on bone after spinal cord injury. Int. J. Mol. Sci. 2022, 23, 608.
- Castello, F.; Louis, B.; Cheng, J.F.; Armento, M.; Santos, A.M. The use of functional electrical stimulation cycles in children and adolescents with spinal cord dysfunction: A pilot study. J. Pediatr. Rehabil. Med. 2012, 5, 261–273.
- Hart, D.A. Evidence for a potential “knee-eye-brain axis” involved in mobility and navigation control: Knee injury and obesity may disrupt axis integrity. J. Biomed. Sci. Eng. 2018, 11, 37–44.
- Jiang, S.D.; Jiang, L.S.; Dai, L.Y. Mechanisms of osteoporosis in spinal cord injury. Clin. Endocrinol. 2006, 65, 555–565.
- Lombardi, G.; Mondaini, N.; Macchiarella, A.; Del Popolo, G. Female sexual dysfunction and hormonal status in spinal cord injured (SCI) patients. J. Androl. 2007, 28, 722–726.
- Charls, A.C.; Rawat, N.; Zachariah, K. Menstrual cycle changes after spinal cord injury. Spinal Cord 2022, 60, 712–715.
- Gass, M.L.; Kagan, R.; Kohles, J.D.; Martens, M.S. Bone turnover marker profile in relation to the menstrual cycle of premenopausal healthy women. Menopause 2008, 15, 667.
- Sabour, H.; Javidan, A.N.; Latifi, S.; Larijani, B.; Shidfar, F.; Vafa, M.R.; Heshmat, R.; Razavi, H.E. Bone markers in patients with chronic traumatic spinal cord injury. Spine J. 2014, 14, 1132–1138.
- Edwards, W.B.; Schnitzer, T.J. Bone imaging and fracture risk after spinal cord injury. Curr. Osteoporos. Rep. 2015, 13, 310–317.
- Schulte, L.M.; Scully, R.D.; Kappa, J.E. Management of lower extremity long-bone fractures in spinal cord injury patients. J. Am. Acad. Orthop. Surg. 2017, 25, e204–e213.
- Haider, I.T.; Lobos, S.M.; Simonian, N.; Schnitzer, T.J.; Edwards, W.B. Bone fragility after spinal cord injury: Reductions in stiffness and bone mineral at the distal femur and proximal tibia as a function of time. Osteoporos. Int. 2018, 29, 2703–2715.
- Zleik, N.; Weaver, F.; Harmon, R.I.; Le, B.; Radhakrishnan, R.; Jirau-Rosaly, W.D.; Craven, B.C.; Raiford, M.; Hill, J.N.; Etingen, B.; et al. Prevention and management of ostepporosis and osteoporotic fractures in persons with a spinal cord injury or disorder: A systematic scoping review. J. Spinal Cord Med. 2019, 42, 735–759.
- Wang, L.; Yao, X.; Tang, X.; Ding, H.; Zhang, H.; Yuan, J. The effects of spinal cord injury on bone healing in patients with femoral fractures. J. Spinal Cord Med. 2014, 37, 414–419.
- Grassner, L.; Klein, B.; Maier, D.; Buhren, V.; Vogel, M. Lower extremity fractures in patients with spinal cord injury characteristics, outcome and risk factors for non-unions. J. Spinal Cord Metab. 2018, 41, 676–683.
- Wang, L.; Liu, L.; Pan, Z.; Zeng, Y. Serum leptin, bone mineral density and the healing of long bone fractures in men with spinal cord injury. Bosn J. Basic Med. Sci. 2015, 15, 69–74.
- Aro, H.; Eerola, E.; Aho, A.J.; Penttinen, R. Healing of experimental fractures in the denervated limbs of rats. Clin. Orthop. Relat. Res. 1981, 155, 211–217.
- Nordsletten, L.; Madsen, J.E.; Almaas, R.; Rootwelt, T.; Halse, J.; Konttinen, Y.T.; Hukkanen, M.; Santavirta, S. The neuronal regulation of fracture healing. Effects of sciatic nerve resection in rat tibia. Acta Orthop. Scand. 1994, 65, 299–304.
- Ding, W.-G.; Jiang, S.-D.; Zhang, Y.-H.; Dai, L.-Y. Bone loss and impaired fracture healing in spinal cord injured mice. Osteoporo. Int. 2011, 22, 507–515.
- Zheng, X.Q.; Huang, J.; Lin, J.L.; Song, C.L. Pathological mechanism of acute bone loss after fracture. J. Adv. Res. 2022, 49, 63–80.
- Benassy, J. Ossifications and fracture-healing in paraplegia and brain injuries. Proc. Annu. Clin. Spinal Cord Inj. Conf. 1966, 15, 55–60.
- Garland, D.E. Clinical observations on fractures and heterotopic ossification in the spinal cord and traumatic brain injured populations. Clin. Orthop. Relat. Res. 1988, 233, 86–101.
- Kushwaha, V.P.; Garland, D.G. Extremity fractures in the patient with a traumatic brain injury. J. Am. Acad. Orthop. Surg. 1998, 6, 298–307.
- Morley, J.; Marsh, S.; Drakoulakis, E.; Pape, H.-C.; Giannoudis, P.Y. Does traumatic brain injury result in accelerated fracture healing? Injury 2005, 36, 363–368.
- Morioka, K.; Marmor, Y.; Sacramento, J.A.; Lin, A.; Shao, T.; Miclau, K.; Clark, D.R.; Beattie, M.S.; Marcucio, R.S.; Miclau, T., 3rd; et al. Differential fracture response to traumatic brain injury suggests dominance of neuroinflammatory response in polytrauma. Sci. Rep. 2019, 9, 12199.
- Mollahosseimni, M.; Ahmadirad, H.; Goujani, R.; Khorramdelazad, H. The association between traumatic brain injury and accelerated fracture healing: A study on the effects of growth factors and cytokines. J. Mol. Neurosci. 2021, 8, 162–168.
- Ravi, P.; Nagesaran, J.; Ramanujam, M.; Suriyakumar, S.; Nambi, E.A. Correlation between traumatic brain injuries and callus formation in long bone fractures. Indian J. Orthop. 2022, 56, 837–846.
- Haffner-Luntzer, M.; Wieber, B.; Morioka, K.; Lackner, I.; Fischer, V.; Bahney, C.; Ignatius, A.; Kalbitz, M.; Marcucio, R.; Miclau, T. Altered early immune response after fracture and traumatic brain injury. Front. Immunol. 2023, 14, 1074207.
- Hofman, M.; Koopmans, G.; Kobbe, P.; Poeze, M.; Andruszkow, H.; Brink, P.R.; Pape, H.-C. Improved fracture healing in patients with concomitant brain injury: Proven or not? Mediat. Inflamm. 2015, 2015, 204842.
- Shim, D.W.; Hong, H.; Cho, K.-C.; Kim, S.H.; Lee, J.W.; Sung, S.-Y. Accelerated tibia fracture healing in traumatic brain injury in accordance with increased hematoma formation. BMC Musculoskelet. Discord. 2022, 23, 1110.
- Wildburger, R.; Zarkovic, N.; Tonkovic, G.; Skoric, T.; Frech, S.; Hartleb, M.; Loncaric, I.; Zakovic, K. Post-traumatic hormonal disturbances: Prolactin as a link between head injury and ehanced osteogenesis. J. Endocrinol. Investig. 1998, 21, 78–86.
- Wildburger, R.; Zarkovic, N.; Egger, G.; Petek, W.; Zarkovic, K.; Hofer, H.P. Basic fibroblast growth factor (BFGF) immunoreactivity as a possible link between head injury and impaired bone fracture healing. Bone Miner. 1994, 27, 183–192.
- Wildburger, R.; Zarkovic, N.; Egger, G.; Petek, W.; Meinitzer, A.; Borovic, S.; Zarkovic, K.; Li, L.; Stipancic, I.; Trbojevic-Cepe, M.; et al. Comparison of the values of basic fibroblast growth factor determined by an immunoassay in the sera of patients with traumatic brain injury and enhanced osteogenesis and the effects of the same sera on the fibroblast growth in vitro. Eur. J. Clin. Chem. Clin. Biochem. 1995, 33, 693–698.
- Song, Y.; Bi, L.; Zhang, Z.; Huang, Z.; Hou, W.; Lu, X.; Sun, P.; Han, Y. Increased levels of calcitonin gene-related peptide in serum accelerate fracture healing following traumatic brain injury. Mol. Med. Rep. 2012, 5, 432–438.
- Zhang, R.; Liang, Y.; Wei, S. The expressions of NGF and VEGF in the fracture tissues are closely associated with accelerated clavicle fracture healing in patients with traumatic brain injury. Ther. Clin. Risk Manag. 2018, 14, 2315–2322.
- Khare, G.N.; Gautam, V.K.; Gupta, L.N.; Gupta, A.K. A new hypothesis for faster healing of fractures in head injured patients. Indian J. Med. Sci. 1995, 49, 281–284.
- Cadosch, D.; Gautschi, O.P.; Thyer, M.; Song, S.; Skirving, A.P.; Filgueira, L.; Zellweger, R. Humoral factors enhance fracture-healing and callus formation in patients with traumatic brain injury. J. Bone Joint Surg. Am. 2009, 91, 282–288.
- Xu, Y.-Q.; Qin, M.-L.; Feng, S.-Y.; Huang, Y.; Jia, Z. Expressions and significance of calcitonin gene-related peptide and nerve growth factor in rabbit model of traumatic brain injury complicated with tibial fracture: Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5040–5050.
- Wei, Y.; Wang, L.; Clark, J.C.M.; Dass, C.R.; Choong, P.F.M. Elevated leptin expression in a rat model of fracture and traumatic brain injury. J. Pharm. Pharmacol. 2008, 60, 1667–1672.
- Boes, M.; Kain, M.; Kakar, S.; Nicholls, F.; Cullinane, D.; Gerstenfeld, L.; Einhorn, T.A.; Tornetta, P., 3rd. Osteogenic effects of traumatic brain injury on experimental fracture-healing. J. Bone Joint Surg. Am. 2006, 88, 738–743.
- Arik, M.; Ekinci, Y.; Gurbuz, K.; Batin, S. The effects of focal brain damage on fracture healing: An experimental study. Eklem Hastalik. Cerrahisi. 2019, 30, 267–274.
- Yang, C.; Gao, C.; Liu, N.; Zhu, Y.; Zhu, X.; Su, X.; Zhang, Q.; Wu, Y.; Zhang, C.; Liu, A.; et al. The effect of traumatic brain injury on bone healing from a novel exosome centered perspective in a mice model. J. Orthop. Translat. 2021, 30, 70–81.
- Kesavan, C.; Rundle, C.; Mohan, S. Repeated mild traumatic brain injury impairs fracture healing in male mice. BMC Res. Notes 2022, 15, 25.
- Meyer, M.H.; Etienne, W.; Meyer, R.A., Jr. Altered mRNA expression of genes related to nerve cell activity in the fracture callus of older rats: A randomized, controlled, microarray study. BMC Musculoskelet. Disord. 2004, 5, 24.
- Wang, L.; Tang, X.; Zhang, H.; Yuan, J.; Ding, H.; Wei, Y. Elevated leptin expression in rat model of traumatic spinal cord injury and femoral fracture. J. Spinal Cord Med. 2011, 34, 501–509.
- Yan, H.; Zhang, H.-W.; Fu, P.; Liu, B.-L.; Jin, W.-Z.; Duan, S.-B.; Xue, J.; Liu, K.; Sun, Z.-M.; Zeng, X.-W. Leptin’s effect on accelerated fracture healing after traumatic brain injury. Neurol. Res. 2013, 35, 537–544.
- Garbe, A.; Graef, F.; Appelt, J.; Schmidt-Bleek, K.; Jahn, D.; Lunnemann, T.; Tsitsilonis, S.; Seemann, R. Leptin mediated pathways stabilize posttraumatic insulin and osteocalcin patterns after long bone fracture and concomitant traumatic brain injury and thus influence fracture healing in a combined murine trauma model. Int. J. Mol. Sci. 2020, 21, 9144.
- Graef, F.; Seemann, R.; Garbe, A.; Schmidt-Bleek, K.; Schaser, K.D.; Keller, J.; Duda, G.; Tsitisilonis, S. Impaired fracture healing with high non-union rates remain irreversible after traumatic brain injury in leptin-deficient mice. J. Musculoskelet. Neuronal Interact. 2017, 17, 78–85.
- Seemann, R.; Graef, F.; Garbe, A.; Keller, J.; Huang, F.; Duda, G.; Schmidt-Bleek, K.; Schaser, K.-D.; Tsitsilonis, S. Leptin-deficiency eradicates the positive effect of traumatic brain injury on bone healing: Histological analyses in a combined trauma mouse model. J. Musculoskelet. Neuronal Interact. 2018, 18, 32–41.
- Khallaf, F.G.; Kehinde, E.O.; Hussein, S. Bone healing and hormonal bioassay in patients with long-bone fractures and concomitant head injury. Med. Princ. Pract. 2016, 25, 336–342.
- Imayama, I.; Prasad, B. Role of leptin in obstructive sleep apnea. Ann. Am. Thorac. Soc. 2017, 14, 1607–1621.
- Moore, T.J. Functional outcome following surgical excision of heterotopic ossification in patients with traumatic brain injury. J. Orthop. Trauma. 1993, 7, 11–14.
- Zhao, X.-G.; Zhao, G.-F.; Ma, Y.-F.; Jiang, G.-Y. Research progress in mechanism of traumatic brain injury affecting speed of fracture healing. Clin. J. Traumatol. 2007, 10, 376–380.
- Huang, H.; Cheng, W.-X.; Hu, Y.-P.; Chen, J.-H.; Zheng, Z.-T.; Zhang, P. Relationship between heterotopic ossification and traumatic brain injury: Why severe traumatic brain injury increases risk of heterotopic ossification. J. Orthop. Translat. 2017, 12, 16–25.
- Anthonissen, J.; Steffen, C.T.; Hofmann, A.; Victor, J. The pathogenesis of heterotopic ossification after traumatic brain injury: A review of current literature. Acta Orthop. Belg. 2020, 86, 369–377.
- O’Brien, E.J.O.; Frank, C.B.; Shrive, N.G.; Halgrimsson, B.; Hart, D.A. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int. J. Exp. Pathol. 2012, 93, 319–331.
- O’Brien, E.J.O.; Shrive, N.G.; Rosvold, J.M.; Thornton, G.M.; Frank, C.B.; Hart, D.a. Tendon mineralization is accelerated bilaterally and creep of contralateral tendons is increased after unilateral needle injury of murine Achilles tendon. J. Orthop. Res. 2013, 31, 1520–1528.
- Salles, J.P. Bone metabolism during pregnancy. Ann. Endocrinol. 2016, 77, 163–168.
- Liu, X.S.; Wang, L.; de Bakker, C.M.J.; Lai, X. Mechanical regulation of the maternal skeleton during reproduction and lactation. Curr. Osteoporos. Rep. 2019, 17, 375–386.
- Kovacs, C.S. The skeleton is a storehouse of mineral that is plundered during lactation and (fully?) replenished afterwards. J. Bone Miner. Res. 2017, 32, 676–680.
- Maliha, G.; Morgan, J.; Vranhas, M. Transient osteoporosis of pregnancy. Injury 2012, 43, 1237–1241.
- Cohen, A.; Kamanda-Kosseh, M.; Dempster, D.W.; Zhou, H.; Muller, R.; Goff, E.; Colon, I.; Bucovsky, M.; Stubby, J.; Nickolas, T.L.; et al. Women with pregnancy and lactation-associated osteoporosis (PLO) have low bone remodeling rates at the tissue level. J. Bone Miner. Res. 2019, 34, 1552–1561.
- Hart, D.A. Sex differences in biological systems and the conundrum of menopause: Potential commonalities in post-menopausal disease mechanisms. Int. J. Mol. Sci. 2022, 23, 4119.
- Zhang, W.; Liu, Y.; Xu, J.; Fan, C.; Zhang, B.; Feng, P.; Wang, Y.; Kong, Q. The role of sympathetic nerves in osteoporosis: A narrative review. Biomedicines 2022, 11, 33.
- Schumacher, M.; Sitruk-Ware, R.; De Nicola, A.F. Progesterone and progestins: Neuroprotection and myelin repair. Curr. Opin. Pharmacol. 2008, 8, 740–746.
- Nugent, B.M.; Tobet, S.A.; Lara, H.E.; Lucion, A.B.; Wilson, M.E.; Recabarren, S.E.; Paredes, A.H. Hormonal programing across the lifespan. Horm. Metab. Res. 2012, 44, 577–586.
- Baker, S.E.; Limberg, J.K.; Ranadive, S.M.; Joyner, M.J. Neurovascular control of blood pressure is influenced by aging, sex, and sex hormones. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R1271–R1275.
- Clarkoudian, N.; Hart, E.C.; Barnes, J.N.; Joyner, M.J. Autonomic control of body temperature and blood pressure: Influences of female sex hormones. Clin. Auton. Res. 2017, 27, 149–155.
- Ghoumari, A.M.; Ghanem, C.A.; Asbelaoui, N.; Schaumaker, M.; Hussain, R. Roles of progesterone, testosterone and their nuclear receptors in central nervous system myelination and remyelination. Int. J. Mol. Sci. 2020, 21, 3163.
- Littlejohn, E.L.; Fedorchak, S.; Boychuk, C.R. Sex-steroid-dependent plasticity of brain-stem autonomic circuits. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R60–R68.
- Rosato, E.; Sciarra, F.; Anastasiadou, E.; Lenzi, A.; Venneri, M.A. Revisiting the physiological role of androgens in women. Expert Rev. Endocrinol. Metab. 2022, 17, 547–561.
- Ansdell, P.; Brownstein, C.G.; Skarabot, J.; Hicks, K.M.; Simones, D.C.M.; Thomas, K.; Howatson, G.; Hunter, S.K.; Goodall, S. Menstrual cycle-associated modulations in neuromuscular function and fatigability of the knee extensors in eumenorrheic women. J. Appl. Physiol. 2019, 126, 701–712.
- Hwang, C.L.; Okazaki, K.; Shibata, S.; Liu, Y.L.; Fu, Q. Menstrual cycle effects on sympathetic neural burst amplitude distribution during orthostasis in young women. Clin. Auton. Res. 2021, 31, 767–773.
- Pellegrino, A.; Ticlus, P.M.; Vandenboom, R. Mechanisms of estrogen influence on skeletal muscle: Mass, regeneration, and mitochondrial function. Sports Med. 2022, 52, 2853–2869.
- Oosthuyse, T.; Strauss, J.A.; Hackney, A.C. Understanding the female athlete: Molecular mechanisms underpinning menstrual phase differences in exercise metabolism. Eur. J. Appl. Physiol. 2023, 123, 423–450.
- Bilke, D.D. The free hormone hypothesis: When, why, and how to measure the free hormone levels to assess vitamin D, thyroid, sex hormone, and cortisol status. JBMR Plus 2020, 5, e10418.
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis due to hormonal imbalance: An overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. Int. J. Mol. Sci. 2022, 23, 1376.
- Albright, F.; Smith, P.H.; Richardson, A.M. Postmenopausal osteoporosis. JAMA 1941, 116, 2465–2474.
- Forbes, A.P. Fuller Albright. His concept of postmenopausal osteoporosis and what came of it. Clin. Orthop. Relat. Res. 1991, 269, 128–141.
- Hart, D.A. Are secondary effects of bisphosphonates on the vascular system of bone contributing to increased risk for atypical femoral fractures in osteoporosis? BioEssays 2023, 45, 2200206.
- Seeman, E. During aging, men lose less bone than women because they gain more periosteal bone, not because they resorb less endosteal bone. Calif. Tissue Int. 2001, 69, 205–208.
- Seeman, E. Periosteal bone formation—A neglected determinant of bone strength. N. Engl. J. Med. 2003, 349, 320–323.
- Seeman, E. The periosteum- a surface for all seasons. Osteoporo. Int. 2007, 18, 123–128.
- Seeman, E. Structural basis of growth-related gain and age-related loss of bone strength. Rheumatology 2008, 47 (Suppl. S4), iv2–iv8.
- Courties, A.; Sellam, J.; Berenbaum, F. Role of the autonomic nervous system in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 661–675.
- Plack, C.J.; Barker, D.; Predergast, G. Perceptual consequences of “hidden” hearing loss. Trends Hear. 2014, 18, 2331216514550621.
- Pollock, N.; Staunton, C.A.; Vasilaki, A.; McArdle, A.; Jackson, M.J. Denervated muscle fibers induce mitochondrial peroxide generation in neighboring innervated fibers: Role in muscle aging. Free Radic. Biol. Med. 2017, 112, 84–92.
- Maryanovich, M.; Zahalka, A.H.; Pierce, H.; Pinho, S.; Nakahara, F.; Asada, N.; Wei, Q.; Wang, X.; Ciero, P.; Xu, J.; et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 2018, 24, 782–791.
- Vincent, K.; Mohanty, S.; Pinelli, R.; Bonavita, R.; Pricop, P.; Albert, T.J.; Dahia, C.L. Aging of mouse intervertebral disc and association with back pain. Bone 2019, 123, 246–259.
- Fogarty, M.J.; Porras, M.A.G.; Mantilla, C.B.; Sieck, G.C. Diaphragm neuromuscular transmission failure in aged rats. J. Neurophysiol. 2019, 122, 93–104.
- Blaszkiewicz, M.; Willows, J.W.; Dubois, A.L.; Waible, S.; DiBello, K.; Lyons, L.L.; Johnson, C.P.; Paradie, E.; Banks, N.; Motyl, K.; et al. Neuropathy and neural plasticity in the subcutaneous white adipose depot. PLoS ONE 2019, 14, e0221766.
- Kos, O.; Hughson, R.L.; Hart, D.A.; Clement, G.; Frings-Meuthen, P.; Linnarsson, D.; Paloski, W.H.; Rittweger, J.; Wuyts, F.; Zange, J.; et al. Elevated serum soluble CD200 and CD200R as surrogate markers of bone loss under bed rest conditions. Bone 2014, 60, 33–40.
- Hart, D.A. Homo sapiens-A species not designed for space flight: Health risks in low Earth orbit and beyond, including potential risks when traveling beyond the geomagnetic field of Earth. Life 2023, 13, 757.
- Endo, I.; Matsumoto, T. Space flight/bedrest immobilization and bone. Bisphosphonate and the loss of bone mineral due to space flight or prolonged bed rest. Clin. Calcium. 2012, 22, 1863–1870. (In Japanese)
- Baran, R.; Wehland, M.; Schultz, H.; Heer, M.; Infanger, M.; Grimm, D. Microgravity-related changes in bone density and treatment options: A systematic review. Int. J. Mol. Sci. 2022, 23, 8650.
- Rengel, A.; Tran, V.; Toh, L.S. Denosumab as a pharmacological countermeasure against osteopenia in long duration spaceflight. Aerosp. Med. Hum. Perform. 2023, 94, 389–395.
- Acconcia, F.; Fiocchetti, M.; Busonero, C.; Fernandez, V.S.; Montalesi, E.; Cipolletti, M.; Pallottini, V.; Marino, M. The extra-nuclear interactome of the estrogen receptors: Implications for physiological functions. Mol. Cell Endocrinol. 2021, 538, 111452.
- Trevino, L.S.; Gorelick, D.A. The interface of nuclear and membrane steroid signaling. Endocrinology 2021, 162, bqab107.
- Thomas, P. Membrane progesterone receptors (mPRs, PAQRs): Review of structural and signaling characteristics. Cells 2022, 11, 1785.
- Thomas, P.; Pang, Y.; Camilletti, M.A.; Castelnovo, L.F. Functions of membrane progesterone receptors (mPRs, PAQRs) in nonreproductive tissues. Endocrinology 2022, 163, bqac147.
- Koszegi, Z.; Cheong, R.Y. Targeting the non-classical estrogen pathway in neurodegenerative diseases and brain injury disorders. Front. Endocrinol. 2022, 13, 999236.
- Goncalves, F.J.; Soares, F.A.; Pouso, M.R.; Longo, M.; Cairrao, E. Non-genomic effect of estradiol on the neurovascular unit and possible involvement in the cerebral vascular accident. Mol. Neurobiol. 2023, 60, 1964–1985.
- Schumacher, M.; Baulieu, E.E. neurosteroids: Synthesis and functions in the central and peripheral nervous systems. Ciba Found. Symp. 1995, 191, 90–106.
- Blacklock, A.D.; Johnson, M.S.; Krizsan-Agbas, D.; Smith, P.G. Estrogen increases sensory nociceptor neuritogenesis in vitro by a direct, nerve growth factor-independent mechanism. Eur. J. Neurosci. 2005, 21, 2320–2328.
- Krizsan-Agbas, D.; Pedchenko, T.; Smith, P.G. Neurotrimmin is an estrogen-regulated determinant of peripheral sympathetic innervation. J. Neurosci. Res. 2008, 86, 3086–3095.
- Vongpatanasin, W. Autonomic regulation of blood pressure in menopause. Semin. Reprod. Med. 2009, 27, 338–345.
- Wang, Q.; Cao, J.; Hu, F.; Lu, R.; Wang, J.; Ding, H.; Gao, R.; Xiao, H. Effects of estradiol on voltage-gated sodium channels in mouse dorsal root ganglion neurons. Brain Res. 2013, 1512, 1–8.
- Sample, S.J.; Racette, M.A.; Hao, Z.; Thomas, C.F.; Behan, M.; Muir, P. Functional adaptation in female rats: The role of estrogen signaling. PLoS ONE 2012, 7, e43215.
- Chen, Y.; Sun, Y.; Xue, X.; Ma, H. Comprehensive analysis of epigenetics mechanisms in osteoporosis. Front. Genet. 2023, 14, 1153585.
- Hart, D.A. Sex differences in musculoskeletal injury and disease risks across the lifespan: Are there unique subsets of females at higher risk than males for these conditions at distinct stages of the life cycle? Front. Physiol. 2023, 14.
- Seeman, E. Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1218–1225.
- Ghasem-Zadeh, A.; Burghardt, A.; Wang, X.F.; Iuliano, S.; Bonaretti, S.; Bui, M.; Zebaze, R.; Seeman, E. Quantifying sex, race, and age specific differences in bone microstructure requires measurement of anatomically equivalent regions. Bone 2017, 101, 206–213.
- Wulf, M.J.; Tom, V.J. Consequences of spinal cord injury on the sympathetic nervous system. Front. Cell Neurosci. 2023, 17, 999253.
- Madden, K.S.; Felton, S.Y.; Felton, D.L.; Hardy, C.A.; Livant, S. Sympathetic nervous system modulation of the immune system. II. Induction of lymphocyte proliferation and migration in vivo by chemical sympathectomy. J. Neuroimmunol. 1994, 49, 67–75.
- Madden, K.S.; Moyihan, J.A.; Brenner, G.J.; Felton, S.Y.; Felton, D.L.; Livant, S. Sympathectic nervous system modulation of the immune system. III. Alterations in T and B cell proliferation and differentiation in vitro following chemical sympathectomy. J. Neuroimmunol. 1994, 49, 77–87.
- Grebe, K.M. Editorial: Regulation of the regulator: Sympathetic nervous system control of regulatory T cells. J. Leukoc. Biol. 2009, 86, 1269–1270.
- Wu, K.; Li, R.; Zhang, Y.; Liu, Y.M.; Wang, M.C.; Huang, J.; Zhu, C.; Zhang, J.; Yuan, X.; Liu, Q. The discovery of a new type of innervation in lymphoid organs. Physiol. Rep. 2023, 11, 15604.
- Kruszewska, B.; Felton, S.Y.; Moyihan, J.A. Alterations in cytokine and antibody production following chemical sympathectomy in two strains of mice. J. Immunol. 1995, 155, 4613–4620.
- Kirkland, L.G.; Barbe, C.G.; Hadaya, J.; Benson, P.V.; Wagner, B.M.; Tankovic, S.; Hoover, D.B. Sympathetic innervation of human and porcine spleens: Implications for between species variation in function. Bioelectron. Med. 2022, 8, 20.
- De Virgliis, F.; Oliva, V.M.; Kizil, B.; Scheiermann, C. Control of lymph node activity by direct local innervation. Trends Neurosci. 2022, 45, 704–712.
- Gonzalez-Ariki, S.; Husband, A.J. The role of sympathetic innervation of the gut in regulating mucosal immune responses. Brain Behav. Immun. 1998, 12, 53–63.
- Veny, M.; Grases, D.; Kucharova, K.; Lin, W.W.; Nguyen, J.; Huang, S.; Ware, C.F.; Ranscht, B.; Sedy, J.R. Contactin-1 is required for peripheral innervation and immune homeostatsis within the intestinal mucosa. Front. Immunol. 2020, 11, 1268.
- Madden, K.S.; Rajan, S.; Bellinger, D.L.; Felton, S.Y.; Felton, D.L. Age-associated alterations in sympathetic neural interactions with the immune system. Dev. Comp. Immunol. 1997, 21, 479–485.
- Afan, A.M.; Broome, C.S.; Nicholls, S.E.; Whetton, A.D.; Miyan, J.A. Bone marrow innervation regulates cellular retention in the murine haemopoietic system. Br. J. Haematol. 1997, 98, 569–577.
- Wang, X.; Xu, J.; Kang, Q. Neuromodulation of bone: Role of different peptides and their interactions (review). Mol. Med. Rep. 2021, 23, 32.
