Over the past century and a half, the taxonomic placement of Eriophyoidea has been in flux. For much of this period, this group has been treated as a subtaxon within Trombidiformes. However, the vast majority of recent phylogenetic analyses, including almost all phylogenomic analyses, place this group outside Trombidiformes. The few studies that still place Eriophyoidea within Trombidiformes are likely to be biased by incomplete taxon/gene sampling, long branch attraction, the omission of RNA secondary structure in sequence alignment, and the inclusion of hypervariable expansion–contraction rRNA regions. Based on the agreement among a number of independent analyses that use a range of different datasets (morphology; multiple genes; mitochondrial/whole genomes), Eriophyoidea are almost certain to be closely related to Nematalycidae, a family of vermiform mites within Endeostigmata, a basal acariform grade. Much of the morphological evidence in support of this relationship was apparent after the discovery of Nematalycidae in the middle of the 20th century.
- Acariformes
- Eriophyoidea
- Nematalycidae
- Phylogenomics
- Trombidiformes
1. Introduction
2. The Morphological Era (1877–2015)
Two very early classification schemes for mites, which both date to 1877 [15[12][13],16], placed Eriophyoidea within Acaridae (=Astigmata). This was due, at least in part, to the absence of stigmata. Shortly afterward, in 1884, Claus and Moquin-Tandon [17][14] treated Eriophyoidea as a separate and distinct group from all the other main branches of mites recognized at the time. Eriophyoids have also been grouped with Demodicidae in Vermiformia based on their shared worm-like body form [18][15], but this placement was not followed in the classification schemes of Oudemans [19][16] and Reuter [20][17]. Due to a shared feeding ecology, Oudemans suspected that Eriophyoidea were closely affiliated with phytophagous taxa within Trombidiformes. However, Reuter rejected this idea based on the morphology of the digestive system. Trombidiformes and Oribatida have a diverticulum (caecum) comprising a pair of cavities that branch away from the main passage of the gut [21][18]. Baker and Wharton [22][19] treated Eriophyoidea as one of three basal lineages within Trombidiformes, the others being Tarsonemini and Prostigmata. Somewhat confusingly, they also inferred that eriophyoids share a recent common ancestry with Tetranychidae and Phytoptipalpidae (=Tenuipalpidae), which are in a relatively derived position within Trombidiformes [4,5][4][5]. They based this inference on the following characters: stylet-like chelicerae, phytophagy, rayed empodia (Eriophyoidea and Tenuipalpidae), and elongate bodies (Eriophyoidea and some Tenuipalpidae) [22,23][19][20]. It is remarkable that a close relationship between Eriophyoidea and Nematalycidae was never proposed as likely until very recently [11]. These taxa share a number of similarities that are readily apparent without the need for a detailed morphological analysis, including an annulated and worm-shaped body, unpaired vi (the rostral seta on the prodorsum) when present, and the absence of prodorsal trichobothria and body lyrifissures (cupules). Keifer [29][21] noted that both taxa have genitalia that are relatively anteriorly positioned on a worm-like body. However, he used these similarities, probably assumed by him to be convergent, only to explain the loss of legs III and IV in Eriophyoidea rather than infer a close phylogenetic relationship. In the period leading up to the relocation of Eriophyoidea to a more basal position within Trombidiformes, the position of Nematalycidae was also in flux. Whereas Nematalycidae were originally thought to belong in Endeostigmata [31][22], Cunliffe [32][23] hypothesized that this family is more closely related to Tydeoidea, but he provided no arguments to support this hypothesis. In accordance with Cunliffe, in the following decades, most authors placed this family within Tydeoidea [33,34][24][25] or as a separate superfamily that is allied to Tydeoidea [35][26]. Nematalycidae and Tydeoidea share a number of characters that are either plesiomorphic (indirect sperm transfer and the retention of fundamental setae on coxisterna II) or homoplastic (undivided femora, fusion of the palpal femur with the palpal genu). Other characters strongly suggest that Nematalycidae fall outside Trombidiformes. For example, nematalycids clearly bear rutella [36[27][28][29],37,38], structures that are widely thought to be lost throughout Trombidiformes [36,39][27][30]. Eriophyoids bear infracapitular guides, which are possible homologues of rutella [38,40][29][31]. Although Kethley treated Nematalycidae as allied to Tydeoidea [35][26], soon afterwards he hypothesized that Nematalycidae group with Micropsammidae and Proteonematalycidae within Nematalycoidea [41][32]. However, in his discussion of the support for this relationship, the only character he listed that is shared by all three families is the absence of trichobothria. He made no mention of where Eriophyoidea belonged, and there is no evidence that he considered the possibility that Eriophyoidea is affiliated with Nematalycidae. Lindquist clearly did consider the possibility of a close relationship between Eriophyoidea and Nematalycidae, but he largely rejected this idea in favor of a sister relationship between Eriophyoidea and Tydeoidea [27][33]. One of his main reasons for doing so was that Nematalycoidea, which is almost certain to be an artificial group [4[4][11],11], was hypothesized to be in a basal position, outside of Trombidiformes (largely because of the shared possession of rutella). By this time, Lindquist had also undertaken an unpublished cladistic analysis of Trombidiformes, in which Eriophyoidea was recovered as sister to Tydeoidea (Lindquist, pers. comm. 10 April 2023). A single cladogram from this analysis was published without any associated data [28][34]. Therefore, although Nematalycidae were relocated outside Trombidiformes, the same treatment was apparently not considered for Eriophyoidea. This was somewhat remedied by Lindquist when he suggested that Eriophyoidea may be closely affiliated with Alycidae [40][31]. However, later classification schemes followed Lindquist’s earlier work [39,42][30][35]. Lindquist contended that the consensus of morphological evidence favored a sister relationship between Eriophyoidea and Tydeoidea [27][33], although many of the characters that he used in support of this relationship show an equal or greater resemblance between Eriophyoidea and Nematalycidae [11]. Of the characters used by Lindquist [27][33] to argue for a sister relationship between Eriophyoidea and Tydeoidea, the only ones that show a greater degree of resemblance between eriophyoids and tydeoids than between eriophyoids and nematalycids are a suite of strongly interdependent and homoplastic characters pertaining to paedomorphisms, including the partial or complete suppression of anamorphosis, the loss of urstigmata, and the suppression of genital papillae and the nymphal progenital chamber [11].3. Molecular Era (2016–Present)
The first molecular phylogenetic analysis to address the position of Eriophyoidea was based on a mitogenomic analysis and showed strong support for the placement of Eriophyoidea outside of Trombidiformes [2]. Subsequent mitogenomic analyses also recovered that result [6,7,8,9][6][7][8][9]. Only a single mitogenomic analysis, which is undermined by the undersampling of non-trombidiform taxa, has recovered Eriophyoidea from within Trombidiformes [13][36]. Whole-genomic analyses have also recovered Eriophyoidea from outside of Trombidiformes [3,10][3][10]. Therefore, there is near unanimity among whole-genomic and mitogenomic analyses. A substantial proportion of phylogenetic analyses provided support for a close relationship between Eriophyoidea and Nematalycidae; Eriophyoidea are either sister to Nematalycidae [4,5][4][5] or nested within Nematalycidae [4,11][4][11]. These analyses are based on morphology [11], Sanger sequencing [4[4][5],5], and whole genomes [3]. The whole genome analysis, which is the only phylogenomic analysis to date to have included endeostigmatic taxa, revealed that Eriophyoidea and Nematalycidae share a suite of highly conserved nuclear proteins [3]. With respect to all the phylogenetic analyses that recovered Eriophyoidea outside of Trombidiformes, their congruence with a close relationship between Eriophyoidea and Nematalycidae is strong support for this relationship and also for the placement of Eriophyoidea outside of Trombidiformes (Figure 21).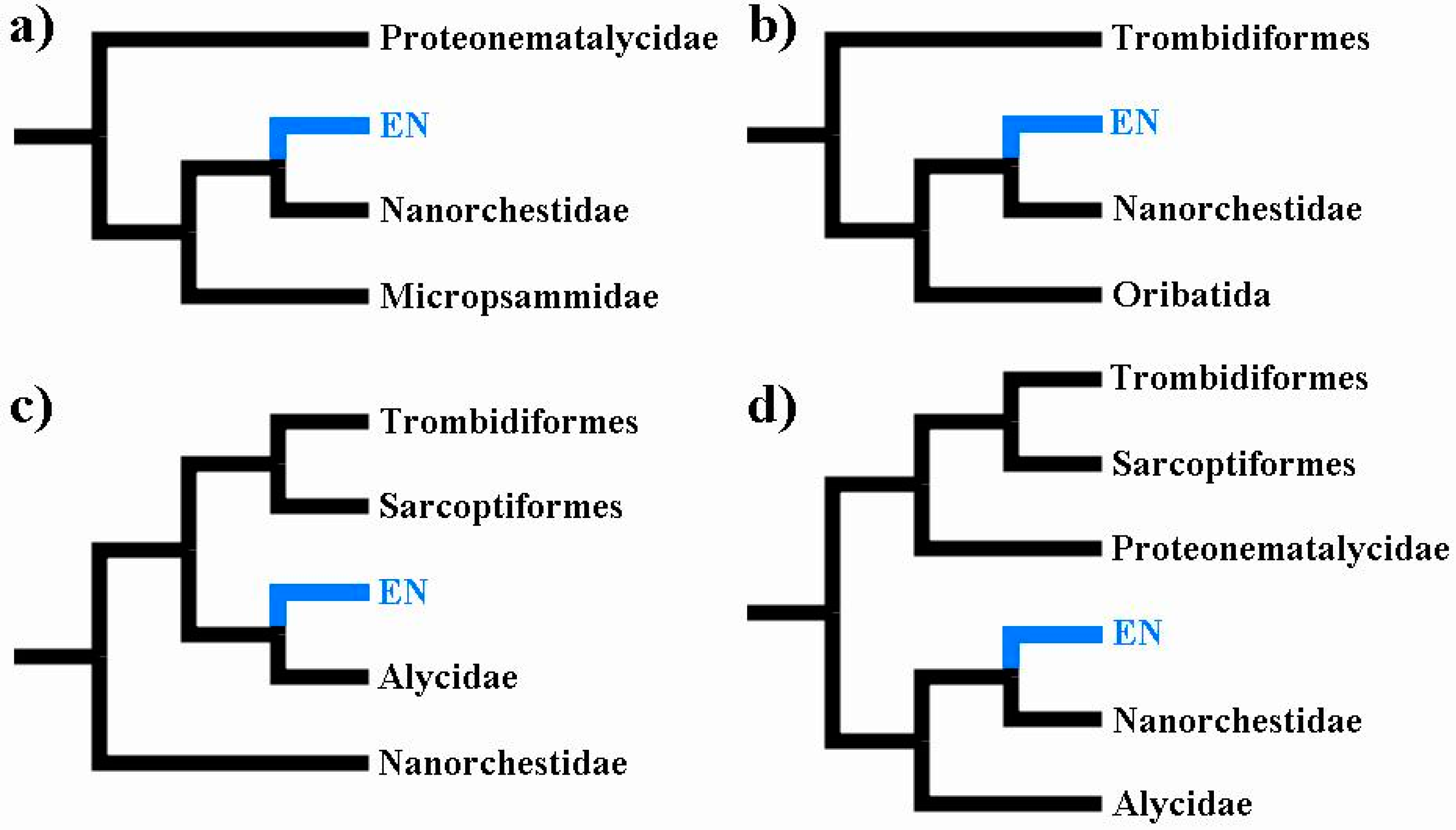
4. Are Eriophyoidea Nested within Tydeoidea?
References
- Zhang, Z.-Q. Eriophyoidea and Allies: Where Do They Belong? Syst. Appl. Acarol. 2017, 22, 1091–1095.
- Xue, X.-F.; Guo, J.-F.; Dong, Y.; Hong, X.-Y.; Shao, R. Mitochondrial Genome Evolution and tRNA Truncation in Acariformes Mites: New Evidence from Eriophyoid Mites. Sci. Rep. 2016, 6, 18920.
- Klimov, P.B.; Chetverikov, P.E.; Dodueva, I.E.; Vishnyakov, A.E.; Bolton, S.J.; Paponova, S.S.; Lutova, L.A.; Tolstikov, A.V. Symbiotic Bacteria of the Gall-Inducing Mite Fragariocoptes Setiger (Eriophyoidea) and Phylogenomic Resolution of the Eriophyoid Position among Acari. Sci. Rep. 2022, 12, 3811.
- Pepato, A.R.; Costa, S.G.D.S.; Harvey, M.S.; Klimov, P.B. One-Way Ticket to the Blue: A Large-Scale, Dated Phylogeny Revealed Asymmetric Land-to-Water Transitions in Acariform Mites (Acari: Acariformes). Mol. Phylogenet. Evol. 2022, 177, 107626.
- Klimov, P.B.; OConnor, B.M.; Chetverikov, P.E.; Bolton, S.J.; Pepato, A.R.; Mortazavi, A.L.; Tolstikov, A.V.; Bauchan, G.R.; Ochoa, R. Comprehensive Phylogeny of Acariform Mites (Acariformes) Provides Insights on the Origin of the Four-Legged Mites (Eriophyoidea), a Long Branch. Mol. Phylogenet. Evol. 2018, 119, 105–117.
- Arribas, P.; Andújar, C.; Moraza, M.L.; Linard, B.; Emerson, B.C.; Vogler, A.P. Mitochondrial Metagenomics Reveals the Ancient Origin and Phylodiversity of Soil Mites and Provides a Phylogeny of the Acari. Mol. Biol. Evol. 2020, 37, 683–694.
- Li, W.-N.; Xue, X.-F. Mitochondrial Genome Reorganization Provides Insights into the Relationship between Oribatid Mites and Astigmatid Mites (Acari: Sarcoptiformes: Oribatida). Zool. J. Linn. Soc. 2019, 187, 585–598.
- Xue, X.-F.; Dong, Y.; Deng, W.; Hong, X.-Y.; Shao, R. The Phylogenetic Position of Eriophyoid Mites (superfamily Eriophyoidea) in Acariformes Inferred from the Sequences of Mitochondrial Genomes and Nuclear Small Subunit (18S) rRNA Gene. Mol. Phylogenet. Evol. 2017, 109, 271–282.
- Fang, Y.; Fang, Y.; Chu, L.; Zuo, Z.; Liu, L.; Feng, R.; Zhang, Z.; Zhan, X.; Li, F.; Hu, C.; et al. The First Complete Mitochondrial Genome of Bdelloidea (Trombidiformes, Eupodina) and Comparative Genomics Provide Insights into Gene Rearrangement and Evolution of Trombidiform Mites. J. Stored Prod. Res. 2022, 98, 102009.
- Greenhalgh, R.; Dermauw, W.; Glas, J.J.; Rombauts, S.; Wybouw, N.; Thomas, J.; Alba, J.M.; Pritham, E.J.; Legarrea, S.; Feyereisen, R.; et al. Genome Streamlining in a Minute Herbivore That Manipulates Its Host Plant. eLife 2020, 9, e56689.
- Bolton, S.J.; Chetverikov, P.E.; Klompen, H. Morphological Support for a Clade Comprising Two Vermiform Mite Lineages: Eriophyoidea (Acariformes) and Nematalycidae (Acariformes). Syst. Appl. Acarol. 2017, 22, 1096–1131.
- Murray, A. Economic Entomology: Aptera; Chapman & Hall: London, UK, 1877.
- Kramer, P. Grundzüge Zur Systematik Der Milben. Arch. Naturgesch. 1877, 43, 215–247.
- Claus, C.; Moquin-Tandon, G. Traité De Zoologie, Traduction Française, 2nd ed.; Librairie F. Savy: Paris, France, 1884.
- Canestrini, G. Abbozzo del Sistema Acarologico. In Atti del Reale Istituto Veneto di Scienzem Lettere ed Arti; University of Bern: Bern, Switzerland, 1891; Volume 7, pp. 699–725.
- Oudemans, A.C. Nieuwe Classificatie Der Acari. Entomolog. Ber. 1906, 2, 43–46.
- Reuter, E. Zur Morphology Und Ontogenie Der Acariden Mit Besonderer Berücksichtigung von Pendiculopsis Graminum. Acta Soc. Scient. Fenn. 1909, 36, 1–288.
- Evans, G.O. Principles of Acarology; CAB International: Wallingford, UK, 1992.
- Baker, E.W.; Wharton, G.W. An Introduction to Acarology; The Macmillan Co.: New. York, NY, USA, 1952; 465p.
- Baker, E.W. A New Trichadenid Mite Which Further Indicates a Phylogenetic Relationship between the Tetranychidae and Eriophyidae. Proc. Entomol. Soc. Wash. 1948, 50, 59–60.
- Keifer, H.H. The Eriophyoidea Nalepa. In Mites Injurious Eriophyoid Mites; Jeppson, L.R., Keifer, H.H., Baker, E.W., Eds.; University of California Press: Berkley, CA, USA, 1975; pp. 327–587.
- Strenzke, K. Nematalycus Nematoides N. Gen. N. Sp. (Acarina Trombidiformes) Aus Dem Grundwasser Der Algerischen Kuste. Vie Milieu Paris 1953, 4, 638–647.
- Cunliffe, F. A New Species of Nematalycus Strenzke with Notes on the Family (Acarina, Nematalycidae). Proc. Entomol. Soc. Wash. 1956, 58, 353–355.
- Wainstein, B.A. The System of the Aquatic Mites and Their Place in the Suborder Trombidiformes. Tr. Inst. Biol. Vnutr. Vod 1965, 8, 66–83.
- Krantz, G.W. A Manual of Acarology; Oregon State University Book Stores: Corvallis, OR, USA, 1971; 335p.
- Kethley, J. Acariformes-Prostigmata. In Synopsis and Classification of Living Organisms; Stony Brook University: Stony Brook, NY, USA, 1982; Volume 2, pp. 117–145.
- Kethley, J.B. Acarina: Prostigmata (Actinedida). In Soil Biology Guide; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1990; pp. 667–756.
- Bolton, S.J.; Bauchan, G.R.; Ochoa, R.; Klompen, H. A Novel Fluid-Feeding Mechanism for Microbivory in the Acariformes (Arachnida: Acari). Arthropod Struct. Dev. 2015, 44, 313–325.
- Bolton, S.J.; Bauchan, G.R.; Chetverikov, P.E.; Ochoa, R.; Klompen, H. A Rudimentary Sheath for the Smallest of “biting” Chelicerae: The Mouthparts of Cunliffea (Nematalycidae) and a New Hypothesis on the Origin of the Stylet Sheath of Eriophyoidea (Acariformes). Int. J. Acarol. 2018, 44, 374–381.
- Lindquist, E.E.; Krantz, G.W.; Walter, D.E. Classification. In A Manual of Acarology: Third Edition; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 97–103.
- Lindquist, E.E. Evolution of Phytophagy in Trombidiform Mites. Exp. Appl. Acarol. 1998, 22, 81–100.
- Kethley, J. Proteonematalycidae (Acari: Acariformes), a New Mite Family from Fore-Dune Sand of Lake Michigan. Int. J. Acarol. 1989, 15, 209–217.
- Lindquist, E.E. 1.5.2 Phylogenetic Relationships. In World Crop Pests; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 6, pp. 301–327. ISBN 9780444886286.
- Nuzzaci, G.; de Lillo, E. Linee Evolutive Dello Gnatosoma in Alcuni Acari Prostigmata. In Atti XVI Congresso Nazionale Italiano di Entomologia; Scientific Press: Christchurch, New Zealand, 1991.
- Zhang, Z.; Fan, Q.H.; Pesic, V.; Smit, H. Order Trombidiformes Reuter, 1909. In Zhang, Z.-Q. (Ed.) Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness. Zootaxa 2011, 3148, 129.
- Thia, J.A.; Young, N.D.; Korhnen, P.K.; Yang, Q.; Gasser, R.B.; Umina, P.A.; Hoffmann, A.A. The Mitogenome of Halotydeus Destructor (Tucker) and Its Relationships with Other Trombidiform Mites as Inferred from Nucleotide Sequences and Gene Arrangements. Ecol. Evol. 2021, 11, 14162–14174.
- Szudarek-Trepto, N.; Kaźmierski, A.; Skoracka, A.; Lewandowski, M.; Dabert, J. Molecular Phylogeny Supports the Monophyly of the Mite Supercohort Eupodides (Acariformes: Trombidiformes) and Greatly Coincides with Traditional Morphological Definition of the Taxon. Annal. Zool. 2022, 72, 757–786.
- Norton, R.A.; Kethley, J.B.; Johnston, D.E.; OConnor, B.M. Phylogenetic Perspectives on Genetic Systems and Reproductive Modes of Mites. In Evolution and Diversity of Sex Ratio in Insects and Mites; Wrensch, D.L., Ebbert, M.A., Eds.; Chapman & Hall: New York, NY, USA, 1993; pp. 8–99.
- Gillespie, J.J.; Johnston, J.S.; Cannone, J.J.; Gutell, R.R. Characteristics of the Nuclear (18S, 5.8S, 28S and 5S) and Mitochondrial (12S and 16S) rRNA Genes of Apis Mellifera (Insecta: Hymenoptera): Structure, Organization, and Retrotransposable Elements. Insect Mol. Biol. 2006, 15, 657–686.
- Gillespie, J.J.; Yoder, M.J.; Wharton, R.A. Predicted Secondary Structure for 28S and 18S rRNA from Ichneumonoidea (Insecta: Hymenoptera: Apocrita): Impact on Sequence Alignment and Phylogeny Estimation. J. Mol. Evol. 2005, 61, 114–137.
- Pepato, A.R.; Klimov, P.B. Origin and Higher-Level Diversification of Acariform Mites—Evidence from Nuclear Ribosomal Genes, Extensive Taxon Sampling, and Secondary Structure Alignment. BMC Evol. Biol. 2015, 15, 178.
- Chetverikov, P.E.; Craemer, C.; Cvrković, T.; Klimov, P.B.; Petanović, R.U.; Romanovich, A.E.; Sukhareva, S.I.; Zukoff, S.N.; Bolton, S.; Amrine, J. Molecular Phylogeny of the Phytoparasitic Mite Family Phytoptidae (Acariformes: Eriophyoidea) Identified the Female Genitalic Anatomy as a Major Macroevolutionary Factor and Revealed Multiple Origins of Gall Induction. Exp. Appl. Acarol. 2021, 83, 31–68.
- Chetverikov, P.E.; Petanović, R.U. Description of a New Early-Derivative Mite, Pentasetacus Plicatus N. Sp. (Acariformes, Eriophyoidea), and Remarks on the Systematic Position of Pentasetacines. Zootaxa 2016, 4144, 211–226.
- Chetverikov, P.E.; Craemer, C. Gnathosomal Interlocking Apparatus and Remarks on Functional Morphology of Frontal Lobes of Eriophyoid Mites (Acariformes, Eriophyoidea). Exp. Appl. Acarol. 2015, 66, 187–202.
- Nuzzaci, G.; Di Palma, A. Mouthparts of a Tydeid Mite: An Ultrastructural and Functional Investigation. Entomologica 2002, 36, 71–91.
