You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Tarek A. Ebeid.
Heat stress is a major environmental stress that causes substantial economic loss in the rabbit industry. Compared to other agricultural animals, rabbits are more sensitive to heat stress as they have fewer sweat glands and a thicker coat of fur, increasing the heat dissipation complexity.
- Oryctolagus cuniculus
- high ambient temperature
- nutrition
1. Introduction
Rabbits are raised primarily for their meat, hair, and fur. Rabbit meat is characterized by low contents of fat, cholesterol, and sodium. At the same time, it is rich in protein, polyunsaturated fatty acids (PUFA), minerals (potassium, phosphorus, and selenium), and vitamins (B12 and niacin) [1]. With the worsening of climate change and the global warming phenomena, heat stress (HS) has become one of the most important types of stress that are challenging the rabbit industry, especially in hot and semi-hot regions of the world. The whole world is now suffering from high ambient temperatures, which have recently reached levels not seen before. HS is the highest serious stress and threat to the rabbit and poultry industry [2,3][2][3]. Rabbits are more susceptible to HS than other agricultural animals as they own fewer sweat glands and a thicker coat of fur, increasing the complexity of heat scattering [4,5][4][5]. Moreover, the genetically improved rabbits are characterized by rapid growth and higher metabolic rates, increasing their susceptibility to HS [6]. Thus, HS leads to significant economic losses in rabbit production as it elevates body temperature and disturbs normal physiological status, deteriorating growth performance, meat characteristics, reproductive traits, antioxidative properties, and immune responsiveness [7,8][7][8] (Figure 1).
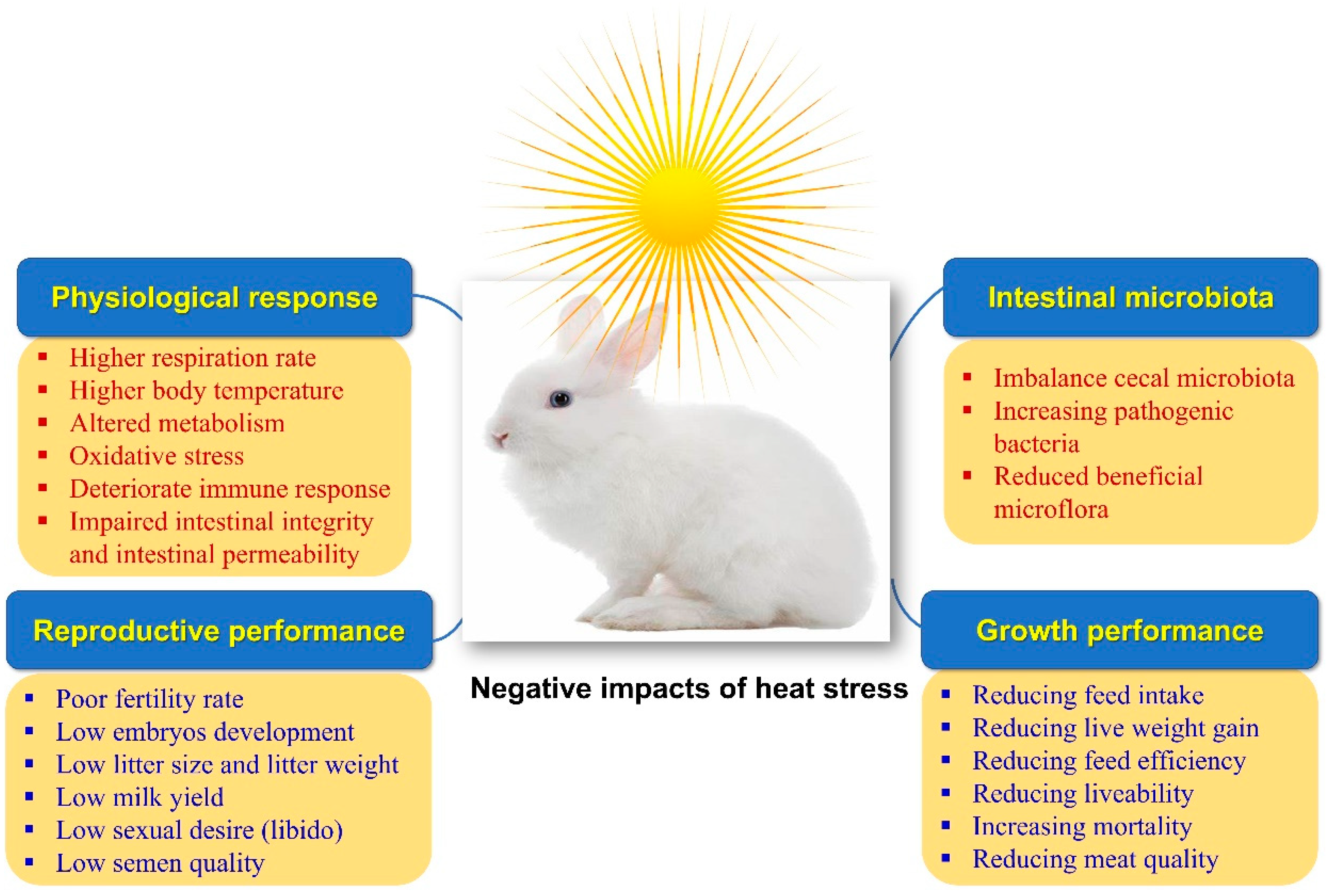
Figure 1.
Impacts of heat stress on rabbits’ physiological response, productive and growth performance, and intestinal microbiota.
Moreover, HS hurts the intestinal histomorphology and microbiome in rabbits [9]. The negative effects of HS on rabbit productivity can be mitigated with the help of cooling systems, ventilation, and management strategies. Implementing cutting-edge technology into the building infrastructure can be challenging under extreme conditions. Thus, nutritional manipulation to relieve the unfavorable influences of HS is an effective additional approach [10]. Nutraceuticals are dietary components that offer additional health benefits that override their nutritional benefits. Due to their potential impacts on maintaining normal physiological situations, strengthening the immune system, and preventing illness—which ultimately lead to an increase in productivity—nutraceuticals have recently attracted a lot of attention in rabbit farms (Table 1 and Figure 2). Nutraceuticals include vitamins, minerals, antioxidants, organic acids, fatty acids, probiotics, prebiotics, synbiotics, enzymes, medicinal plants, etc. [11]. Natural antioxidants are crucial in safeguarding the animal against the damage caused by free radicals. Weaned rabbits become extremely vulnerable to enteric infections due to the prevention of using antibiotics as growth enhancers because they have a very complicated and distinctive digestive system [12,13][12][13].
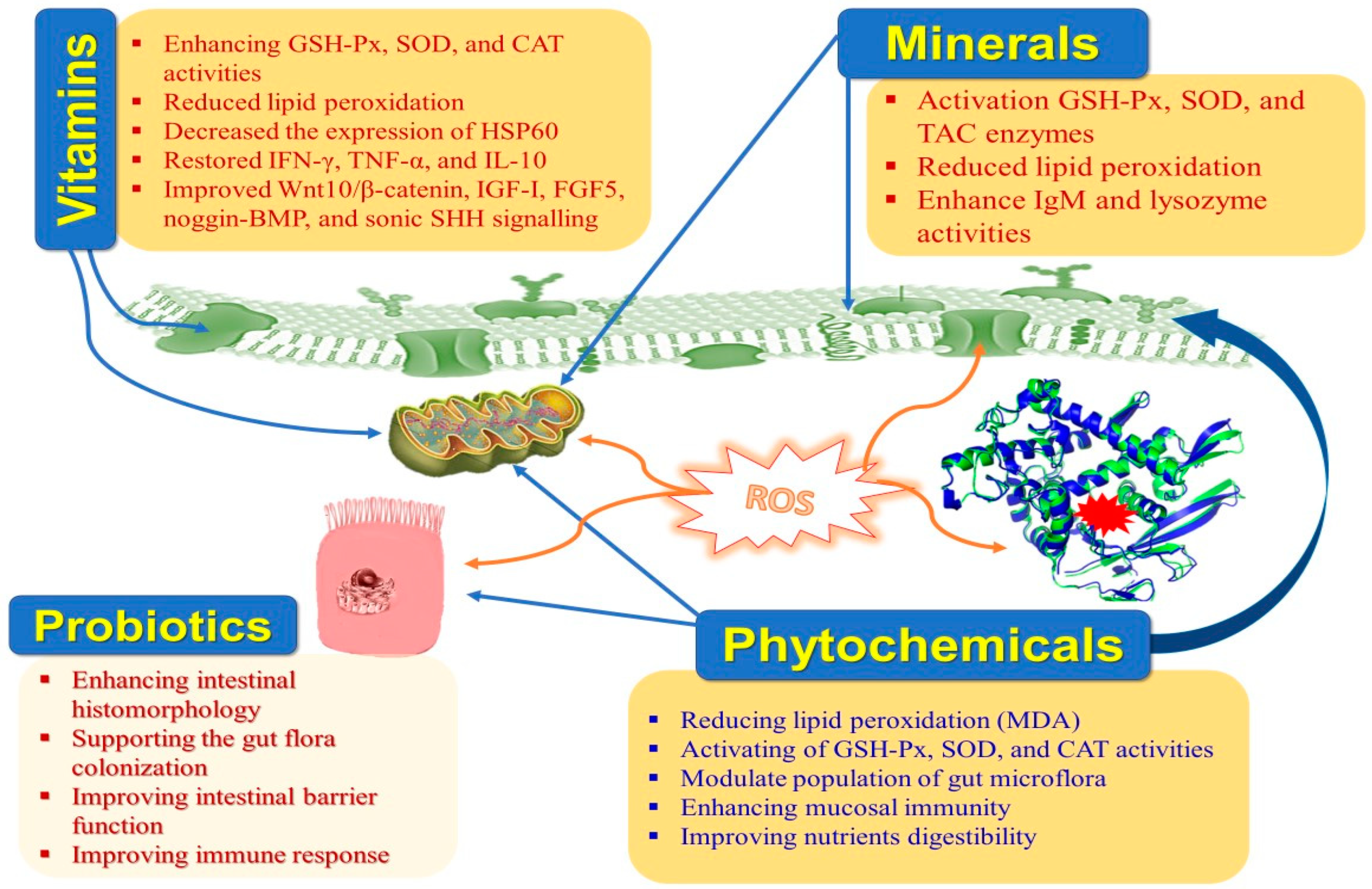
Figure 2.
Main impacts of nutraceuticals on heat-stressed rabbits.
Moreover, there is a growing interest in natural alternatives to antibiotics that could be used in rabbit production and antibiotic-free rabbit meat. Several dietary supplements—including vitamins, minerals, and enzymes—are already utilized to preserve the normal physiological status, support immunological responsiveness, and improve rabbit productivity in thermo-neutral and HS circumstances [14,15,16,17][14][15][16][17]. It has been suggested that probiotics, prebiotics, synbiotics, and organic acids could replace antibiotic growth enhancers in rabbit production because they promote a healthy intestinal environment [18,19,20,21][18][19][20][21]. Furthermore, phytobiotics or phytogenics are being utilized more frequently in rabbit nutrition as antioxidants, physiological stimulants, flavorings, digestive aids, and colorants, and for protecting and treating different pathological troubles [22,23,24][22][23][24].
2. Effect of HS on Growth Performance
It is well known that HS is the highest hazard factor deteriorating growing rabbits’ growth performance and viability [4,8,25,26,27,28,29][4][8][25][26][27][28][29]. Farghly et al. [8] reported that rabbits were susceptible to HS, which resulted in deteriorating growth performance indicators in terms of body weight (BW), body weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR). Similarly, Matics et al. [3] noted that HS negatively influenced the growing rabbits’ FI, BWG, BW, and fat deposits. During HS circumstances, rabbits attempt to disperse the extra heat generated inside the body by reducing FI, and this FI decline could be about 28–38% [4,25][4][25]. Besides, under HS conditions, growing rabbits prefer to direct energy toward heat dissipation rather than the growth and building of muscles and tissues [30]. Moreover, HS suppresses the hypothalamus’s appetite–satiety center and enhances leptin and adiponectin secretion, decreasing FI [30,31][30][31]. Furthermore, elevating the ambient temperature reduced digestion [32] and absorption of nutrients [33], which, coupled with decreasing FI, almost resulted in reducing the supply of essential nutrients, leading to a deteriorating growth rate, final BW, meat quality traits, antioxidative status, and immune responsiveness in fattening rabbits [4,34][4][34]. Sirotkin et al. [26] pointed out that exposure to HS resulted in suppressing growth parameters (FI, FCR, and viability), reducing serum insulin-like growth factor 1 (IGF-I) content, and increasing serum corticosterone concentration and mortality of growing rabbits. From another point of view, several studies elucidated that HS harmed thyroid activity in the form of reducing serum concentrations of triiodothyronine (T3) and thyroxine (T4), which resulted in retardation of protein synthesis and an increase of protein destruction, leading to the suppression of the growth rate in growing rabbits [29,35][29][35].3. Effect of HS on Reproductive Performance
In general, HS negatively impacted reproductive performance in female and male rabbits, which posed a danger to the rabbit business in hot and semi-hot climates [36,37][36][37]. Rabbits subjected to HS conditions had a decrease in fertility, embryo development, litter size, litter weight, and milk production [28,37][28][37]. Marco-Jimenez et al. [37] reported that maternal exposure to high environmental temperatures decreased litter weight, litter size, and kit weight at birth, while the stillborn rate was greater in heat-stressed does during pregnancy.Table 1.
Nutraceuticals’ potential to alleviate heat stress impacts in rabbit production.
| Additives | Level | Heat Stress Conditions | Animal | Main Impacts | References |
|---|---|---|---|---|---|
| Vitamin C | 0.5 g/kg dietfrom 5 to 14 wk of age | 32.44 °C and 84.67% relative humidity | Giant Flander male growing rabbits |
|
[38] |
| Vitamin C | 200 mg/kg diet from 6 to 12 wk of age | 28–39 °C and 60% relative humidity | New Zealand White growing rabbits |
|
[39] |
| Vitamin E | 0.25 g/kg diet from 7 to 14 wk of age | 36.4 °C and 97% relative humidity | Californian unsexed growing rabbits |
|
[40] |
| Vitamin E | 100 mg/kg diet for 3 months | 32.9 °C and 80.38% relative humidity | New Zealand White rabbits does |
|
[41] |
| Vitamin A | 12,000 IU/kg diet | 30–34 °C | Rex rabbits |
|
[42] |
| Selenium | 25 and 50 mg of nano-Se/kg diet from 7 to 13 wk of age | 33 °C and 90% relative humidity | Domestic rowing rabbits |
|
[43] |
| Selenium | 0.3 mg organic Se/kg diet for 12 wk | 31 °C and 75% relative humidity | Adult V-line male rabbits |
|
[44] |
| Zinc | 20, 40, 60, and 80 mg nano-Zn/kg dietfor 60 d | 38.20–40.10 °C and 45–50% relative humidity | New Zealand White male growing rabbits |
|
[16] |
| Zinc | 75 mg ZnSO4/kg diet or 75 mg Zn picolinate/kg diet from 32 to 42 wk of age | 30.7–37.6 °C and 70–80% relative humidity | New Zealand White rabbit bucks |
|
[45] |
| Copper | 200 mg Cu-methionine/kg diet or 200 mg copper-glycine/kg diet for 5 wk | 30.12 °C and 82.40% relative humidity | V line unsexed growing rabbits |
|
[46] |
| Copper | 100 mg Cu-acetate/kg diet or 50 mg nano-Cu/kg diet from 5 to 14 wk of age | 17 and 22 °C | New Zealand White unsexed growing rabbits |
|
[47] |
| Chromium | 0.4–1.6 mg organic Cr/kg diet | 30 °C and 80% relative humidity | Growing rabbits |
|
[48] |
| Chromium | 2.5 mg Cr-yeast/kg diet for 198 d | 29.3 °C and 71% relative humidity | New Zealand White male rabbits |
|
[49] |
| Probiotics | 5 × 106 CFU Clostridium butyricum, 2 × 108 CFU Enterococcus faecium, or 2.5 × 106 CFU C. butyricum + 1 × 108 CFU E. faecium/kg diet from 35 to 91 d of age | 31.78 °C and 60.19% relative humidity | New Zealand White male growing rabbits |
|
[50] |
| Probiotics | 3 × 109 CFU Saccharomyces cerevisiae/kg or 3 × 109 CFU Lactobacillus acidophilus/kg from 5 to 13 wk of age | 33 °C and 81% relative humidity | New Zealand White growing rabbits |
|
[51] |
| Prebiotics | 0.3% mannan-oligosaccharides or 0.05% isomalto-oligosaccharide from 6 to 16 wk of age | 19 °C | New Zealand White male growing rabbits |
|
[19] |
| Prebiotics | 3 g Bio-Mos®/kg diet from 4 to 12 wk of age | 31.50 °C and 79.07% relative humidity | New Zealand White male growing rabbits |
|
[7] |
| Moringa oleifera | 200 mg Moringa oleifera leaves powder/kg BW daily for 4 wk (from 32 to 32 wk of age) | 35 °C and 80% relative humidity | New Zealand White male rabbits |
|
[52,53][52][53] |
| Moringa oleifera | 50 mg Moringa oleifera leaves ethanolic extract/kg BW for 12 consecutive weeks | 31.11 °C and 87% relative humidity | V-line rabbit bucks |
|
[54] |
| Ginger | 7.5 g ginger powder/kg diet from 5 to 13 wk of age | 33 °C and 74.5% relative humidity | APRI growing rabbits |
|
[55] |
| Ginger | 250 mg ginger/doe/d for 8 wk | 35 °C and 80% relative humidity | New Zealand White virgin female rabbits |
|
[56] |
| Thyme | 16 g thyme/kg diet for 90 d | 39 °C and 30–35% relative humidity | New Zealand White male rabbits |
|
[57] |
| Thyme | 100 thyme essential oil mg/kg diet from 6 to 9 months of age | 33 °C and 80% relative humidity | APRI rabbit does |
|
[58] |
| Turmeric | 2.5 g turmeric nanoparticles/kg diet from 5 to 13 wk of age | 32.77 °C and 43.23% relative humidity | APRI growing rabbits |
|
[59] |
| Maca (Lepidium meyenii) | 400 or 600 mg maca extract/head twice weekly | 33 °C and 80% relative humidity | V-line rabbit bucks |
|
[24] |
| Pumpkin | 2 mL pumpkin seed essential oil/kg diet from 5 to 13 wk of age | 38 °C and 60% relative humidity | New Zealand White male growing rabbits |
|
[60] |
4. Effect of HS on Carcass Traits and Meat Quality
Carcass characteristics and meat quality parameters of rabbits are very important criteria for consumer acceptance. Several studies elucidated that HS negatively affected carcass and meat quality traits [3,66][3][66]. Matics et al. [4] noted that a high ambient temperature hurt slaughter weight, hot carcass weight, chilled carcass weight, and reference carcass weight in growing rabbits. Zeferino et al. [66] observed that HS reduced slaughter weight, carcass weight, and relative weights of internal organs (thoracic viscera, liver, and kidneys), decreasing meat juiciness and meat color (redness and yellowness) while increasing cooking loss. HS did not influence other meat quality characteristics, including pH (24 h and 48 h), water-holding capacity, and the Warner–Bratzler force [66]. Contrarily, Dahmani et al. [27] postulated that HS did not significantly influence the carcass yield %, forelegs %, hind legs %, and loin %. In contrast, the liver %, kidney %, peritoneal fat %, and inter-scapular fat % were reduced in fattening rabbits. Similarly, Matics et al. [4] noted that HS harmed perirenal and scapular fat percentages in growing rabbits. Additionally, Zeferino et al. [66] concluded that heat-stressed rabbits had lower fat depots. Meanwhile, Liu et al. [29] elucidated that chronic HS decreased the liver index (%), while the shoulder fat % and kidney fat % were increased.5. Effect of HS on the Intestinal Microbiome
The intestinal microbiome performs a vital task in gut action and health and is involved in nutrient digestion, immune response, and productiveness [75,76][75][76]. The highest percentage of digestive disorders is noticed in juvenile rabbits, mostly during the weaning period (feed transmission, handling, stressful factors, etc.). Such disturbances might be linked to unbalance and instability in intestinal microbiota and the inability of nonspecific and specific immune responses to combat harmful pathogens effectively [77]. Environmental stressors, mainly HS, can modify the balance of the gut flora in growing rabbits [78,79,80][78][79][80]. Bai et al. [79] postulated that thermal stress increased the number of Firmicutes, Proteobacteria, and Verrucomicrobiota at the phylum class, while reducing the Bacteriodota number in growing rabbits. Liu et al. [29] reported that HS affected the cecal microflora and increased the number of cecal Proteobacteria of Proteus, and reduced the number of Lachnospiraceae, Ruminococcaceae, and Candidatus saccharimonas, which may lead to inflammatory diseases in growing rabbits. Yasoob et al. [81] demonstrated that HS induced an imbalance in cecal microbiota by increasing the quantity of Proteobacteria in growing rabbits. El-Badawi et al. [9] noted that HS increased the total count of pathogenic bacteria such as Salmonella, E. coli, Staphylococcus aureus, Clostridium perfringens, and molds in the small intestine and cecum of growing rabbits. Moreover, Patra and Kar [82] documented that HS causes injury to the mucosal epithelia’s structure and deteriorates the intestinal barrier function, increasing intestinal permeability to toxins and pathogens in farm animals. This damage increased the sensitivity to oxidative stress insults and inflammation.6. Effect of HS on Antioxidative Properties
Under thermoneutral conditions, there is an equilibrium between the generation and elimination of free radicals (e.g., ROS) by the antioxidative system. Enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) scavenge reactive oxygen species (ROS), which are neutralized by the antioxidative defence system [83]. While under HS conditions, the redox balance is disturbed, and consequently, the ROS generation is elevated, leading to oxidative stress in rabbits [62,79,84][62][79][84]. Saghir et al. [34] indicated that exposure to HS resulted in reducing the activities of antioxidative enzymes, including GSH-Px, SOD, and CAT, and increasing serum oxidative markers such as protein carbonyl (as an index of amino acids oxidation) and malondialdehyde (MDA, as an index of lipids peroxidation) in growing rabbits. Similarly, several studies revealed that the plasma concentration of GSH-Px, SOD, and CAT was significantly minimized, while the plasma level of MDA was elevated in heat-stressed rabbits [84,85][84][85]. Likewise, Madkour et al. [86] observed that HS at 36 °C lessened the amounts of SOD, GSH, and CAT, and elevated the MDA concentration in the blood plasma and muscle of broiler rabbits. Bai et al. [79] postulated that the plasma total antioxidant capacity (TAC) concentration was reduced in growing rabbits under HS conditions. Moreover, thermal stress hurts metabolism by increasing the contents of 4-pyridoxic acid, kynurenine, 20-OH-leukotriene B4, and dopamine. It reduces pyridoxal’s value, making rabbits susceptible to inflammatory and oxidative stress [79]. Yasoob et al. [81] noted that HS generated cecal oxidative stress, whereas the MDA concentration in cecal mucosa was elevated in growing rabbits subjected to HS conditions. Madkour et al. [87] elucidated that HS had an unfavorable effect on hepatic antioxidative status, including reduced glutathione (GSH), SOD, and CAT concentrations, while the MDA concentration was increased in fattening rabbits. In female rabbits, Mutwedu et al. [62] demonstrated that exposing rabbits to HS (35–36 °C) caused oxidative stress, reduced the renal values of CAT, SOD, and GSH-Px, and raised the renal content of MDA.7. Effect of HS on Immune Responsiveness
Inhibition of humoral and cell-mediated immune responses was seen in rabbits exposed to cyclic or chronic HS [87,88][87][88]. HS inhibits the immune system components and disturbed homeostasis in rabbits [89]. Liu et al. [29] noted that HS harmed growing rabbits’ thymus index (%). Saghir et al. [34] indicated that HS induced the raising of pro-inflammatory cytokines containing tumor necrosis factor-α (TNF-α), IL-1β, and interferon-gamma (IFNγ) in growing rabbits. These results conform with reports postulated that HS stimulated inflammatory signaling, including TNF-α, IL-1β, and IFNγ in heat-stressed rabbits [43,79,87,89][43][79][87][89]. Yasoob et al. [81] noted that HS adversely affected the mucosal immune response and increased cecal concentrations of TNF-α, IL-1α, and IL-1β as markers of cecal mucosa inflammation in growing rabbits. Additionally, in fattening rabbits exposed to HS, the serum lysosome activity and nitric oxide levels were reduced [34]. Moreover, HS disturbed the equilibrium between anti-inflammatory and pro-inflammatory cytokines [34], which might be connected with a progressive inflammation response [86]. Abdel-Latif et al. [39] observed that HS had a negative influence on IFN-γ, TNF-α, and heat shock protein 70 (HSP70) expression leading to affect the infiltration of regulatory T cells adversely and NK cells in New Zealand White (NZW) growing rabbits. From another point of view, normal thyroid hormone concentrations are essential for the proper function of the immune system [35]. Exposure to HS suppresses the hypothalamic–pituitary–thyroid axis and reduces the serum concentrations of T3 and T4 [29,61][29][61], and, finally, depressing the immune response in growing rabbits. Furthermore, considering the link between oxidative stress and inflammation, it might be indicated that rabbits exposed to HS are under a penalty of oxidative stress, which might adversely affect their health status.References
- Dalle Zotte, A.; Szendrő, Z. The Role of Rabbit Meat as Functional Food. Meat Sci. 2011, 88, 319–931.
- Abdel-Moneim, A.E.; Shehata, A.M.; Khidr, R.E.; Paswan, V.K.; Ibrahim, N.S.; El-Ghoul, A.A.; Aldhumri, S.A.; Gabr, S.A.; Mesalam, N.M.; Elbaz, A.M.; et al. Nutritional Manipulation to Combat Heat Stress in Poultry—A Comprehensive Review. J. Therm. Biol. 2021, 98, 102915.
- Liang, Z.L.; Chen, F.; Park, S.; Balasubramanian, B.; Liu, W.C. Impacts of Heat Stress on Rabbit Immune Function, Endocrine, Blood Biochemical Changes, Antioxidant Capacity and Production Performance, and the Potential Mitigation Strategies of Nutritional Intervention. Front. Vet. Sci. 2022, 9, 906084.
- Matics, Z.; Gerencsér, Z.; Kasza, R.; Terhes, K.; Nagy, I.; Radnai, I.; Dalle Zotte, A.; Cullere, M.; Szendrő, Z. Effect of Ambient Temperature on the Productive and Carcass Traits of Growing Rabbits Divergently Selected for Body Fat Content. Animal 2021, 15, 100096.
- Oladimeji, A.M.; Johnson, T.G.; Metwally, K.; Farghly, M.; Mahrose, K.M. Environmental Heat Stress in Rabbits: Implications and Ameliorations. Int. J. Biometeorol. 2022, 66, 1–11.
- Marai, I.F.M.; Haeeb, A.A.M.; Gad, A.E. Biological Functions in Young Pregnant Rabbit Does as Affected by Heat Stress and Lighting Regime under Subtropical Conditions of Egypt. Trop. Subtrop. Agroecosyst. 2007, 7, 165–176.
- Ayyat, M.S.; Al-Sagheer, A.A.; Abd El-Latif, K.M.; Khalil, B.A. Organic Selenium, Probiotics, and Prebiotics Effects on Growth, Blood Biochemistry, and Carcass Traits of Growing Rabbits During Summer and Winter Seasons. Biol. Trace. Elem. Res. 2018, 186, 162–173.
- Farghly, M.F.A.; Mahrose, K.M.; Mahmoud, G.B.; Ali, R.M.; Daghash, W.; Metwally, K.A.; Abougaba, M.S. Lighting Programs as an Appliance to Improve Growing New Zealand White Rabbit’s Performance. Inter. J. Biometeorol. 2020, 64, 1295–1303.
- El-Badawi, A.Y.; El-Wardany, I.; Abd El-Moez, S.I.; Helal, F.I.S.; Ali, N.G.M.; Shourrap, M.I.; Aboelazab, O.M. Impact of Dietary Moringa oleifera Leaves on Intestinal Pathogenic Load and Histological Structure of Growing Rabbits Raised under Heat-Stress Conditions. Anim. Prod. Sci. 2018, 58, 1901–1907.
- Abdelsalam, M.; Al-Homidan, I.; Ebeid, T.; Abou-Emera, O.; Mostafa, M.; Abd El-Razik, M.; Shehab-El-Deen, M.; Abdel Ghani, S.; Fathi, M. Effect of Silver Nanoparticles Administration on Productive Performance, Blood Parameters, Antioxidative Status, and Silver Residues in Growing Rabbits under Hot Climate. Animals 2019, 9, 845.
- Ebeid, T.A.; Ketta, M.; Al-Homidan, I.H.; Barakat, H.; Abdel-Moneim, E.A.M. In Ovo Feeding of Nutraceuticals and its Role in Adjusting the Gastrointestinal Tract, Antioxidative Properties, Immunological Response, and Performance in Poultry: An Updated Review. Czech J. Anim. Sci. 2023, 68, 1–16.
- Tůmová, E.; Chodová, D.; Volek, Z.; Ebeid, T.A.; Ketta, M.; Skřivanová, V. A Comparative Study on the Effect of Quantitative Feed Restriction in Males and Females of Broiler Chickens, Rabbits and Nutrias. I. Performance and Carcass Composition. Czech J. Anim. Sci. 2022, 67, 47–54.
- Tůmová, E.; Chodová, D.; Volek, Z.; Ebeid, T.A.; Ketta, M.; Skřivanová, V. A Comparative Study on the Effect of Quantitative Feed Restriction in Males and Females of Broiler Chickens, Rabbits and Nutrias. II. Meat Quality. Czech J. Anim. Sci. 2022, 67, 55–64.
- Dalle Zotte, A.; Cullere, M.; Gleeson, E.Y.; Cossu, M.E. Animal Fat and Vitamin E in Rabbit Diets: Total Tract Apparent Digestibility, Growth Performance, Carcass and Meat Quality Traits. Czech J. Anim. Sci. 2020, 65, 380–388.
- Al-Homidan, I.; Fathi, M.; Abdelsalam, M.; Ebied, T.; Abou-Emera, O.; Mostafa, M.; Abd El-Razik, M.; Shehab-El-Deen, M. Effect of Propolis Supplementation and Breed on Growth Performance, Immunity, Blood Parameters and Cecal Microbiota in Growing Rabbits. Anim. Biosci. 2022, 35, 1606–1615.
- Abdel-Wareth, A.A.A.; Amer, S.A.; Mobashar, M.; El-Sayed, H.G.M. Use of Zinc Oxide Nanoparticles in the Growing Rabbit Diets to Mitigate Hot Environmental Conditions for Sustainable Production and Improved Meat Quality. BMC Vet. Res. 2022, 18, 354.
- Abdel-Wareth, A.A.A.; Mohamed, E.M.H.; Hassan, H.A.; Eldeek, A.A.; Lohakare, J. Effect of Substituting Hydroponic Barley Forage with or without Enzymes on Performance of Growing Rabbits. Sci. Rep. 2023, 13, 943.
- El-Deep, M.H.; Dawood, M.A.O.; Assar, M.H.; Ahamad Paray, B. Aspergillus awamori Positively Impacts the Growth Performance, Nutrient Digestibility, Antioxidative Activity and Immune Responses of Growing Rabbits. Vet. Med. Sci. 2021, 7, 226–235.
- Abd El-Aziz, A.H.; El-Kasrawy, N.I.; Abd El-Hack, M.E.; Kamel, S.Z.; Mahrous, U.E.; El-Deeb, E.M.; Atta, M.S.; Amer, M.S.; Naiel, M.A.E.; Khafaga, A.F.; et al. Growth, Immunity, Relative Gene Expression, Carcass Traits and Economic Efficiency of Two Rabbit Breeds Fed Prebiotic Supplemented Diets. Anim. Biotechnol. 2022, 33, 417–428.
- Abd El-Aziz, A.H.; Abo Ghanima, M.M.; Alsanie, W.F.; Gaber, A.; Alsenosy, A.E.; Easa, A.A.; Moawed, S.A.; Raza, S.H.A.; Elfadadny, A.; Yossef, H.A.; et al. Fructooligosaccharide Supplementation Boosts Growth Performance, Antioxidant Status, and Cecal Microbiota Differently in Two Rabbit Breeds. Animals 2022, 12, 1528.
- Nwachukwu, C.U.; Aliyu, K.I.; Ewuola, E.O. Growth Indices, Intestinal Histomorphology, and Blood Profile of Rabbits Fed Probiotics- and Prebiotics-Supplemented Diets. Transl. Anim. Sci. 2021, 5, txab096.
- Fathi, M.; Abdelsalam, M.; Al-Homidan, I.; Ebeid, T.; Shehab-El-Deen, M.; Abd El-Razik, M.; Abou-Emera, O.; Mostafa, M. Supplemental Effects of Eucalyptus (Eucalyptus camaldulensis) Leaves on Growth Performance, Carcass Characteristics, Blood Biochemistry and Immune Response of Growing Rabbits. Ann. Anim. Sci. 2019, 19, 779–791.
- Mutwedu, V.B.; Nyongesa, A.W.; Kitaa, J.M.; Ayagirwe, R.B.B.; Baharanyi, C.; Mbaria, J.M. Effects of Moringa oleifera Aqueous Seed Extracts on Reproductive Traits of Heat-Stressed New Zealand White Female Rabbits. Front. Vet. Sci. 2022, 9, 883976.
- Ragab, M.A.; Hassan, M.A.E.; Shazly, S.A.; El-Kholany, M.E.; Ahmed, M.E.; El-Raghi, A.A. The Benefits of Maca (Lepidium meyenii) Extract Administration for Male Rabbits Affected by Environmental Heat Stress. J. Anim. Physiol. Anim. Nutr. 2023, 107, 286–297.
- Szendrő, Z.; Papp, Z.; Kustos, K. Effect of Ambient Temperature and Restricted Feeding on the Production of Rabbit Does and Their Kits. Acta Agraria. Kaposváriensis 2018, 22, 1–17.
- Sirotkin, A.V.; Parkanyi, V.; Pivko, J. High Temperature Impairs Rabbit Viability, Feed Consumption, Growth and Fecundity: Examination of Endocrine Mechanisms. Domest. Anim. Endocrinol. 2021, 74, 106478.
- Dahmani, Y.; Benali, N.; Saidj, D.; Chirane, M.; Ainbaziz, H.; Temim, S. Effects of Heat Stress on Growth Performance, Carcass Traits, Physiological Components, and Biochemical Parameters in Local Algerian Growing Rabbits. World Vet. J. 2022, 12, 405–417.
- Ragab, M.A.; Shazly, S.A.; Ibrahem, M.A.; El-Kholany, M.E.; Khalil, W.A. Black Maca (Lepidium meyenii Walp.) Hydroalcoholic Extract as an Ameliorating Agent Against Heat Stress Conditions of V-Line Rabbit Does. Sustainability 2022, 14, 15154.
- Liu, H.; Zhang, B.; Li, F.; Liu, L.; Yang, T.; Zhang, H.; Li, F. Effects of Heat Stress on Growth Performance, Carcass Traits, Serum Metabolism, and Intestinal Microflora of Meat Rabbits. Front. Microbiol. 2022, 13, 998095.
- Slimen, B.I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412.
- Bakr, M.H.; Tusell, L.; Rafel, O.; Terré, M.; Sánchez, J.P.; Piles, M. Lactating Performance, Water and Feed Consumption of Rabbit Does Reared under a Mediterranean Summer Circadian Cycle of Temperature v. Comfort Temperature Conditions. Animal 2015, 9, 1203–1209.
- Al-Sagheer, A.A.; Daader, A.H.; Gabr, H.A.; Abd El-Moniem, E.A. Palliative Effects of Extra Virgin Olive Oil, Gallic Acid, and Lemongrass Oil Dietary Supplementation on Growth Performance, Digestibility, Carcass Traits, and Antioxidant Status of Heat-Stressed Growing New Zealand White Rabbits. Environ. Sci. Pollut. Res. 2017, 24, 6807–6818.
- Ayyat, M.S.; El-Latif, A.; Khaled, M.; Helal, A.A.; Al-Sagheer, A.A. Interaction of Supplementary L-Carnitine and Dietary Energy Levels on Feed Utilization and Blood Constituents in New Zealand White Rabbits Reared under Summer Conditions. Trop. Anim. Health. Prod. 2021, 53, 279.
- Saghir, S.A.M.; Hroob, A.M.; Majrashi, K.A.; Jaber, F.A.; Abduh, M.S.; Al-Gabri, N.; Albaqami, N.M.; Abdelnour, S.A.; Alqhtani, A.H.; Abd El-Hack, M.E.; et al. Effects of Alginates on the Growth, Haematological, Immunity, Antioxidant and Pro-inflammatory Responses of Rabbits under High Temperature. Res. Vet. Sci. 2023, 155, 36–43.
- Hassan, R.A.; Ebeid, T.A.; Abd El-Lateif, A.I.; Ismail, N.B. Effect of Dietary Betaine Supplementation on Growth, Carcass and Immunity of New Zealand White Rabbits under High Ambient Temperature. Livest. Sci. 2011, 135, 103–109.
- Marai, I.F.M.; Habee, A.A.M.; Gad, A.E. Rabbits’ Productive, Reproductive and Physiological Performance Traits as Affected by Heat Stress: A Review. Livest. Prod. Sci. 2002, 78, 71–90.
- Marco-Jiménez, F.; García-Diego, F.J.; Vicente, J.S. Effect of Gestational and Lactational Exposure to Heat Stress on PERFORMANCE in Rabbits. World Rabbit Sci. 2017, 25, 17–25.
- Hassan, F.A.; Shalaby, A.G.; Elkassas, N.E.M.; El-Medany, S.A.; Hamdi Rabie, A.; Mahrose, K.; Abd El-Aziz, A.; Bassiony, S. Efficacy of Ascorbic Acid and Different Sources of Orange Peel on Growth Performance, Gene Expression, Anti-Oxidant Status and Microbial Activity of Growing Rabbits under Hot Conditions. Anim. Biotechnol. 2022, 23, 1–12.
- Abdel-Latif, M.; Sakran, T.; Badawi, Y.K.; Abdel-Hady, D.S. Influence of Moringa oleifera Extract, Vitamin C, and Sodium Bicarbonate on Heat Stress-Induced Hsp70 Expression and Cellular Immune Response in Rabbits. Cell Stress Chaperone. 2018, 23, 975–984.
- Sherif, S.K. Response of Growing Rabbits to Stoking Density and Dietary Supplementation with Ascorbic Acid and Vitamin E under Summer Conditions. Egypt. Poult. Sci. J. 2018, 38, 831–846.
- El-Ratel, I.; Gabr, A.A. Effect of Spirulina and Vitamin E on Reproduction and in vitro Embryo Production in Heat-Stressed Rabbits. Pak. J. Biol. Sci. 2019, 22, 545–553.
- Yue, Z.; Li, C.; Liu, Y.; Liu, M.; Zhao, M.; Li, F.; Liu, L. Vitamin A alleviates Heat Stress-Induced Damage to Hair Follicle Development in Rex Rabbits. J. Sci. Food Agric. 2022, 102, 2291–2299.
- Sheiha, A.M.; Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Metwally, K.A.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; El-Saadony, M.T. Effects of Dietary Biological or Chemical-Synthesized Nano-Selenium Supplementation on Growing Rabbits Exposed to Thermal Stress. Animals 2020, 10, 430.
- Hosny, N.S.; Hashem, N.M.; Morsy, A.S.; Abo-Elezz, Z.R. Effects of Organic Selenium on the Physiological Response, Blood Metabolites, Redox Status, Semen Quality, and Fertility of Rabbit Bucks Kept under Natural Heat Stress Conditions. Front. Vet. Sci. 2020, 7, 290.
- El-Kholy, M.S.; El-Mekkawy, M.M.; Madkour, M.; Abd El-Azeem, N.; Di Cerbo, A.; Mohamed, L.A.; Alagawany, M.; Selim, D.A. The Role of Different Dietary Zn Sources in Modulating Heat Stress-related Effects on SomeThermoregulatory Parameters of New Zealand White Rabbit Bucks. Anim. Biotechnol. 2021, 23, 1–10.
- Goodb, F.; Soliman, F.; Elghalid, O.; Abd El-Hady, A.M. Can copper amino acid chelates reduce the physiological strain of growing rabbits under summer conditions? World Rabbit Sci. 2022, 30, 277–286.
- Al-Sagheer, A.A.; Abdel-Rahman, G.; Elsisi, G.F.; Ayyat, M.S. Comparative Effects of Supplementary Different Copper Forms on Performance, Protein Efficiency, Digestibility of Nutrients, Immune Function and Architecture of Liver and Kidney in Growing Rabbits. Anim. Biotechnol. 2022, 22, 1–11.
- Huang, C.B.; Tang, L.; Guo, Z.Q.; Yan, J.Y.; Xie, X.H.; Lei, M. Effects of Organic Chromium on the Production Performance and Immune Function of Heat-Stressed Rabbits. Chin. J. Anim. Husb. 2017, 53, 93–95.
- El-Kholy, K.H.; Shabaan, H.M.A.; Gad-Alla, S.Z.; Abdel-Kafy, E.M.; Ghazal, M.N. Productive and Physiological Responses of New Zealand White Rabbit Males to Dietary Organic Chromium Addition. Egypt. J. Rabbit Sci. 2014, 24, 1–18.
- Bassiony, S.S.; Al-Sagheer, A.A.; El-Kholy, M.S.; Elwakeel, E.A.; Helal, A.A.; Alagawany, M. Evaluation of Enterococcus faecium NCIMB 11181 and Clostridium butyricum Probiotic Supplements in Post-Weaning Rabbits Reared under Thermal Stress Conditions. Ital. J. Anim. Sci. 2021, 20, 1232–1243.
- Hegab, I.M.; Attia, E.A.; Hassan, R.A.; El-Azzazi, F.E. Effect of Probiotics on Productive, Physiological and Microbiological Parameters of New Zealand White Rabbits Reared under Hot Summer Conditions. Egypt. Poult. Sci. 2019, 39, 599–614.
- Khalid, A.R.; Yasoob, T.B.; Zhang, Z.; Yu, D.; Feng, J.; Zhu, X.; Hang, S. Supplementation of Moringa oleifera Leaf Powder Orally Improved Productive Performance by Enhancing the Intestinal Health in Rabbits under Chronic Heat Stress. J. Therm. Biol. 2020, 93, 102680.
- Khalid, A.R.; Yasoob, T.B.; Zhang, Z.; Zhu, X.; Hang, S. Dietary Moringa oleifera Leaf Powder Improves Jejunal Permeability and Digestive Function by Modulating The Microbiota Composition and Mucosal Immunity in Heat Stressed Rabbits. Environ. Sci. Pollut. Res. Int. 2022, 29, 80952–80967.
- El-Desoky, N.I.; Hashem, N.M.; Elkomy, A.; Abo-Elezz, Z.R. Physiological Response and Semen Quality of Rabbit Bucks Supplemented with Moringa Leaves Ethanolic Extract During Summer Season. Animal 2017, 11, 1549–1557.
- Amber, K.; Badawy, N.A.; El-Sayd, A.E.A.; Morsy, W.A.; Hassan, A.M.; Dawood, M.A.O. Ginger Root Powder Enhanced the Growth Productivity, Digestibility, and Antioxidative Capacity to Cope with the Impacts of Heat Stress in Rabbits. J. Therm. Biol. 2021, 100, 103075.
- Habeeb, A.A.; El-Darawany, A.A.; Nasr, A.S.; Sharaf, A.K. Impact of Some Medicinal Plants Supplement on Pregnant Rabbits Diet During Hot Summer Season. Res. J. Med. Plants 2019, 13, 145–154.
- Ahmed, A.E.; Alkahtani, M.A.; Abdel-Wareth, A.A.A. Thyme Leaves as an Eco-Friendly Feed Additive Improves Both the Productive and Reproductive Performance of Rabbits under Hot Climatic Conditions. Vet. Med. Czech. 2020, 65, 65553–65563.
- Abdelnour, S.A.; El-Ratel, I.T.; Peris, S.I.; El-Raghi, A.A.; Fouda, S. Effects of Dietary Thyme essential oil, on Blood Haematobiochemical, Redox Status, Immunological and Reproductive Variables of Rabbit Does Exposed to High Environmental Temperature. Ital. J. Anim. Sci. 2022, 21, 51–61.
- El-Ratel, I.T.; Tag El-Din, T.E.H.; Bedier, M.M. Beneficial Effects of Curcumin as A Native or Nanoparticles Form on Productive Efficiency, Liver and Kidney Functions, Antioxidative Status and Immunity of Heat-Stressed Growing Rabbits. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1778–1787.
- Abdelnour, S.A.; Metwally, M.G.E.; Bahgat, L.B.; Naiel, M.A.E. Pumpkin Seed Oil–Supplemented Diets Promoted the Growth Productivity, Antioxidative Capacity, and Immune Response in Heat-Stressed Growing Rabbits. Trop. Anim. Health. Prod. 2023, 55, 55.
- García, M.L.; Argente, M.J. Exposure to High Ambient Temperatures Alters Embryology in Rabbits. Int. J. Biometeorol. 2017, 61, 1555–1560.
- Mutwedu, V.; Nyongesa, A.; Oduma, J.; Kitaa, J.; Mbaria, J. Thermal Stress Causes Oxidative Stress and Physiological Changes in Female Rabbits. J. Therm Biol. 2021, 95, 102780.
- Tang, L.; Bai, X.; Xie, X.; Chen, G.; Jia, X.; Lei, M.; Li, C.; Lai, S. Negative Effects of Heat Stress on Ovarian Tissue in Female Rabbit. Front. Vet. Sci. 2022, 9, 1009182.
- Somuncu, S.; Cakmak, M.; Dikmen, G.; Akman, H.; Kaya, M. Ischemia-Reperfusion Injury of Rabbit Ovary and Protective Effect of Trapidil: An Experimental Study. Pediatr. Surg. Int. 2008, 24, 315–318.
- Marco-Jiménez, F.; Naturil-Alfonso, C.; Peñaranda, D.S.; Jiménez-Trigos, E.; García-Diego, F.J.; Vicente, J.S. Maternal Exposure to High Temperatures Disrupts OCT4 mRNA Expression of Rabbit Pre-Implantation Embryos and Endometrial Tissue. Reprod. Domest. Anim. 2013, 48, 429–434.
- Zeferino, C.P.; Komiyama, C.M.; Fernandes, S.; Sartori, J.R.; Teixeira, P.S.; Moura, A.S. Carcass and Meat Quality Traits of Rabbits under Heat Stress. Animal 2013, 7, 518–523.
- Maya-Soriano, M.J.; Taberner, E.; Sabes-Alsina, M.; Ramon, J.; Rafel, O.; Tusell, L.; Piles, M.; Lopez-Bejar, M. Daily Exposure to Summer Temperatures Affects the Motile Subpopulation Structure of Epididymal Sperm Cells but Not Male Fertility in an In Vivo Rabbit Model. Theriogenology 2015, 84, 384–389.
- Sabés-Alsina, M.; Tallo-Parra, O.; Mogas, M.T.; Morrell, J.M.; Lopez-Bejar, M. Heat Stress Has an Effect on Motility and Metabolic Activity of Rabbit Spermatozoa. Anim. Reprod. Sci. 2016, 173, 18–23.
- Abdelnour, S.A.; Al-Gabri, N.A.; Hashem, N.M.; Gonzalez-Bulnes, A. Supplementation With Proline Improves Haemato-Biochemical and Reproductive Indicators in Male Rabbits Affected by Environmental Heat-Stress. Animals 2021, 11, 373.
- Sabés-Alsina, M.; Planell, N.; Torres-Mejia, E.; Taberner, E.; Maya-Soriano, M.J.; Tusell, L.; Ramon, J.; Dalmau, A.; Piles, M.; López-Béjar, M. Daily Exposure to Summer Circadian Cycles Affects Spermatogenesis, But Not Fertility in an in vivo Rabbit Model. Theriogenology 2015, 83, 246–252.
- Pei, Y.; Wu, Y.; Cao, J.; Qin, Y. Effects of Chronic Heat Stress on The Reproductive Capacity of Male Rex Rabbits. Livest. Sci. 2012, 146, 13–21.
- Jie, Z.; Chao, Y.; Min, L.; Li, T.; Zhang, X.Y.; Xie, X.H. The Effect of Heat Stress on the Reproductive Performance of Rabbits and the Research Progress of Related Heat Shock Proteins. Rabbit Rais. China 2020, 235, 19–22.
- Durairajanayagam, D.; Agarwal, A.; Ong, C. Causes, Effects and Molecular Mechanisms of Testicular Heat Stress. Reprod. Biomed. Online 2015, 30, 14–27.
- Zheng, J.; Xie, X.H.; Lei, M.; Tang, L.; Zhang, X.Y.; Yang, C. Research Progress on the Effect of Heat Stress on the Semen Quality of Male Rabbits and its Mechanism. Chin. Rabbit Rais. 2018, 6, 24–28.
- Fathi, M.; Abdelsalam, M.; Al-Homidan, I.; Ebeid, T.; El-Zarei, M.; Abou-Emera, O. Effect of Probiotic Supplementation and Genotype on Growth Performance, Carcass Traits, Hematological Parameters and Immunity of Growing Rabbits under Hot Environmental Conditions. Anim. Sci. J. 2017, 88, 1644–1650.
- Ebeid, T.A.; Al-Homidan, I.H.; Fathi, M.M. Physiological and Immunological Benefits of Probiotics and Their Impacts in Poultry Productivity. World’s Poul. Sci. J. 2021, 77, 883–899.
- Ebeid, T.A.; Tůmová, E.; Al-Homidan, I.H.; Ketta, M.; Chodová, D. The Potential Role of Feed Restriction on Productivity, Carcass Composition, Meat Quality, and Muscle Fibre Properties of Growing Rabbits: A Review. Meat Sci. 2022, 191, 108845.
- Blas, J.C.D. Nutritional Impact On Health and Performance in Intensively Reared Rabbits. Animal 2013, 7, 102–111.
- Bai, X.; Shi, Y.; Tang, L.; Chen, L.; Fan, H.; Wang, H.; Wang, J.; Jia, X.; Chen, S.; Lai, S. Heat Stress Affects Faecal Microbial and Metabolic Alterations of Rabbits. Front. Microbiol. 2022, 12, 817615.
- Li, F.; Liu, H.; Wu, X.; Liu, M.; Yue, Z.; Liu, L.; Li, F. Copper Modulates Mitochondrial Oxidative Phosphorylation to Enhance Dermal Papilla Cells Proliferation in Rex Rabbits. Int. J. Mol. Sci. 2022, 23, 6209.
- Yasoob, T.B.; Yu, D.; Khalid, A.R.; Zhang, Z.; Zhu, X.; Saad, H.M.; Hang, S. Oral Administration of Moringa oleifera Leaf Powder Relieves Oxidative Stress, Modulates Mucosal Immune Response and Cecal Microbiota After Exposure to Heat Stress in New Zealand White Rabbits. J. Anim. Sci. Biotechnol. 2021, 12, 66.
- Patra, A.K.; Kar, I. Heat Stress on Microbiota Composition, Barrier Integrity, and Nutrient Transport in Gut, Production Performance, and its Amelioration in Farm Animals. J. Anim. Sci. Technol. 2021, 63, 211–247.
- Ebeid, T.A.; Zeweil, H.S.; Basyony, M.M.; Dosoky, W.M.; Badry, H. Fortification of Rabbits Diets with Vitamin E or Selenium Affects Growth Performance, Lipid Peroxidation, Oxidative Status and Immune Response in Growing Rabbits. Livest. Sci. 2013, 155, 323–331.
- Jimoh, O.A.; Ayedun, E.S.; Oyelade, W.A.; Oloruntola, O.D.; Daramola, O.T.; Ayodele, S.O.; Omoniyi, I.S. Protective Effect of Soursop (Annona Muricata Linn.) Juice on Oxidative Stress in Heat Stressed Rabbits. J. Anim. Sci. Technol. 2018, 60, 28.
- Kuang, L.D.; Li, C.Y.; Guo, Z.Q.; Ren, Y.J.; Zheng, J.; Mei, X.L. Effects of Heat Stress on Reproductive Performance, Serum Biochemical Indexes and Reproductive Hormones in Female Rabbit of Qixing. Southwest China J. Agric. Sci. 2021, 34, 1323–1329.
- Madkour, M.; Shakweer, W.M.E.; Hashem, N.M.; Aboelazab, O.; Younis, E.; El-Azeem, N.A.; Shourrap, M. Antioxidants Status and Physiological Responses to Early and Late Heat Stress in Two Rabbit Breeds. J. Anim. Physiol. Anim. Nutr. 2023, 107, 298–307.
- Madkour, M.; Aboelenin, M.M.; Younis, E.; Mohamed, M.A.; Hassan, H.; Alagawany, M.; Shourrap, M. Hepatic Acute-Phase Response, Antioxidant Biomarkers and DNA Fragmentation of Two Rabbit Breeds Subjected to Acute Heat Stress. Ital. J. Anim. Sci. 2020, 19, 1558–1566.
- El-Desoky, N.I.; Hashem, N.M.; Gonzalez-Bulnes, A.; Elkomy, A.G.; Abo-Elezz, Z.R. Effects of A Nanoencapsulated Moringa Leaf Ethanolic Extract on The Physiology, Metabolism, and Reproductive Performance of Rabbit Does During Summer. Antioxidants 2021, 10, 1326.
- Abdelnour, S.A.; Hassan, M.A.E.; Mohammed, A.K.; Alhimaidi, A.R.; Al-Gabri, N.; Al-Khaldi, K.O.; Swelum, A.A. The Effect of Adding Different Levels of Curcumin and its Nanoparticles to Extender on Post-Thaw Quality of Cryopreserved Rabbit Sperm. Animals 2020, 10, 1508.
More
