Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Francesco Rizzetto and Version 2 by Fanny Huang.
Budd–Chiari syndrome (BCS) is a rare clinical entity characterized by hepatic venous outflow obstruction, resulting in liver congestion and subsequent chronic parenchymal damage. This condition often leads to the development of focal liver lesions, including benign focal nodular hyperplasia-like regenerative nodules, hepatocellular carcinoma, and perfusion-related pseudo-lesions. Computed tomography, ultrasound, and magnetic resonance are the commonly employed imaging modalities for the follow-up of BCS patients and for the detection and characterization of new-onset lesions. The accurate differentiation between benign and malignant nodules is crucial for optimal patient management and treatment planning.
- Budd-Chiari syndrome
- regenerative nodules
- imaging
1. Introduction
Budd–Chiari syndrome (BCS) is a rare disorder characterized by obstruction of hepatic venous outflow. The most prevalent form, defined as primary, is related to hypercoagulable conditions that lead to the formation of blood clots in the hepatic veins, affecting either the distal branches or the main vessels [1]. In rarer instances, primary BCS is caused by a membranous web-like obstruction in the inferior vena cava, usually of congenital origin, which may extend to the hepatic veins [2]. Conversely, secondary BCS occurs when the hepatic veins are externally compressed by an expansive mass or invaded by neoplastic growths [3].
Regardless of the underlying cause, BCS results in liver congestion with hepatocellular necrosis and atrophy, leading to the development of portal hypertension and parenchymal fibrosis within a few weeks of the venous obstruction. Consequently, liver regeneration mechanisms activate, including responsive hyperarterialization and hypertrophy in regions with preserved venous drainage [4]. These processes are responsible for hepatocellular proliferation, thereby facilitating the emergence of various types of lesions, such as regenerative nodules and hepatocellular carcinoma (HCC). Accurately distinguishing between benign and malignant lesions is crucial due to its significant implications for patient management and treatment decisions. In this regard, computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound (US) serve as the initial non-invasive diagnostic modalities for the detection and characterization of focal liver lesions in BCS.
2. Focal Nodular Hyperplasia-like Regenerative Nodules
Focal nodular hyperplasia-like regenerative nodules (FNH-like RNs) are the most common liver lesions in patients with BCS, with a reported prevalence of 36–44% [5][6][5,6]. These nodules, documented only in chronic disease, seem to develop because of the combination of impaired liver venous drainage, portal flow deprivation and increased hepatic arterialization. The parenchymal damage due to venous congestion is supposed to induce hepatocellular proliferation in the areas with preserved venous outflow, a process further supported by arterial neoformation and consequent hyperperfusion [7].
Histologically, these nodules are composed of hyperplastic hepatocytes and fibrovascular septa, tend to present scarce-to-absent portal supply, and may display large arteries branching radially from the lesion fibrous center, closely resembling FNH [8][9][10][8,9,10]. However, since FNH typically develops in healthy liver parenchyma [11], the definition of FNH-like RNs was suggested to emphasize their distinct nature [12]. As different nodules in the same liver can show different microscopic findings, and mixed patterns may be observed in the same nodule [13], FNH-like RNs in BCS could even be assumed to express a lesion spectrum between regenerative nodules and FNH. Unusual pathological characteristics include intranodular cholestasis and fatty degeneration [10], whereas hemorrhage and calcification have not been observed [14].
FNH-like RNs can involve all liver segments, with a predilection for the right liver (67–82%) [15][16][15,16] probably secondary to the right lobe atrophy and left lobe hypertrophy that characterize chronic stage BCS. To confirm the role of imbalanced vascular supply, a longitudinal study by Flor et al. [15] found that only 1% of FNH-like RNs were located in the caudate lobe. Indeed, the venous outflow of this segment, the only one to directly drain into the inferior vena cava, is usually preserved in BCS [17].
FNH-like RNs are most often numerous and variably sized, with a diameter usually ranging from 0.5 to 4 cm [18], although the lesions detectable on CT and MR imaging are only a tiny fraction of all the nodules actually present, with most of them being very small [14]. No specific distribution has been identified, although microscopic nodules show preferential localization in the periportal regions [19][20][19,20].
During follow-up the nodules can increase in size and/or number, likely contributing with their expansile growth to portal hypertension through compression of the central vein and portal spaces [7][18][7,18]. New FNH-like RNs may also appear after transjugular intrahepatic portosystemic shunt (TIPS) positioning, as a consequence of the hemodynamic changes that the procedure induces in the liver parenchyma [21]. However, up to 40% of the nodules can disappear over time [6], reflecting their reactive rather than neoplastic nature.
Size increase and changes in imaging characteristics are the main indications for performing liver biopsies in suspicious focal hepatic lesions, especially considering that cirrhosis and deranged venous outflow may induce HCC development. However, these variations in imaging features are not specific indicators of malignancy in patients with BCS. Furthermore, to the best available knowledge, there is no evidence of malignant transformation of FNH-like RNs.
Imaging Features
Being composed of nearly normal hepatocytes, >95% of FNH-like RNs are isodense to surrounding parenchyma on nonenhanced CT imaging (Figure 1A) [14]. In some cases, when located in the subcapsular region, they may be detected due to the alteration of liver contours. More rarely, they may appear hyperdense or hypodense compared to the liver.
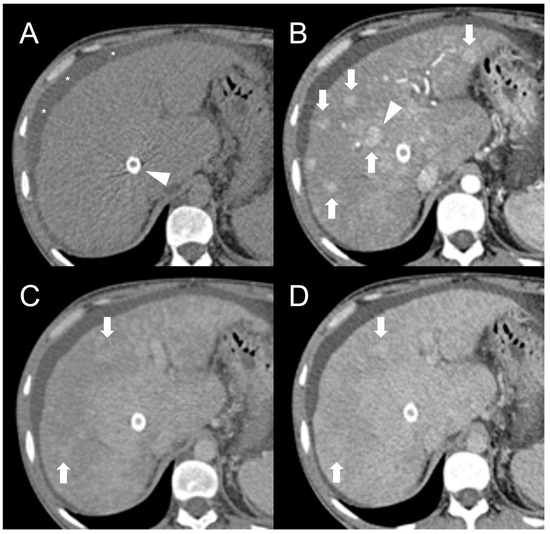
Figure 1. CT examination of 36-years-old male patient with Budd–Chiari syndrome. On unenhanced images (A), the liver parenchyma appears relatively homogeneous, and the presence of TIPS stent (arrowhead) and perihepatic ascites (asterisks) can be observed. On arterial phase (B), multiple homogeneously enhancing FNH-like RNs can be visualized in the liver (arrows), some exhibiting a hypodense perinodular rim (arrowhead) due to atrophic hepatic tissue with congested sinusoids. In both portal venous (C) and delayed phase (D), the regenerative nodules (arrows) become iso-dense or slightly hyperdense compared to the surrounding inhomogeneous liver parenchyma, making them difficult to detect.
After iodine contrast administration, the nodules are well evident on arterial phase images because they are hypervascularized, hence showing marked and homogeneous contrast enhancement in almost all cases (Figure 1B). Hypervascularization is atypical in cirrhotic regenerative nodules, but in FNH-like RNs associated with BCS it probably represents a compensatory response to regional loss of portal flow. On the portal and late venous phases, the FNH-like RNs usually remain isodense or slightly hyperdense (Figure 1C,D). A hypodense perinodular rim may also be observed on contrast enhanced CT imaging because of the presence of atrophic hepatic tissue around the nodule with sinusoidal congestion.
CT features of FNH-like RNs frequently change during follow-up. According to a retrospective longitudinal study by Flor et al. [15], nodules that were small (<15 mm) and homogeneously enhancing in the arterial phase became larger and showed a heterogeneous enhancing pattern, often with washout and a central scar, in 42% of cases after three years from detection.
In MRI imaging, most FNH-like RNs show a typical T1-hyperintensity (75–84%) [5][22][5,22], which is better appreciated on fat suppressed sequences. On T2-weighted images, their appearance has been variously reported [6][10][23][24][6,10,23,24], with most nodules being isointense or hypointense to the liver parenchyma and less frequently (<20%) hyperintense. Some authors suggested that these features of FN-like RNs depend on mineral deposits inside the lesions, especially copper [9][25][9,25]; however, they could also be explained by liver congestion resulting in relative T1-hypointensity and T2-hyperintensity of the perinodular parenchyma [10]. Considering that these nodules are drained by hepatic veins, the few cases of T1-hypointense or T2-hyperintense lesions may also be related to intralesional infarction when the venous outflow is compromised [14].
The presence of a central scar is another common finding in FNH-like RNs, particularly in those larger than 1 cm [10]. This scar typically appears as a hypointense, central stellate area on fat-suppressed T1-weighted imaging and a hyperintense area on T2-weighted imaging (Figure 2). Moreover, since regenerative nodules are made up of normal hepatocytes, they appear isointense or slightly hyperintense to the adjacent liver parenchyma on diffusion-weighted imaging, and there is no significant reduction in the diffusion values observed on the ADC map. These characteristics help to differentiate between regenerative and HCC nodules with reasonable confidence in patients with BCS.
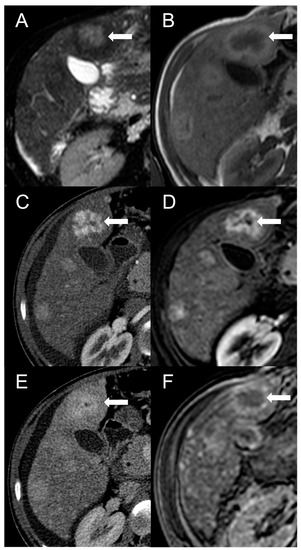
Figure 2. FNH-like RN with central scar (arrow) in a 34-year-old male patient with Budd–Chiari syndrome. In MRI, the scar is typically hyperintense on fat-saturated T2-weighted images (A) and hypointense in T1-weighted images (B). In the arterial phase after contrast agent administration, the scar can be identified as a central stellate area that appears hypodense in CT (C) and hypointense in MRI (D). In the CT delayed phase, the nodule shows increased density, but a central hypodense component representing the scar remains visible (E). In gradient-echo T1-weighted images acquired in the hepatobiliary phase (F), the scar is clearly depicted as a central low-signal area.
As described for CT imaging, FNH-like RNs usually exhibit marked homogeneous enhancement on arterial phase fat-suppressed T1-weighted images following the administration of gadolinium-based contrast agents. In some cases, a peripheral hypointense rim may also be present due to congestion and atrophy of the surrounding liver parenchyma. This enhancement pattern differs from that of cirrhotic hyperplastic nodules, which are T2 hypointense but typically appear hypovascular after contrast administration [26]. It is worth noting that less than 5% of FNH-like RNs are hypointense or isointense in the arterial phase [6].
On portal venous phase and delayed images, FNH-like RNs usually remain slightly hyperintense or isointense compared to the surrounding parenchyma [12]. However, in almost 10% of cases on portal venous phase images and 30–40% on delayed phase images, they may exhibit washout [22]. This finding has been suggested to be a “pseudo-washout” caused by perilesional congestion, resulting in increased signal intensity of the surrounding parenchyma and relative hypointensity of the FNH-like RNs [27]. Consequently, the portal venous and delayed phases do not aid in the characterization of nodules in BCS patients. Furthermore, 18% of FNH-like RNs larger than 1 cm are reported to show hypervascularity on the arterial phase and washout on subsequent phases [16], mimicking HCC. Due to the limited specificity of these features, the Liver Imaging Reporting and Data System (LI-RADS) criteria [28] and those proposed by the American Association for the Study of Liver Diseases (AASLD) [29] and the European Association for the Study of the Liver (EASL) [30] for the diagnosis of HCC in cirrhotic patients are not suitable for patients with BCS.
On the contrary, hepatobiliary phase (HBP) images acquired after administration of hepatobiliary contrast agents can allow us to distinguish the FNH-like RNs from HCC. The benign nodules often contain ductular proliferation [7], hence appearing iso- or more often hyperintense in HBP compared to the normal parenchyma like typical FNH. For this reason, the use of hepatobiliary contrast agents on MRI is mandatory in patients with hepatic nodules associated with BCS.
MRI features of FNH-like RNs are shown in Figure 3.
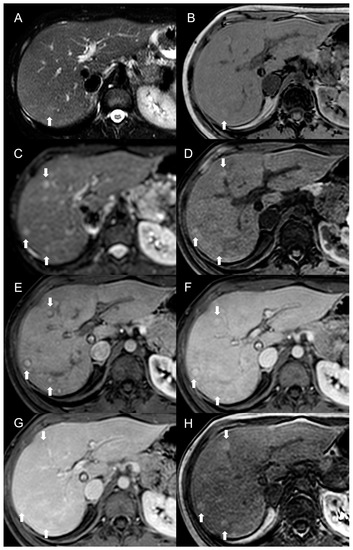
Figure 3. MRI examination of a 29-year-old female with Budd–Chiari syndrome. On fat-saturated T2-weighted images (A) FNH-like RNs are barely discernable as they appear isointense to the liver parenchyma; occasionally, areas of very slightly hyperintense signal (arrow) can be identified, possibly related to congested nodules or central scar. On out-of-phase T1-weighted images (B) the presence of nodular lesions is more appreciable, as FNH-like RNs (arrow) appear slightly hyperintense compared to the surrounding liver parenchyma. On DWI (b-value = 600) (C), FNH-like RNs may show a slight heterogeneous hyperintensity due to central scar or congestion. On unenhanced gradient-echo T1-weighted images (D), the nodules (arrows) appear hyperintense, while on arterial phase (E) they show vivid enhancement with a peripheral hypointense rim due to hepatic tissue congestion. On portal venous (F) and delayed (G) phases, the nodules become progressively isointense to the liver. On the T1-weighted images (flip angle = 30°) in the hepatobiliary phase (H), FNH-like RNs show hyperintensity compared to the surrounding liver parenchyma.
Besides changes in size, as mentioned above, the MRI features of FNH-like RNs can alter during the follow-up period (Figure 4). For example, the T1 and T2 signal intensity may change, more frequently with a shift from hyperintense to isointense on T1-weighted images and from hypointense to isointense on T2-weighted images. The enhancement pattern may vary as well, with washout acquired in 8% of cases and lost in nearly 20% of cases.
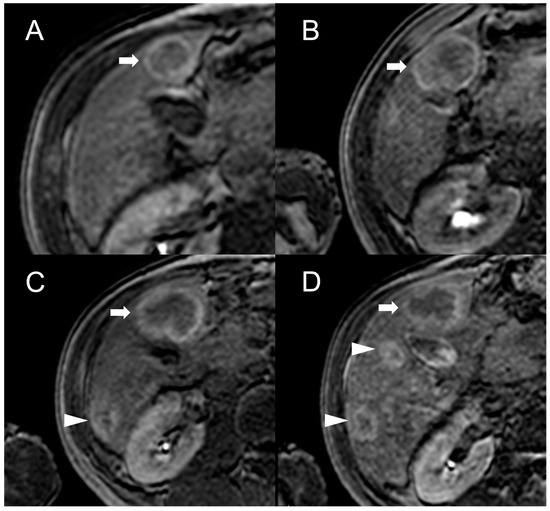
Figure 4. MRI examinations of a 31-year-old male patient with Budd-Chiari syndrome performed in 2016 (A), 2017 (B), 2018 (C), and 2020 (D). Gradient-echo T1-weighted sequences (flip angle = 30°) acquired in the hepatobiliary phase revealed a 25 mm FNH-like RN (arrows) with central scar and peripheral hyperintensity in 2016 (A). The lesion size grew to 41 mm after 1 year (B) and further to 44 mm after 2 years (C), but it reduced to 35 mm in 2020 (D). Additionally, new FNH-like RNs appeared over time (arrowheads in C,D).
In US imaging, FNH-like RNs are often heterogeneous, but they may also appear isoechoic [31] and become challenging to detect in cases where the background liver parenchyma is altered due to congestion or fibrosis (Figure 5). Hypervascularity on Doppler flow imaging and peripheral hypoechoic rim were very common findings, but lack the specificity to distinguish regenerative nodules from malignant lesions. Contrast-enhanced ultrasound (CEUS) offers valuable support in the detection and characterization of focal liver lesions in BCS [32][33][32,33]. After intravenous administration of microbubbles, around 70% of FNH-like RNs demonstrate a rapid center-to-periphery hyperenhancing filling in the arterial phase, with one-third displaying a characteristic “spoke wheel” aspect. In the portal and delayed phases, nearly 90% of FNH-like RNs maintain consistent and homogeneous hyperenhancement, while the rest show enhancement similar to the background liver parenchyma. These features differ from those of HCC nodules, as they more commonly show washout in the portal and venous phases.
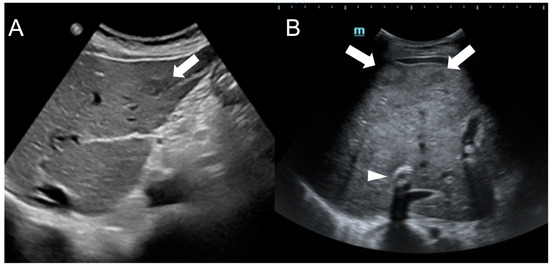
Figure 5. Ultrasound images of a 59-year-old female patient (A) and a 34-year-old male patient (B) with Budd-Chiari syndrome. In both cases, FNH-like RNs appear as hypoechoic lesions (arrows) within the heterogeneous liver parenchyma, occasionally exhibiting a central hyperechoic scar. A TIPS stent is visible in case B (arrowhead).
