Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Tarik Chafik and Version 2 by Alfred Zheng.
Supercapacitors (SCs), also known as “ultracapacitors”, are used in applications requiring rapid energy storage or high-power delivery. Carbon-based materials for supercapacitors derived from affordable coal deposits or crop waste with appropriate characteristics in terms of specific surface area, electrical conductivity, and charge/discharge stability. In addition, the substitution of organic liquids electrolytes with less dangerous solutions, such as aqueous electrolytes containing high concentrations of salt, is a valuable strategy for the design of green devices.
- energy storage
- supercapacitors
- carbon-based electrodes
- aqueous electrolytes
1. Introduction
Currently, more than 70% of the world’s primary energy demand is dominated by fossil fuels. This scenario is expected to change in the near future with the increasing deployment of renewable energy sources as an urgent response to climate change [1]. The alternative energy sources produce energy from sun and wind that typically are not constant [2]. To ensure the reliability and consistency of renewable energy source output, energy storage devices (ESDs) are required as buffers for the intermittent sources. Furthermore, the use of ESDs could power off-grid energy in remote locations [3][4][5][3,4,5]. Hence, ESDs with high energy and power densities will be crucial in the future to ensure the integration of renewable energy sources into existing power systems and boost the potential for applications related to electric mobility.
ESDs based on batteries and electrochemical supercapacitors (SCs) are in rapid development [6][7][8][9][10][11][6,7,8,9,10,11]. Lithium-ion batteries have not yet been widely used as an efficient storage technology and still need to overcome some disadvantages, mainly those associated with safety, cost, and metal availability [8][12][13][14][15][8,12,13,14,15]. SCs have attracted attention in recent years mostly because of their high power density and long life cycle. Notably, these characteristics of SCs allow them to provide interesting hybrid solutions for the automotive sector, where they are coupled with batteries.
In general, in an electrochemical ESD, the energy storage processes occur at the electrodes either by Faradic or non-Faradic modes. The Faradic processes are controlled by the kinetics and activation energies of the electrode reactions as well as by the mass transport of reagents/products, which limit device power density. In addition, the electrode materials may undergo transformations during cycling, like the modification of the chemical composition or structure. Furthermore, the electrochemical reactions involved may have a columbic efficiency lower than 100%. For these reasons, despite the high charge storage capacity, Faradic electrodes can show reduced lifetimes compared to those involving non-Faradic processes. The latter occur through the electrostatic storage of charges at the electrode surfaces. This phenomenon drives energy storage in electrochemical capacitors. It is a rapid surface phenomenon that is completely reversible and does not yield to any chemical or structural changes in the electrodes. The fast kinetics and high reversibility of the electrostatic process enable high SC power density and a theoretically unlimited lifetime.
2. Carbon Materials for Supercapacitors
A variety of carbon materials with different morphologies and structures have been used as EDLC electrodes, including activated carbon (AC) obtained from mineral resources or biomasses, carbon nanotubes (CNTs), and graphene, thanks to their large SSA, high porosity, good electronic conductivity, and chemical stability, as well as their wide range of operating temperatures [16][52].
Other synthetic strategies involve the emulsion-assisted production of polymer nanoparticles that, after calcination under N2 atmosphere, release carbon spheres 200–300 nm in diameter with a single cavity inside and a SSA of about 300 m2 g−1 [17][54].
The large SSA of carbon is generally responsible for the high specific capacity of the electrode. Large SSAs can reach 2500 m2 g−1 and deliver specific capacitance from 100 to 250 F g−1 depending on the electrolyte [18][55], and, in turn, the pore size distribution significantly affects the EDLC charge/discharge rate. According to the International Union of Pure and Applied Chemistry (IUPAC), the porosity classifications for macropores, mesopores, and micropores are as follows [19][56]:
- -
-
Macropores with a diameter greater than 50 nm;
- -
-
Mesopores with a diameter between 2 and 50 nm;
- -
-
Micropores with a diameter of less than 2 nm.
It is worth noting that the IUPAC outlines two subcategories of micropores: supermicropores, with diameters between 0.7 nm and 2 nm, and ultramicropores, with diameter less than 0.7 nm [19][56].
2.1. Activated Carbon (AC)
AC is a promising material for SC electrodes because of its relatively low cost, high conductivity, good thermal stability, and corrosion resistance. Several synthesis routes are for the preparation of activated carbons with high a SSA and a porosity that is suitable for EDLC electrodes have been reported in the literature [20][57]. It should be emphasized that these characteristics are influenced by the precursor used and the synthesis and activation process [21][22][58,59]. Furthermore, activated carbon production processes (carbonization and activation) are generally simple and involve cheap and abundant precursors [23][60]. The activation process consists of oxidation via physical or chemical processes that allow for the creation of a random network of pores (macropores, mesopores, or micropores). Usually, physical activation is carried out through the carbonization of materials (for biomass or hard coal see below) at temperatures ranging from 900 °C to 1100 °C under oxidizing conditions. Such a temperature range induces the sublimation of the lower molecular weight fraction and structural rearrangement, whereas the oxidation of carbon results in the creation and/or enlargement of the pores.
Chemical activation proceeds in the presence of chemical agents (e.g., H3PO4, ZnCl2, KOH, etc.) through the dehydration, carbonization, and structural reorganization of the precursor, inducing the development of micropores and mesopores and the functionalization of their surfaces [24][61]. By carefully controlling the activation parameters, it is possible to reach a specific surface area of 3000 m2·g−1 [16][52].
ACs can be easily produced and, consequently, have been commercially available for a long time for many others applications, including air purification, water treatment, energy storage, etc. Indeed, the global market for AC is growing, and it is expected to be worth up to USD 7 billion by 2028 [25][63]. From a business point of view, the use of AC is of interest because related low-cost and abundant precursors such as biomass are considered renewable resources; thus, in terms of sustainability, AC production is of great importance.
2.2. Carbon Nanotubes (CNTs)
CNTs have been reported to have special features of interest for EDLC electrodes. The development of high-power SCs has been driven by their high electrical conductivity and accessible pore network, along with their good thermal and mechanical stability [16][52]. CNTs are classified as single-walled nanotubes (SWCNTs) and multiwalled nanotubes (MWCNTs) based on the number of graphite-like layers rolled into the cylinder, which, in turn, affects the electrical and mechanical characteristics of the resulting materials [16][26][52,64]. The main methods for CNTs synthesis are laser ablation, arc-discharge, and CVD, all of which are experimentally complex and require expensive equipment. In addition, it seems difficult to achieve high purity and good bulky yields [20][57].
2.3. Graphene
Graphene consists of a two-dimensional single-layer of hexagonal rings of sp2 carbon atoms. This carbon arrangement potentially provides an accessible surface area that is much wider than that of any other carbon material used in EDLCs [16][27][52,65]. Graphene can be produced via CVD; chemical [28][66], electrochemical [29][67], or plasma exfoliation from natural graphite; and mechanical cleavage from natural graphite [30][68]. In one study, a graphene ribbon aerogel monolith with high mass loading (11 g cm−2) exhibited a capacitance density of about 150 F g−1 at 1 A g−1 and about 100% capacitance retention after 10,000 cycles [31][69].
However, despite their great potential, carbon materials based on graphene or CNTs are still far from being commonly used in industrial sectors, mainly because of their high production costs.
2.4. Activated Biochar-Based SCs
This section of the present article focuses on the use of biomass as a sustainable and renewable precursor for the production of ACs. The fabrication process of biomass-derived AC has been demonstrated by the carbonization and activation of a huge variety of raw materials [32][70]. During carbonization, biochar is produced as a result of the precursor being subjected to heat treatment in the absence of oxygen. The development of AC surface area and porosity is achieved through physical or chemical activation using oxidizing gases (e.g., O2, steam, etc.) or other oxidizing agents (e.g., KOH, NaOH, ZnCl2, H3PO4), respectively [33][34][35][71,72,73]. In contrast to physical activation, which partially gasifies the char to CO2 in order to enhance the pores, chemical activation involves dehydrating chemicals to prevent the development of tar and boost the carbon yield [36][74]. Chemical activation is sometimes carried out in one step after pyrolysis and sometimes produces AC with a higher carbon yield, larger SSA, and more developed microporosity than physical activation [37][75].
Using low-cost biomass such as biowaste (e.g., agriculture by-products or food industry waste) to derive ACS for SC electrodes could not only pave the way to solve waste management problems [38][39][76,77] but also generate revenue for farmers in the context of circular economy because it changes a waste product in a secondary raw material into a high-value product that could be used to produce SCs [40][78].
In recent years, interest in producing activated carbon from biomass has steadily grown [40][41][42][43][44][45][78,79,80,81,82,83]. Various sources of biowaste, including waste from plants, animals, and vegetables, have been listed in the literature as raw materials that could be used to produce ACs for use as electrode materials in SCs [40][46][47][48][49][50][51][78,84,85,86,87,88,89]. Figure 1 shows some of those biowaste products, specifically the following: olive seeds, lotus calyx, rice husk, mangosteen peel, chrysopogon zizanioides, lemon peel, eggs shells, and idesia polycarpa fruit oil residue.
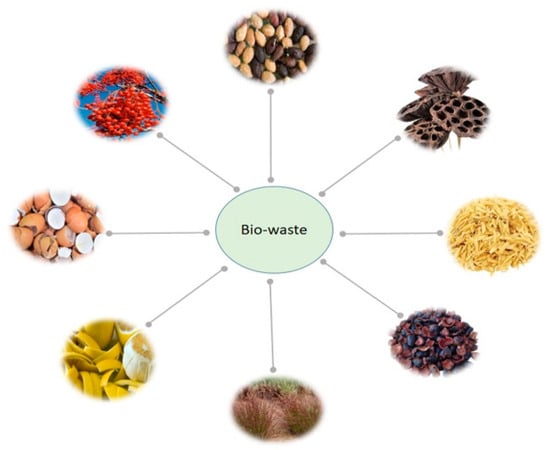
Figure 1.
Some biowaste products that could be used as precursors for carbon materials to generate electrodes for SCs.
For instance, Yang et al. developed a porous carbon with a SSA of 1471.4 m2 g−1 from corncob that provided an EDLC that could deliver an energy density of 20.15 Wh kg−1 in 6 M KOH electrolyte [52][90]. By following the same procedure, Mitravinda et al. investigated EDLCs based on corn silk-derived AC; the EDLCs showed a promising energy density of ~32.28 Wh kg−1 and a power density of 870.68 W kg−1 [53][91]. This was made possible by the AC’s mesoporous fiber-like morphology and texture, which helped to diffuse electrolytes into and out of the pores during the charge/discharge processes. In another study, Yin et al. used coconut fibers to develop three-dimensional hierarchical porous carbon [54][92] with a high SSA of 2898 m2 g−1 and pore volume of 1.59 cm3 g−1 to allow for an EDLC with 6 M KOH to reach an energy density of 53 Wh kg−1 and an impressive power density of 8200 W kg−1. Moreover, Qin et al. synthesized pine nutshell-derived AC using physical activation [54][92], obtaining an interconnected porous structure with different pore size distributions (micro-, meso-, and macropores). This material was used as an electrode in an EDLC with 6 M KOH electrolyte, releasing 98% of the initial capacity after 10,000 cycles [54][92]. Bridget et al. reported the use of lignin residue from biodigestion plants as a precursor for preparing AC [55][93]. The lignin-derived carbon contained mesopores and micropores showing a high SSA of 1879 m2 g−1. A SC with this lignin-derived carbon electrode exhibited a specific energy and specific power density of up to 10 Wh kg−1 and 6.9 kW kg−1, respectively. Durability tests revealed that the device could maintain 84.5% of its capacitance after 15,000 charge/discharge cycles [55][93]. Table 1 shows the electrochemical performance of some investigated biowaste-derived carbon electrodes and SCs using aqueous electrolytes.
Table 1.
Characteristics of biowaste-derived carbon-based electrodes and the related EDLCs with aqueous electrolytes.
| Biowaste | SSA (m | 2 | g | −1 | ) | Specific Capacitance (F/g) | Electrolyte for the Assembled Device | Energy Density (Wh kg | −1 | ) | Power Density (kW kg | −1 | ) | Cyclic Stability (%) |
Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lotus calyx | 798 | 223 (1 A/g) |
1 M Na | 2 | SO | 4 | 17.5 | 0.8 | 95.5 (10,000) |
[56] | [94] | ||||||
| Stem pith of helianthus annuus | 1900.2 | 403.6 (0.5 A/g) |
6 M KOH | 5.8 | 17.3 | 94.5 (10,000) |
[57] | [95] | |||||||||
| Mangosteen peel | 2623 | 357 (1 A/g) |
1 M Li | 2 | SO | 4 | 17.28 | 0.401 | 80 (10,000) |
[58] | [96] | ||||||
| Lemon peel | 744.78 | 152.14 (1 mV/s) |
0.5 M H | 2 | SO | 4 | 4.67 | 8.113 | 95.5 (10,000) |
[59] | [97] | ||||||
| Idesia polycarpa fruit oil residue | 1537.1 | 350.4 (1 A/g) |
6 M KOH | 6.4 | 0.1 | 3305 | 308 (1 A/g) |
6 M KOH | 8.92 | 254.995.4 (10,000) |
[60] | [ | -98] | ||||
| [ | 77 | ] | [ | 115 | ] | Camellia oleifera | shell | 1750 | |||||||||
| Coal-based green needle coke | 807.69 | 259 (1 A/g) |
1 M H | 274.9 (1 A/g) | 2 | SO | 4 | 8.61 | 0.477 | 94 (20,000) |
[61] | [99] | |||||
| 6 M KOH | 20.51 | 1031.42 | 98.5 | (5000) | [ | 78] | [116] | Syzygium cumini | - | 253 (0.5 A/g) |
6 M KOH | 27.22 | 0.2 | 96.5 | |||
| Coal tar pitch | (5000) | 2984 | 320 (0.1 A/g) | [ | 6 M KOH | 62 | ] | [100] | |||||||||
| 10.6 | 50.1 | 94 | (10,000) | [ | 79 | ] | Chrysopogon zizanioides | - | 294 (0.5 A/g) |
6 M KOH | 16.72 | 0.2 | 91.8 (5000) |
[62] | [100] | ||
| [ | 117 | ] | |||||||||||||||
| Anthracite | 2947 | 282 (0.5 A/g) |
6 M KOH | 9.75 | 124.65 | - | [80] | [118] | Baobab fruit shells | 2700.65 | 332 | ||||||
| Coal | (1 A/g) | 6 M KOH | 2168 | 17.7 | 166.4 | 93 | 215 (10,000) |
(20 A/g) | [63] | [101] | |||||||
| 6 M KOH | 7.64 | 50 | 91.9 | (5000) | [ | 81] | [119] | Waste wolfberry fruits | 1423 | 365 (0.2 A/g) |
1 M Li | 2 | SO | 4 | 23.2 | 0.225 | |
| Bituminous coal | 3472.41 | 487 (1 A/g) |
6 M KOH | 96.4 | (10,000) | 249.6 | 10.34 | 96 (10,000) | [64] | [102] | |||||||
| [ | 82 | ] | [ | 120 | ] | Camellia pollen | 810 | 300 (1 A/g) |
6 M KOH | 14.3 | - | 84.5 (20,000) |
[65] | [103] | |||
| Rice husk | 1183 | 163.1 (0.2 A/g) |
6 M KOH | 5.1 | 0.049 | 85 (6000) |
[66] | [104] | |||||||||
| Corn Husk | 1370 | 127 (1 A/g) |
6 M KOH | 4.4 | 0.248 | 90 (5000) |
[67] | [105] | |||||||||
| Olive Seed | 1700 | 224 (0.25 A/g) |
1 M H | 2 | SO | 4 | / 1 M Na | 2 | SO | 4 | 3–5 | 20–30 | 91 (12,500) |
[68] | [106] | ||
| Lignin residue of biodigester | 1879 | 114 (0.5 A/g) |
2.5 M KNO | 3 | 10 | 6.9 | 84.5 (15,000) |
[55] | [93] |
2.5. Coal-Derived AC-Based SCs
Coal is a low-cost carbon-rich material that exists in large natural reserves. In 2020, global coal reserves were estimated to be 1074 billion tons [69][107]. Restrictions regarding CO2 emissions should reduce the use of coal as a fuel and encourage the adoption of other, renewable energy sources with added value due to their applicability to fast-developing zero-emission vehicles [70][108]. There are five different varieties of coal: peat, lignite, subbituminous, bituminous, and anthracite, all of which are classified according to their carbon content. Peat is a soft, crumbly, dark brown substance formed by the decomposition of dead and partially decaying organic matter on the ground in oxygen-poor conditions. Peat contains the least amount of carbon (less than 60%). Lignite, also known as brown coal, has a brown color and preserves the fibrous aspect of the original wood. Its carbon content varies between 65 and 70%. Subbituminous coal, also known as black lignite, is a dark brown or gray-black coal; its carbon content ranges between 70 and 76%. Anthracite is the most high-quality coal because it contains nearly 95% carbon and has a low moisture content [71][109].
Similar to biomass-derived AC production, coal derivatives with a large SSA can be obtained by physical activation in the presence of air, O2, steam, CO2, etc., or by chemical activation using KOH, ZnCl2, NaOH, H3PO4, etc. In recent years, many researchers have investigated different coal-based ACs and their performance as SC electrode materials. Zhao et al. [72][110] used chemical activation by KOH to prepare AC from “hypercoal” with a high surface area of 2540 m2 g−1; Zhao et al. reported a capacitance of 46.0 F g−1. Shi et al. [73][111] assembled a high-performance SC with a specific electrode capacitance of 280 F g−1 and energy density of 38.9 Wh kg−1 at 0.5 A g−1 using an AC produced from anthracite. Zhu et al. [74][112] prepared high-performance coal derivatives via KOH activation, and the optimized sample had a surface area of 2457 m2 g−1 and total pore volume of 1.448 cm3 g−1, which allowed the material to exhibit a specific capacitance of 384 F g−1 in 6 M KOH.
Table 2 summarizes the electrochemical performance of coal-derived AC electrodes, including specific capacitance and power density values, and EDLCs assembled using aqueous electrolytes. Overall, the use of carbon derived from natural resources presents a propitious opportunity to design affordable, cheap, and environmentally friendly SCs.
Table 2. Electrochemical performance of coal-derived carbon-based electrodes (including specific surface area and specific capacitance) and EDLCs (including energy density, power density, and cycling stability) assembled using aqueous electrolytes.
| Materials | SSA (m | 2 | g | −1 | ) | Specific Capacitance (F g | −1 | ) | Electrolyte for the Assembled Device |
Energy Density (Wh kg | −1 | ) | Power Density (W kg | −1 | ) | Cyclic Stability (%) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub-bituminous coal | 1021 | 227 (0.5 A/g) |
6 M KOH | 25 | 12.952 | 82 (10,000) |
[75] | [113] | |||||||||
| Anthracite | 3550.7 | 433 (0.5 A/g) |
6 M KOH | 38.9 | 1000 | 99 (10,000) |
[73] | [111] | |||||||||
| Coal | 2129 | 323 (0.5 A/g) |
6 M KOH | 10 | 250 | 93.7 (10,000) |
[76] | [114] | |||||||||
| Coal tar pitch | 3305 | 308 (1 A/g) |
1 M Na | 2 | SO | 4 | 21.9 | 461.6 | - | [77] | [115] | ||||||
| Coal tar pitch |
