As greenhouse gas emissions are continuously increasing, research is now privileging greener and more sustainable human activities. An attractive strategy in the pursuit of sustainability is the valorization of lignocellulosic biomasses for the production of bioethanol. This approach relies on the bioconversion of wood and agricultural waste, which are abundant globally. They represent considerable sources of fermentable sugars that can be recovered through enzymatic hydrolysis. However, the presence of lignin in wood waste makes it more recalcitrant to enzymatic hydrolysis, and reduces the efficiency of the bioconversion process. Therefore, a pretreatment preceding hydrolysis is highly necessary in order to disrupt the resistant structure of woody biomass. The type and severity of the pretreatment affect the outcomes of the hydrolysis and fermentation steps, just as they strongly influence the overall process costs. Given this context, bioenergy production from this biomass is a promising alternative method of sustainably responding to energy demands while reducing the amounts of waste left in nature.
- bioconversion
- enzymatic hydrolysis
- fermentation
1. Introduction
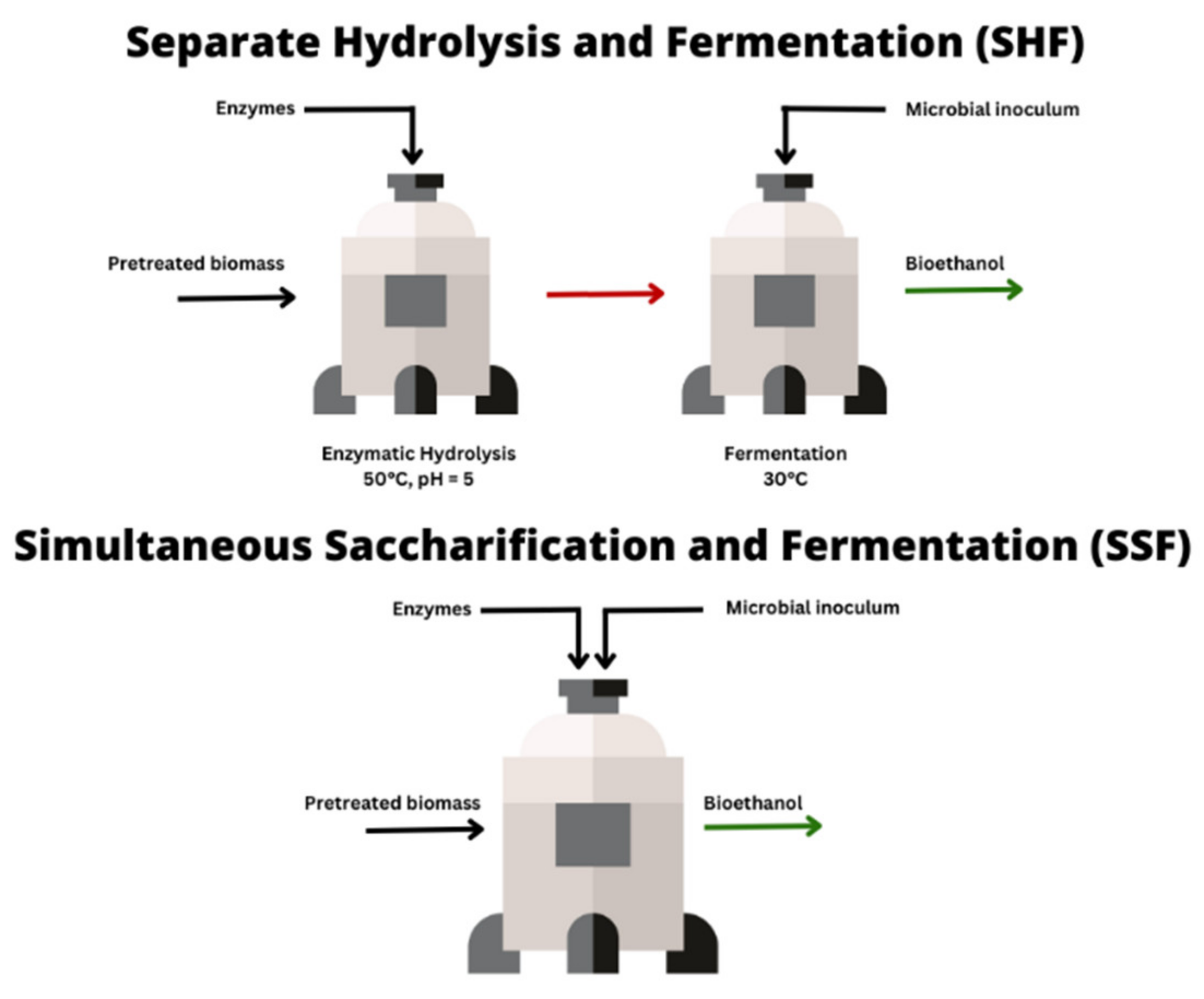
2. Enzymatic Hydrolysis of Woody Biomass after Thermomechanical PT
3. Enzymatic Hydrolysis of Woody Biomass after Chemical PT
4. Enzymatic Hydrolysis of Woody Biomass after Thermal/Thermochemical PT
References
- Fan, Z. Consolidated Bioprocessing for Ethanol Production. In Biorefineries Integrated Biochemical Processes for Liquid Biofuels; Elsevier: Amsterdam, The Netherlands, 2014; pp. 141–160.
- Stickel, J.J.; Elander, R.T.; MCmillan, J.D.; Brunecky, R. Enzymatic Hydrolysis of Lignocellulosic Biomass. In Bioprocessing of Renewable Resources to Commodity Bioproducts; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 77–103. ISBN 9781118175835.
- Al-Ghanayem, A.A.; Joseph, B.; Alhussaini, M.S.; Ramteke, P.W. Current Applications and Future Trends of Extremozymes in Detergent Industries. Microb. Extrem. 2022, 223–230.
- Yi, Y. Tiny Bugs Play Big Role: Microorganisms’ Contribution to Biofuel Production. In Advances in 2nd Generation of Bioethanol Production; Elsevier: Amsterdam, The Netherlands, 2021; pp. 113–136.
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753.
- Meena, M.; Zehra, A.; Dubey, M.K.; Aamir, M.; Upadhyay, R.S. Penicillium Enzymes for the Food Industries. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 167–186.
- Purkait, M.K.; Haldar, D. Enzymatic Hydrolysis of Lignocellulosic Biomass: Mechanistic Insight and Advancement. In Lignocellulosic Biomass to Value-Added Products: Fundamental Strategies and Technological Advancements; Elsevier: Amsterdam, The Netherlands, 2021; pp. 79–94.
- Van Dyk, J.S.; Pletschke, B.I. A Review of Lignocellulose Bioconversion Using Enzymatic Hydrolysis and Synergistic Cooperation between Enzymes--Factors Affecting Enzymes, Conversion and Synergy. Biotechnol. Adv. 2012, 30, 1458–1480.
- Laureano-Perez, L.; Teymouri, F.; Alizadeh, H.; Dale, B.E. Understanding Factors That Limit Enzymatic Hydrolysis of Biomass. In Twenty-Sixth Symposium on Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 2009; Volume 121, pp. 1081–1099.
- Mussatto, S.I.; Dragone, G.; Fernandes, M.; Milagres, A.M.F.; Roberto, I.C. The Effect of Agitation Speed, Enzyme Loading and Substrate Concentration on Enzymatic Hydrolysis of Cellulose from Brewer’s Spent Grain. Cellulose 2008, 15, 711–721.
- Shiva; Climent Barba, F.; Rodríguez-Jasso, R.M.; Sukumaran, R.K.; Ruiz, H.A. High-Solids Loading Processing for an Integrated Lignocellulosic Biorefinery: Effects of Transport Phenomena and Rheology—A Review. Bioresour. Technol. 2022, 351, 127044.
- Zanuso, E.; Ruiz, H.A.; Domingues, L.; Teixeira, J.A. Oscillatory Flow Bioreactor Operating at High Solids Loading for Enzymatic Hydrolysis of Lignocellulosic Biomass. Biochem. Eng. J. 2022, 187, 108632.
- Lu, M.; He, D.; Li, J.; Han, L.; Xiao, W. Rheological Characterization of Ball-Milled Corn Stover with Different Fragmentation Scales at High-Solids Loading. Ind. Crops Prod. 2021, 167, 113517.
- Olofsson, K.; Bertilsson, M.; Lidén, G. A Short Review on SSF—An Interesting Process Option for Ethanol Production from Lignocellulosic Feedstocks. Biotechnol. Biofuels 2008, 1, 7.
- Rana, V.; Eckard, A.D.; Ahring, B.K. Comparison of SHF and SSF of Wet Exploded Corn Stover and Loblolly Pine Using In-House Enzymes Produced from T. Reesei RUT C30 and A. Saccharolyticus. Springerplus 2014, 3, 516.
- Dahnum, D.; Tasum, S.O.; Triwahyuni, E.; Nurdin, M.; Abimanyu, H. Comparison of SHF and SSF Processes Using Enzyme and Dry Yeast for Optimization of Bioethanol Production from Empty Fruit Bunch. Energy Procedia 2015, 68, 107–116.
- Nijland, J.G.; Driessen, A.J.M. Engineering of Pentose Transport in Saccharomyces Cerevisiae for Biotechnological Applications. Front. Bioeng. Biotechnol. 2020, 7, 464.
- Rochón, E.; Cabrera, M.N.; Scutari, V.; Cagno, M.; Guibaud, A.; Martínez, S.; Böthig, S.; Guchin, N.; Ferrari, M.D.; Lareo, C. Co-Production of Bioethanol and Xylosaccharides from Steam-Exploded Eucalyptus Sawdust Using High Solid Loads in Enzymatic Hydrolysis: Effect of Alkaline Impregnation. Ind. Crops Prod. 2022, 175, 114253.
- Romaní, A.; Garrote, G.; Ballesteros, I.; Ballesteros, M. Second Generation Bioethanol from Steam Exploded Eucalyptus Globulus Wood. Fuel 2013, 111, 66–74.
- Schneider, W.D.H.; Fontana, R.C.; Baudel, H.M.; de Siqueira, F.G.; Rencoret, J.; Gutiérrez, A.; de Eugenio, L.I.; Prieto, A.; Martínez, M.J.; Martínez, Á.T.; et al. Lignin Degradation and Detoxification of Eucalyptus Wastes by On-Site Manufacturing Fungal Enzymes to Enhance Second-Generation Ethanol Yield. Appl. Energy 2020, 262, 114493.
- Pažitný, A.; Russ, A.; Boháček, Š.; Stankovská, M.; Ihnát, V. Effect of Steam Explosion on Enzymatic Hydrolysis of Various Parts of Poplar Tree. Wood Res. 2020, 65, 579–590.
- Pielhop, T.; Amgarten, J.; Von Rohr, P.R.; Studer, M.H. Steam Explosion Pretreatment of Softwood: The Effect of the Explosive Decompression on Enzymatic Digestibility. Biotechnol. Biofuels 2016, 9, 152.
- Besserer, A.; Obame, S.N.; Safou-Tchima, R.; Saker, S.; Ziegler-Devin, I.; Brosse, N. Biorefining of Aucoumea Klaineana Wood: Impact of Steam Explosion on the Composition and Ultrastructure the Cell Wall. Ind. Crops Prod. 2022, 177, 114432.
- Barbanera, M.; Lascaro, E.; Foschini, D.; Cotana, F.; Buratti, C. Optimization of Bioethanol Production from Steam Exploded Hornbeam Wood (Ostrya Carpinifolia) by Enzymatic Hydrolysis. Renew. Energy 2018, 124, 136–143.
- Mihiretu, G.T.; Chimphango, A.F.; Görgens, J.F. Steam Explosion Pre-Treatment of Alkali-Impregnated Lignocelluloses for Hemicelluloses Extraction and Improved Digestibility. Bioresour. Technol. 2019, 294, 122121.
- Semhaoui, I. Etude de la Bioconversion de la Chènevotte (Cannabis Sativa) et de l’alfa (Stipa Tenacissima) par Prétraitement Thermomécanique en Présence d’un Catalyseur Acide ou Alcalin. Ph.D. Thesis, Université de La Rochelle, La Rochelle, France, 2019.
- Zhou, Y.; Li, Y.; Wan, C.; Li, D.; Mao, Z. Effect of Hot Water Pretreatment Severity on the Degradation and Enzymatic Hydrolysis of Corn Stover. Trans. ASABE 2010, 53, 1929–1934.
- Nitsos, C.K.; Choli-Papadopoulou, T.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Hardwood and Softwood Lignocellulosic Residues for Selective Hemicellulose Recovery and Improved Cellulose Enzymatic Hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4529–4544.
- Bardak, S.; Nemli, G.; Bardak, T.; Bardak, S.; Nemli, G.; Bardak, T. The Quality Comparison of Particleboards Produced from Heartwood and Sapwood of European Larch. Maderas. Cienc. Tecnol. 2019, 21, 511–520.
- Benouadah, N.; Aliouche, D.; Pranovich, A.; Willför, S. Chemical Characterization of Pinus Halepensis Sapwood and Heartwood. Wood Mater. Sci. Eng. 2018, 14, 157–164.
- Chitambar, J. Marasmiellus Palmivorus. Pest Rating Proposals and Final Ratings. Available online: https://blogs.cdfa.ca.gov/Section3162/?p=4525 (accessed on 4 April 2023).
- Cavka, A.; Jönsson, L.J. Detoxification of Lignocellulosic Hydrolysates Using Sodium Borohydride. Bioresour. Technol. 2013, 136, 368–376.
- Ujor, V.C.; Okonkwo, C.C. Microbial Detoxification of Lignocellulosic Biomass Hydrolysates: Biochemical and Molecular Aspects, Challenges, Exploits and Future Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 1061667.
- Zhang, J.; Zhu, Z.; Wang, X.; Wang, N.; Wang, W.; Bao, J. Biodetoxification of Toxins Generated from Lignocellulose Pretreatment Using a Newly Isolated Fungus, Amorphotheca Resinae ZN1, and the Consequent Ethanol Fermentation. Biotechnol. Biofuels 2010, 3, 26.
- Bay, M.S.; Eslami, F.; Karimi, K. The Relationship between Structural Features of Lignocellulosic Materials and Ethanol Production Yield. Designs 2022, 6, 119.
- Abdou Alio, M.; Tugui, O.C.; Rusu, L.; Pons, A.; Vial, C. Hydrolysis and Fermentation Steps of a Pretreated Sawmill Mixed Feedstock for Bioethanol Production in a Wood Biorefinery. Bioresour. Technol. 2020, 310, 123412.
- Matsakas, L.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. Lignin-First Biomass Fractionation Using a Hybrid Organosolv—Steam Explosion Pretreatment Technology Improves the Saccharification and Fermentability of Spruce Biomass. Bioresour. Technol. 2019, 273, 521–528.
- Najafi, E.; Castro, E.; Karimi, K. Biorefining for Olive Wastes Management and Efficient Bioenergy Production. Energy Convers. Manag. 2021, 244, 114467.
- Ajayo, P.C.; Huang, M.; Zhao, L.; Tian, D.; He, J.; Zou, J.; Fang, D.; Zeng, Y.; Shen, F. High Yield of Fermentable Sugar from Paper Mulberry Woods Using Phosphoric Acid plus Hydrogen Peroxide Pretreatment: Multifactorial Investigation and Optimization. Ind. Crops Prod. 2022, 180, 114771.
- Wu, P.; Li, L.; Zhou, Y.; Wang, W.; Sun, Y.; Guo, Y.; Kang, X. Biorefining of Ethanol and Methane from NaOH Pretreated Poplar Residues: Mass Balance and Energy Flow Analyses. Fuel 2023, 333, 126293.
- Wan, X.; Liu, J.; Zhang, Y.; Tian, D.; Liu, Y.; Zhao, L.; Huang, M.; Hu, J.; Shen, F. Conversion of Agricultural and Forestry Biomass into Bioethanol, Water-Soluble Polysaccharides, and Lignin Nanoparticles by an Integrated Phosphoric Acid plus Hydrogen Peroxide Process. Ind. Crops Prod. 2023, 191, 115969.
- Zhao, J.; Tian, D.; Shen, F.; Hu, J.; Zeng, Y.; Huang, C. Valorizing Waste Lignocellulose-Based Furniture Boards by Phosphoric Acid and Hydrogen Peroxide (Php) Pretreatment for Bioethanol Production and High-Value Lignin Recovery. Sustainability 2019, 11, 6175.
- Cheng, Y.; Mondal, A.K.; Wu, S.; Xu, D.; Ning, D.; Ni, Y.; Huang, F. Study on the Anti-Biodegradation Property of Tunicate Cellulose. Polymers 2020, 12, 3071.
- Zhao, X.; Zhang, L.; Liu, D. Biomass Recalcitrance. Part II: Fundamentals of Different Pre-Treatments to Increase the Enzymatic Digestibility of Lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 561–579.
- Singh, N.; Devi, A.; Bishnoi, M.B.; Jaryal, R.; Dahiya, A.; Tashyrev, O.; Hovorukha, V.; Singh, N.; Devi, A.; Bishnoi, M.B.; et al. Overview of the Process of Enzymatic Transformation of Biomass. In Elements of Bioeconomy; Biernat, K., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-862-4.
- Kumar, D.; Murthy, G.S. Stochastic Molecular Model of Enzymatic Hydrolysis of Cellulose for Ethanol Production. Biotechnol. Biofuels 2013, 6, 63.
- Daicho, K.; Saito, T.; Fujisawa, S.; Isogai, A. The Crystallinity of Nanocellulose: Dispersion-Induced Disordering of the Grain Boundary in Biologically Structured Cellulose. ACS Appl. Nano Mater. 2018, 1, 5774–5785.
- Xu, H.; Che, X.; Ding, Y.; Kong, Y.; Li, B.; Tian, W. Effect of Crystallinity on Pretreatment and Enzymatic Hydrolysis of Lignocellulosic Biomass Based on Multivariate Analysis. Bioresour. Technol. 2019, 279, 271–280.
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141.
- Han, L.; Feng, J.; Zhang, S.; Ma, Z.; Wang, Y.; Zhang, X. Alkali Pretreated of Wheat Straw and Its Enzymatic Hydrolysis. Braz. J. Microbiol. 2012, 43, 53–61.
- Eriksson, T.; Börjesson, J.; Tjerneld, F. Mechanism of Surfactant Effect in Enzymatic Hydrolysis of Lignocellulose. Enzyme Microb. Technol. 2002, 31, 353–364.
- Wang, W.; Wang, C.; Zahoor; Chen, X.; Yu, Q.; Wang, Z.; Zhuang, X.; Yuan, Z. Effect of a Nonionic Surfactant on Enzymatic Hydrolysis of Lignocellulose Based on Lignocellulosic Features and Enzyme Adsorption. ACS Omega 2020, 5, 15812–15820.
- Huang, C.; Zhao, X.; Zheng, Y.; Lin, W.; Lai, C.; Yong, Q.; Ragauskas, A.J.; Meng, X. Revealing the Mechanism of Surfactant-Promoted Enzymatic Hydrolysis of Dilute Acid Pretreated Bamboo. Bioresour. Technol. 2022, 360, 127524.
- Oliva-Taravilla, A.; Carrasco, C.; Jönsson, L.J.; Martín, C. Effects of Biosurfactants on Enzymatic Saccharification and Fermentation of Pretreated Softwood. Molecules 2020, 25, 3559.
- Muñoz, S.S.; Balbino, T.R.; Alba, E.M.; Barbosa, F.C.; de Pier, F.T.; de Almeida, A.L.M.; Zilla, A.H.B.; Antunes, F.A.F.; Hilares, R.T.; Balagurusamy, N.; et al. Surfactants in Biorefineries: Role, Challenges & Perspectives. Bioresour. Technol. 2022, 345, 126477.
- Sjulander, N.; Kikas, T. Two-Step Pretreatment of Lignocellulosic Biomass for High-Sugar Recovery from the Structural Plant Polymers Cellulose and Hemicellulose. Energies 2022, 15, 8898.
- Mikulski, D.; Kłosowski, G. High-Pressure Microwave-Assisted Pretreatment of Softwood, Hardwood and Non-Wood Biomass Using Different Solvents in the Production of Cellulosic Ethanol. Biotechnol. Biofuels Bioprod. 2023, 16, 19.
- Sierra-Ibarra, E.; Alcaraz-Cienfuegos, J.; Vargas-Tah, A.; Rosas-Aburto, A.; Valdivia-López, Á.; Hernández-Luna, M.G.; Vivaldo-Lima, E.; Martinez, A. Ethanol Production by Escherichia Coli from Detoxified Lignocellulosic Teak Wood Hydrolysates with High Concentration of Phenolic Compounds. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab077.
- Dessie, W.; Tang, J.; Wang, M.; Luo, X.; Liu, X.; Qin, Z. One-Pot Conversion of Industrial Hemp Residue into Fermentable Feedstocks Using Green Catalyst and Enzyme Cocktails Generated by Solid-State Fermentation. Ind. Crops Prod. 2022, 182, 114885.
- Raina, N.; Slathia, P.S.; Sharma, P. Experimental Optimization of Thermochemical Pretreatment of Sal (Shorea Robusta) Sawdust by Central Composite Design Study for Bioethanol Production by Co-Fermentation Using Saccharomyces Cerevisiae (MTCC-36) and Pichia Stipitis (NCIM-3498). Biomass Bioenergy 2020, 143, 105819.
- Lee, I.; Yu, J.H. The Production of Fermentable Sugar and Bioethanol from Acacia Wood by Optimizing Dilute Sulfuric Acid Pretreatment and Post Treatment. Fuel 2020, 275, 117943.
- Singh, S. Carbohydrates 10. Available online: https://www.uou.ac.in/lecturenotes/science/MSCCH-17/CHEMISTRY%20LN%204%20CARBOHYDRATES-converted%20(1).pdf (accessed on 22 June 2023).
- Frankó, B.; Carlqvist, K.; Galbe, M.; Lidén, G.; Wallberg, O. Removal of Water-Soluble Extractives Improves the Enzymatic Digestibility of Steam-Pretreated Softwood Barks. Appl. Biochem. Biotechnol. 2018, 184, 599.
- Shi, J.; Gladden, J.M.; Sathitsuksanoh, N.; Kambam, P.; Sandoval, L.; Mitra, D.; Zhang, S.; George, A.; Singer, S.W.; Simmons, B.A.; et al. One-Pot Ionic Liquid Pretreatment and Saccharification of Switchgrass. Green Chem. 2013, 15, 2579–2589.
- Sriariyanun, M.; Kitiborwornkul, N.; Tantayotai, P.; Rattanaporn, K.; Show, P.L. One-Pot Ionic Liquid-Mediated Bioprocess for Pretreatment and Enzymatic Hydrolysis of Rice Straw with Ionic Liquid-Tolerance Bacterial Cellulase. Bioengineering 2022, 9, 17.
- Florencio, C.; Badino, A.C.; Farinas, C.S. Soybean Protein as a Cost-Effective Lignin-Blocking Additive for the Saccharification of Sugarcane Bagasse. Bioresour. Technol. 2016, 221, 172–180.
- Simões, I.R.; Brondi, M.G.; Farinas, C.S. In-House Extracted Soybean Protein Can Reduce the Enzyme Dosage in Biomass Saccharification. Fermentation 2023, 9, 142.
