Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Andrea Gabai and Version 2 by Jason Zhu.
Innovative and new drug delivery systems (DDSs) have recently been developed to vehicle treatments and drugs to the ocular posterior segment and the retina. New formulations and technological developments, such as nanotechnology, novel matrices, and non-traditional treatment strategies, open new perspectives in this field.
- drug delivery systems (DDSs)
- nanotechnology
- matrices
- ocular posterior segment
1. Introduction
New drug delivery methods to target specific ocular tissues and treat debilitating ocular diseases have been proposed in the past decade. Of all ocular diseases, 55% originate from the posterior segment [1]. Retinal diseases, such as age-related macular degeneration (AMD), diabetic retinopathy (DR), diabetic macular edema (DME), endophthalmitis, viral retinitis, proliferative vitreoretinopathy (PVR), posterior uveitis, retinal vascular occlusions, retinitis pigmentosa (RP), and inherited retinal diseases (IRDs), represent the leading cause of vision impairment [2][3][2,3].
The presence of static barriers (different layers of the cornea, sclera, retina, blood–aqueous (BAB) and (BRB) blood–retinal barriers) (Figure 1) and dynamic barriers (conjunctival and choroidal blood flow, tear turnover, and lymphatic clearance) represent an obstacle to the delivery of a drug to a particular ocular tissue and to treating these retinal diseases [4]. The research field based on ocular “drug delivery”, which refers to numerous innovative techniques to target retinal tissue and overcome ocular barriers, has witnessed huge growth in recent years.
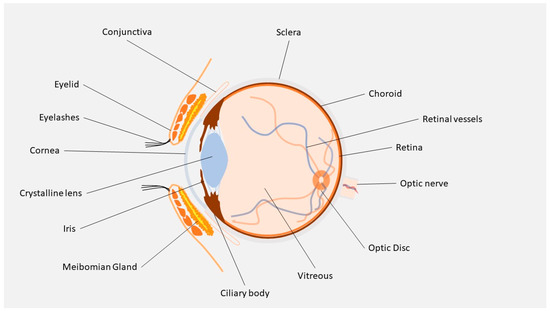
Figure 1.
Ocular anatomy.
Depending on disease type, drug property, and target site, drugs can be administered through different routes, including topical, intravitreal, periocular, and systemic. Several types of ophthalmic drug delivery systems (DDSs) are currently available on the market, and range from eyedrops, eye ointments, gels, and ocular inserts, such as eye dosage formulations that are created to increase the holding time of drugs in the eye. Topical drug instillation on the ocular surface is a common and non-invasive application method for the treatment modalities of eye disorders. This route, however, offers suboptimal ocular bioavailability. Studies have shown that 90% of eyedrops available on the market only provide 5% of drug bioavailability, while the rest of the drug gets washed away through different elimination routes, such as tear fluid, nasolacrimal secretion, protein binding, enzymatic degradation, or metabolism by protease and esterase enzyme [5]. Despite the limits of topical treatments, current studies have reported promising results for substances delivered to the retina, which suggests that topical treatment of retinal diseases might be possible in the future [6][7][6,7].
Injection into the globe is very often recommended for drug delivery into the posterior region, but it is painful, causes patient non-compliance, has risks of infection, and is associated with various side effects. Over the past decade, thanks to advances in nanotechnology and the development of many drug delivery systems, such as implants, in situ gel, contact lenses, microneedles, liposomes, nanomicelles, dendrimers, and other nanoparticles (NPs), numerous drug-delivery solutions have been proposed. The polymeric nature of these various technologies includes the distinction between microparticles, which are polymeric systems larger than 1 µm, and nanoparticles that have a smaller diameter. Structurally, these particles can be broadly described as micro/nanospheres, when the loaded drug is integrated in the matrix and nano/microcapsules, when the drug is enclosed in a polymeric shell. Although most of the studies in this field are still in the preclinical stages, micro/nanotechnology-based DDSs possess great potential to solve the shortcomings of currently available drug delivery systems. These new strategies can potentially allow safe and effective drug administration to the ocular posterior segment and retina [8][9][10][11][8,9,10,11].
