Ultrasound is known as a green novel technology due to its role in environmental sustainability. Ultrasound waves are classified into four different categories based on the mode of vibration of the particles in the medium, with respect to the direction of propagation of the wave, viz., longitudinal waves, transverse waves, surface waves, and plate waves.
- ultrasound
- food processing
1. Background
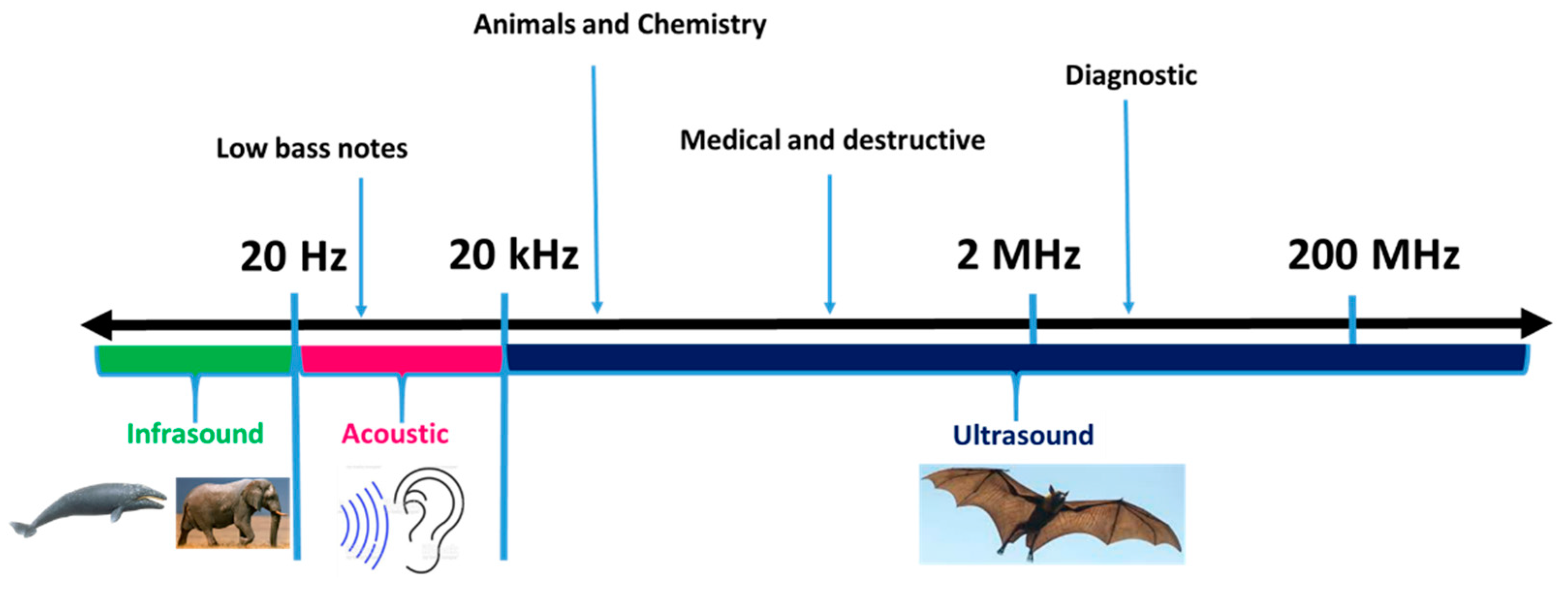
Ultrasound, as a non-thermal food processing technology, is applied to bring positive effects in food processing, such as food preservation, improvement in mass transfer, the assistance of thermal treatments, the alteration of texture, and food analysis [4][1]. Ultrasonic waves (also called supersonic waves) are sound waves in the frequency ranges of 20 kHz to 100 kHz [5][2]. Ultrasound produces ‘cavitation’ in liquids, pressure variations in gas media, and liquid movement in solid media, respectively [6][3]. It can be viewed as a form of high-frequency vibration that generates fluid mixing and shear forces on a micro scale [7][4].
2. Classification of Ultrasound Applications
3. Applications of Ultrasonic Waves in Food Processing
3.1. Microbial Inactivation
Thermal treatments such as pasteurization are mostly considered useful for bacterial inactivation, but undesirable effects such as unwanted flavours and nutrient losses have encouraged research on novel processing techniques. Ultrasound treatment is applied as a processing aid to inactivate microbes. Various textural and physiological changes, i.e., the thinning of cell membranes and the production of free radicals, are the main mechanisms by which microorganism inactivation takes place [28][13]. Transient cavitation will produce localized hot spots up to 4500–5000 K, and pressures > 199 MPa produce shock waves and free radicals, whereas stable cavitation will produce micro streaming accompanied by high shear [29][14]. All these contribute to damage of the cell wall and membrane, resulting in cell death. It was reported that ruptured and disintegrated cells cannot be reviewed, which is advantageous over some other techniques in which damaged cells can recover if they encounter the right environmental conditions (temperature, pH, water activity, and nutrients) [29][14]. The resistance offered by five groups of microorganisms to the ultrasonic inactivation is in the order of spores > fungi > yeast > gram-positive cells > gram-negative cells. The resistance of viruses to ultrasound is high, but not enough data is yet available to compare it with the other microorganisms. Microbial destruction can also be accomplished by combining ultrasound with other treatments, such as heat (thermosonication), low static pressure (monosonication), ultraviolet light, or antimicrobials. Sonication combined with high pressures and temperatures (manothermosonication at 400 kPa/59 °C) was applied by Lee et al. [30][15] for the control of Escherichia coli (E. coli) K12 populations in apple cider, and they achieved a 5-log reduction in 1.4 min and in 3.7 min when sonication was combined with a lethal temperature—whereas treatment via sonication alone takes 15.9 min for the same E. Coli reduction. A sonication of yeast cells in Saccharomyces cerevisiae 2200 strain using a 20-kHz horn-type sonicator was carried out by Fabiszewska [31][16]. After sonication, the count of live yeast cells decreases by 100 to 1000 times, compared to their initial count, expressed as Colony Forming Units CFU/cm3. The efficacy of microbial destruction is governed by amplitude and frequency of the ultrasonic waves, the exposure/contact time, the composition and volume of the food to be processed, and the conditions [28][13]. It was observed that the number of bubbles undergoing cavitation per unit of time increases at higher amplitudes, which resulted in a higher inactivation rate of the microorganisms. The majority of the research has been conducted to determine the effect of ultrasound on the microbial inactivation of fruits and vegetables [32,33,34,35][17][18][19][20]. The inactivation of microorganisms using heat treatments and ultrasound and their D values (time for 90% reduction of microorganisms) are summarized in Table 1.| Temperature (°C) | Organism | D Value (min) | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Heat | Ultrasound | Thermosonication | Monosonication/Manothermosonication (MTS) | ||||||
| 60 | Saccharomyces cerevisiae | 3.53 | 3.1(20 kHz, 124 μm) | Indirect sonication: 25 kHz, 1.75 kW Osmotic solution: 70° Brix Immersion time: 60 min Air drying: 70 °C1.9 (1 min US, 55 °C) 0.73 (1 min US, 60 °C) | - | Guava slices | Initial moisture content: 91.3 ± 0.6% wet basis (w.b.) Final moisture content: 19.5 ± 3 (w.b.) Total dehydration time: 300 min. Drying time reduced by 33%. | [57][42][36][21] | |
| 61 | Escherichia coli K12 | 0.79 | 1.01 | ||||||
| Ultrasound-assisted convective drying | Ultrasound: 21.8 kHz, 60 W Air drying: 70 °C | Strawberry | Initial moisture content: 90.5 ± 0.27 (w.b.) Final moisture content: 23.07% (w.b.). Total drying time: 2.2 h. Drying time reduced by 44%. | 0.44 (100 kPa) | [58]0.40 (300 kPa)/0.27 (MTS 300 kPa 61 °C) | [43][37][22] | |||
| 56 | Cronabacter sakazakii | 0.86 | |||||||
| Ultrasound-assisted osmotic dehydration | - | - | Ultrasound: 25 kHz, 700 W | 0.28 (MTS-20 kHz, 117 μm, 200 kPa, 56 °C) | Cherries | It was proved that intermittent drying of cherries preceded by ultrasonic-assisted osmotic dehydration contributes to shorter drying time, better colour preservation, and smaller water activity. | [59][44][38][23] | ||
| 55 | Aspergillus flavus | 17.40 | - | 5.06 (120 μm) 4.94 (120 μm, 500 ppm vanillin (Vi)) 1.09 (120 μm, 500 ppm potassium sorbate (KS)) | [39 | ||||
| Ultrasound-assisted convective drying | Ultrasound: 21.8 kHz, 30.8 W Air drying: 70 °C | ] | [ | 24 | ] | ||||
| Passion fruit peel | Initial moisture content: 87.5± 1.9 (w.b.) Final moisture content: 32% (w.b.) Total drying time: 3.9 ± 0.7 h. | [ | 60 | ][45] | 60 | 2.60 | - | 1.20 (120 μm) < 0.5 (120 μm, 500 ppm Vi) < 0.5 (120 μm, 500 ppm KS) | |
| 50 | Penicillium digitatum | 25.42 | - | 9.59 (120 μm) 8.57 (120 μm, 500 ppm Vi) 7.15 (120 μm, 500 ppm KS) | [39][24] | ||||
| 52.5 | 13.30 | - | 5.33 (120 μm) 6.47 (120 μm, 500 ppm Vi) 4.19 (120 μm, 500 ppm KS) | ||||||
| 63 | Listeria innocua | 30 | - | 10 (400 W, 24 kHz, 120 μm) | [40][25] | ||||
| Ambient | Mesophilic aerobic, Lactic acid bacteria, Coliform bacteria, yeast | 750 W, 20 kHz, 6.8–126 μm | [28][13] | ||||||
| 25 | Salmonella Typhimurium, Escherichia coli | 80 and 37 kHz, (330 W), pulsed modes, frequency amplitude (40% and 100%) | [41][26] | ||||||
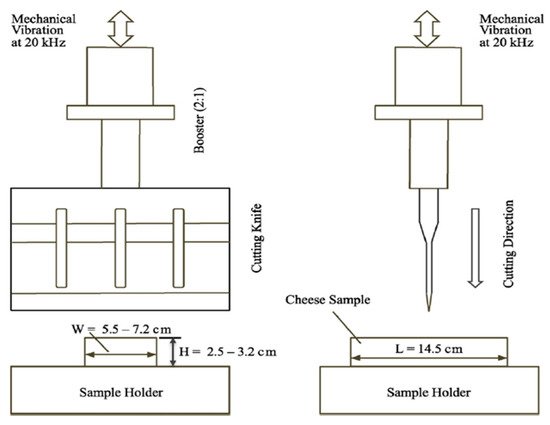
3.2. Ultrasonic Cutting
3.3. Ultrasonic Drying
Drying of the food materials is the most common and promising method for stabilizing the product. Traditional drying techniques cause adverse effects, such as shrinkage, discolouration, and the oxidation of vitamins. Additionally, rising energy costs, increasing quality requirements at reduced nutritional losses, and adverse environmental impacts have resulted in an increased interest in the development of modern food-drying technologies [47,48][32][33]. The use of ultrasound accelerates the drying process without causing a severe temperature change. It has been suggested that mass transport kinetics can be increased by using high-intensity ultrasound. Moreover, ultrasound can decrease the boundary layer thus resulting in a decrease in resistance to mass transfer required for the drying process [49][34]. An experimental study was carried out by Ortuno et al. [50][35] on the convective drying kinetics of orange peel slabs at 40 °C and 1 m/s with and without power ultrasound, and they interpreted that oscillating velocities, pressure variations, and microstreaming created by ultrasound not only reduced the boundary layer thickness but also increased the water transfer to the air phase. In another study, ultrasound-assisted convective drying of apple shortened the drying time to 160 min, which incurred purely under convective drying [51][36], whereas combining the treatments of convective drying (50 °C), microwave (100 W), and ultrasound (200 W) reduced the drying time by 79%, as compared to convective drying alone for raspberries [52][37]. An ultrasonic pretreatment on Andean blackberry was applied before convective drying by Romero and Yépez [53][38], who found greater antioxidant retention than the control due to a reduced processing time and lower drying temperatures. Recently, the application of ultrasound in food-drying methods has gained popularity among researchers for the drying of hydrophilic and lipophilic nutrients for microencapsulation [54][39], the drying of deformable porous materials [55][40], and ultrasound-assisted infrared drying of jackfruit [56][41]. The different techniques of ultrasound-assisted drying of various fruit and vegetable crops have been explained in Table 2.| Drying Technique | Ultrasound Processing Parameters | Sample | Inference | Reference |
|---|---|---|---|---|
| Ultrasound-assisted osmotic drying | ||||
| Ultrasound-assisted radiation drying | ||||
| Ultrasound: 1200 W, 20 kHz Sonication time: 5 s Drying: 62 °C | Carrot slices | Final moisture content: 10 ± 0.5% (d.b.) Drying time increased with increasing ultrasonic power levels. | [ | 61][46] |
| Ultrasound-assisted vacuum drying | Sonication time: 10 s Drying: 65–75 °C | Carrot slices | Final moisture content: 12–13% dry basis (d.b) Drying time was decreased by 53%. | [62][47] |
| Ultrasound-assisted heating | 1000 W and 50 ℃ | Ham slices | Decrease of 0.65-fold in adhesiveness values. Population of free water increased from 2.71% to 11.35%. Decreased the content of rancid and sour compounds. Accelerated the formation of esters. | [63][48] |
| Ultrasound-assisted microwave dryer | 28 kHz, 70 W, 30 min | Carrot slices | Reduction in drying time by 63%. Least-specific energy consumption: 23.75 ±2.22 MJ/kg Lowest shrinkage: 31.8 ±1.1% |
[64][49] |
References
- Firouz, M.S.; Farahmandi, A.; Hosseinpour, S. Recent advances in ultrasound application as a novel technique in analysis, processing and quality control of fruits, juices and dairy products industries: A review. Ultrason. Sonochem. 2019, 57, 73–88.
- Singla, M.; Sit, N. Application of ultrasound in combination with other technologies in food processing: A review. Ultrason. Sonochem. 2021, 73, 105506.
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of Ultrasound in Food Science and Technology: A Perspective. Foods 2018, 7, 164.
- Khadhraoui, B.; Ummat, V.; Tiwari, B.; Fabiano-Tixier, A.; Chemat, F. Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason. Sonochem. 2021, 76, 105625.
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293.
- Kentish, S.; Feng, H. Applications of Power Ultrasound in Food Processing. Annu. Rev. Food Sci. Technol. 2014, 5, 263–284.
- Qiu, L.; Zhang, M.; Chitrakar, B.; Bhandari, B. Application of power ultrasound in freezing and thawing Processes: Effect on process efficiency and product quality. Ultrason. Sonochem. 2020, 68, 105230.
- Charoux, C.M.; Inguglia, E.S.; O’Donnell, C.P.; Tiwari, B.K. Ultrasonic Waves: Inactivation of foodborne microorganisms using power ultrasound. In Innovative Food Processing Technologies; Knoerzer, K., Juliano, P., Smithers, G., Eds.; Elsevier: Cambridge, UK, 2021.
- Chew, S.C.; Ali, M.A. Recent advances in ultrasound technology applications of vegetable oil refining. Trends Food Sci. Technol. 2021, 116, 468–479.
- Yao, Y.; Pan, Y.; Liu, S. Power ultrasound and its applications: A state-of-the-art review. Ultrason. Sonochem. 2019, 62, 104722.
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835.
- Ravikumar, M. Ultrasonication: An Advanced Technology for Food Preservation. Int. J. Pure Appl. Biosci. 2017, 5, 363–371.
- Starek, A.; Kobus, Z.; Sagan, A.; Chudzik, B.; Pawłat, J.; Kwiatkowski, M.; Terebun, P.; Andrejko, D. Influence of ultrasound on selected microorganisms, chemical and structural changes in fresh tomato juice. Sci. Rep. 2021, 11, 1–12.
- Evelyn; Silva, F.V. Ultrasound assisted thermal inactivation of spores in foods: Pathogenic and spoilage bacteria, molds and yeasts. Trends Food Sci. Technol. 2020, 105, 402–415.
- Lee, H.; Kim, H.; Cadwallader, K.R.; Feng, H.; Martin, S.E. Sonication in combination with heat and low pressure as an alternative pasteurization treatment—Effect on Escherichia coli K12 inactivation and quality of apple cider. Ultrason. Sonochem. 2013, 20, 1131–1138.
- Kapturowska, A.; Stolarzewicz, I.; Chmielewska, I.; Bialecka-Florjanczyk, E. Ultrasounds—A tool to inactivate yeast and to extract intracellular protein. Food Sci. Technol. Qual. 2011, 18, 160–171.
- Sulaiman, A.; Soo, M.J.; Farid, M.; Silva, F.V. Thermosonication for polyphenoloxidase inactivation in fruits: Modeling the ultrasound and thermal kinetics in pear, apple and strawberry purees at different temperatures. J. Food Eng. 2015, 165, 133–140.
- Park, S.Y.; Mizan, F.R.; Ha, S.-D. Inactivation of Cronobacter sakazakii in head lettuce by using a combination of ultrasound and sodium hypochlorite. Food Control 2016, 60, 582–587.
- Muñoz, R.; Viveros, N.; Bevilacqua, A.; Pérez, M.S.; Arévalo-Villena, M. Effects of ultrasound treatments on wine microorganisms. Ultrason. Sonochem. 2021, 79, 105775.
- Costello, K.M.; Velliou, E.; Gutierrez-Merino, J.; Smet, C.; El Kadri, H.; Van Impe, J.F.; Bussemaker, M. The effect of ultrasound treatment in combination with nisin on the inactivation of Listeria innocua and Escherichia coli. Ultrason. Sonochem. 2021, 79, 105776.
- Wordon, B.; Mortimer, B.; McMaster, L. Comparative real-time analysis of Saccharomyces cerevisiae cell viability, injury and death induced by ultrasound (20 kHz) and heat for the application of hurdle technology. Food Res. Int. 2012, 47, 134–139.
- Lee, H.; Zhou, B.; Liang, W.; Feng, H.; Martin, S.E. Inactivation of Escherichia coli cells with sonication, manosonication, thermosonication, and manothermosonication: Microbial responses and kinetics modeling. J. Food Eng. 2009, 93, 354–364.
- Arroyo, C.; Cebrián, G.; Pagán, R.; Condón, S. Synergistic combination of heat and ultrasonic waves under pressure for Cronobacter sakazakii inactivation in apple juice. Food Control 2012, 25, 342–348.
- Lopez-Malo, A.; Palou, E.; Jimenez-Fernandez, M.; Al-Zamora, S.M.; Guerrero, S. Multifactorial fungal inactivation combining thermosonication and antimicrobials. J. Food Eng. 2005, 67, 87–93.
- Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V. Study of butter fat content in milk on the inactivation of Listeria innocua ATCC 51742 by thermo-sonication. Innov. Food Sci. Emerg. Technol. 2008, 9, 176–185.
- Rossi, A.P.; Kalschne, D.L.; Byler, A.P.I.; Flores, E.L.D.M.; Leite, O.D.; dos Santos, D.; Barin, J.S.; Canan, C. Effect of ultrasound and chlorine dioxide on Salmonella Typhimurium and Escherichia coli inactivation in poultry chiller tank water. Ultrason. Sonochem. 2021, 80, 105815.
- Liu, L.; Jia, W.; Xu, D.; Li, R. Applications of Ultrasonic Cutting in Food Processing. J. Food Process. Preserv. 2014, 39, 1762–1769.
- Yildiz, G.; Rababah, T.M.; Feng, H. Ultrasound-assisted cutting of cheddar, mozzarella and Swiss cheeses—Effects on quality attributes during storage. Innov. Food Sci. Emerg. Technol. 2016, 37, 1–9.
- Arnold, G.; Zahn, S.; Legler, A.; Rohm, H. Ultrasonic cutting of foods with inclined moving blades. J. Food Eng. 2011, 103, 394–400.
- McCulloch, E. Experimental and Finite Element Modelling of Ultrasonic Cutting of Food. Doctoral Dissertation, University of Glasgow, Glasgow, UK, 2008.
- Zahn, S.; Schneider, Y.; Rohm, H. Ultrasonic cutting of foods: Effects of excitation magnitude and cutting velocity on the reduction of cutting work. Innov. Food Sci. Emerg. Technol. 2006, 7, 288–293.
- Deng, L.-Z.; Mujumdar, A.S.; Zhang, Q.; Yang, X.-H.; Wang, J.; Zheng, Z.-A.; Gao, Z.-J.; Xiao, H.-W. Chemical and physical pretreatments of fruits and vegetables: Effects on drying characteristics and quality attributes—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1408–1432.
- Liu, Y.; Pu, H.; Sun, D.-W. Hyperspectral imaging technique for evaluating food quality and safety during various processes: A review of recent applications. Trends Food Sci. Technol. 2017, 69, 25–35.
- Mar, V.; Riera, E.; Pérez, G.; García-Pérez, J.V. The use of ultrasound for drying, degassing and defoaming of foods. Innov. Food Processing Technol. Compr. Rev. 2021, 415–438.
- Ortuño, C.; Pérez-Munuera, I.; Puig, A.; Riera, E.; Garcia-Perez, J. Influence of power ultrasound application on mass transport and microstructure of orange peel during hot air drying. Phys. Procedia 2010, 3, 153–159.
- Kowalski, S.J.; Pawłowski, A. Intensification of apple drying due to ultrasound enhancement. J. Food Eng. 2015, 156, 1–9.
- Kowalski, S.J.; Pawłowski, A.; Szadzińska, J.; Łechtańska, J.; Stasiak, M. High power airborne ultrasound assist in combined drying of raspberries. Innov. Food Sci. Emerg. Technol. 2016, 34, 225–233.
- Romero, J.C.; Yépez, V.B. Ultrasound as pretreatment to convective drying of Andean blackberry (Rubus glaucus Benth). Ultrason. Sonochem. 2015, 22, 205–210.
- Rojas, M.L.; Alvim, I.D.; Augusto, P.E.D. Incorporation of microencapsulated hydrophilic and lipophilic nutrients into foods by using ultrasound as a pre-treatment for drying: A prospective study. Ultrason. Sonochem. 2019, 54, 153–161.
- Mou, X.; Chen, Z. Study on the ultrasound-assisted drying process of deformable porous materials. J. Food Eng. 2021, 306, 110612.
- Wu, B.; Guo, X.; Guo, Y.; Ma, H.; Zhou, C. Enhancing jackfruit infrared drying by combining ultrasound treatments: Effect on drying characteristics, quality properties and microstructure. Food Chem. 2021, 358, 129845.
- Kek, S.; Chin, N.; Yusof, Y. Direct and indirect power ultrasound assisted pre-osmotic treatments in convective drying of guava slices. Food Bioprod. Process. 2013, 91, 495–506.
- Gamboa-Santos, J.; Montilla, A.; Cárcel, J.A.; Villamiel, M.; Garcia-Perez, J.V. Air-borne ultrasound application in the convective drying of strawberry. J. Food Eng. 2014, 128, 132–139.
- Kowalski, S.; Szadzińska, J. Convective-intermittent drying of cherries preceded by ultrasonic assisted osmotic dehydration. Chem. Eng. Process. Process. Intensif. 2014, 82, 65–70.
- do Nascimento, E.M.G.C.; Mulet, A.; Ascheri, J.L.R.; de Carvalho, C.W.P.; Cárcel, J.A. Effects of high-intensity ultrasound on drying kinetics and antioxidant properties of passion fruit peel. J. Food Eng. 2016, 170, 108–118.
- Wang, L.; Xu, B.; Wei, B.; Zeng, R. Low frequency ultrasound pretreatment of carrot slices: Effect on the moisture migration and quality attributes by intermediate-wave infrared radiation drying. Ultrason. Sonochem. 2018, 40, 619–628.
- Chen, Z.-G.; Guo, X.-Y.; Wu, T. A novel dehydration technique for carrot slices implementing ultrasound and vacuum drying methods. Ultrason. Sonochem. 2016, 30, 28–34.
- Zhou, C.-Y.; Xia, Q.; He, J.; Sun, Y.-Y.; Dang, Y.-L.; Ou, C.-R.; Pan, D.-D.; Cao, J.-X.; Zhou, G.-H. Improvement of ultrasound-assisted thermal treatment on organoleptic quality, rheological behavior and flavor of defective dry-cured ham. Food Biosci. 2021, 43, 101310.
- Abbaspour-Gilandeh, Y.; Kaveh, M.; Aziz, M. Ultrasonic-Microwave and Infrared Assisted Convective Drying of Carrot: Drying Kinetic, Quality and Energy Consumption. Appl. Sci. 2020, 10, 6309.
